Abstract
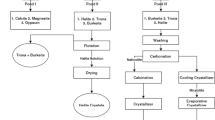
Desalination plants produce huge amounts of reject brine water (RBW), which are usually sent back to the sea, where they could, in the long run, result in detrimental effects on the aquatic life. The Phosphogypsum waste (PG) is a raw material, mostly discharged into the sea or deposited in large stockpiles without any pretreatment, which represents serious disposal problems with the phosphoric acid production units. This work tries to manage two types of wastes: (1) Reject brine water (RBW) from the sea water desalination plants and (2) Phosphogypsum waste (PG) from the H3PO4 production units, for commercial products preparation (such as Ca(OH)2, Na2SO4, and CaCO3), reduction in salinity of RBW and CO2 capture. Different alkali bases (Ca(OH)2 and NaOH) were dissolved into RBW solution under continuous CO2 flow bubbling. The structure and the morphology of all the samples and prepared materials were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM). The precipitated phases were calcite and a mixture of calcite and aragonite when we use Ca(OH)2 prepared from PG and pure Ca(OH)2, respectively. In addition, the treatment of RBW with these bases could minimize the salinity of the RBW to produce useful water for possible application in the agriculture especially in the irrigation operations. Treating RBW with Ca(OH)2 prepared from PG may reduce the salinity up to 19%, whereas this value decreased to 12 and 9% for commercial Ca(OH)2 and NaOH, respectively. This work was able to calculate the amount of CO2 removed as well as the cost analysis, salinity reduction, final products purity and the profits related to the extracted products. The process gain ratio (output cost/ input cost) was calculated as 1.66.
Graphic abstract


















Similar content being viewed by others
References
Herrero-Gonzalez M, Admon N, Dominguez-Ramos A, Ibañez R, Wolfson A, Irabien A (2020) Environmental sustainability assessment of seawater reverse osmosis brine valorization by means of electrodialysis with bipolar membranes. Environ Sci Pollut Res 27:1256–1266. https://doi.org/10.1007/s11356-019-04788-w
Gilron J, Folkman RY, Savliev R, Waisman M, Kedem O (2003) WAIV-wind aided intensified evaporation for desalination brine volume. Desalination 158:205–214
Sethi S, Walker S, Drewes J, Xu P (2006) Existing & emerging concentrate minimization & disposal practices for membrane systems. Water Resour, 40–45
Danoun R (2007) Desalination Plants: Potential impacts of brine discharge on marine life. Final Proj. Ocean Technol Gr, 59
Dawoud MA (2012) Environmental impacts of seawater desalination: Arabian Gulf Case Study. Int J Environ Sustain 1:22–37. https://doi.org/10.24102/ijes.v1i3.96
Lattemann S, Rodriguez SGS, Kennedy MD, Schippers JC, Amy GL (2012) Environmental and performance aspects of pretreatment and desalination technologies. In: Advances in Water Desalination, pp 79–195 (2012)
Dindi A, Quang DV, AlNashef I, Abu-Zahra MRM (2018) A process for combined CO2 utilization and treatment of desalination reject brine. Desalination 442:62–74. https://doi.org/10.1016/j.desal.2018.05.014
Elazhar F, Tahaikat M, El Azhar M, Jalili Z, Zouahri A, Hafsi M, Tahri K, El Midaoui A (2013) Performances of remineralization post for reverse osmosis (RO)-desalted water. Int J Chem Sci 11:1595–1606
Biyoune MG, Atbir A, Bari H, Mongach E, Khadir A, Hassnaoui L, Boukbir L, El Hadek M (2014) Reminéralisation de l’eau osmosée par l’ajout de la chaux Ca(OH)2 dans la station de dessalement de Laâyoune (SDL). J Mater Environ Sci 5:2359–2364
Biyoune MG, Atbir A, Mongach E, Bari H, Khadir A, Hasnaoui L, Boukbir L, Elhadek M (2015) Etude comparative entre trois méthodes de reminéralisation de l’eau osmosée des stations de dessalement et de déminéralisation au sud Marocain. J Mater Environ Sci 6:1243–1252
Biyoune, M.G., Bouargane, B., Bari, H., Marrouche, A., Bellajrou, R., Atbir, A., Boukbir, L., Mançour Billah, S.: Water quality depends on remineralization’s method in the desalination plant. Mediterr. J. Chem. 10, 162–170 (2020). https://doi.org/10.13171/mjc10202002141228mgb
Al-Subaie KZ (2007) Precise way to select a desalination technology. Desalination 206:29–35. https://doi.org/10.1016/j.desal.2006.04.049
Del Bene JV, Jirka G, Largier J (1994) Ocean brine disposal. Desalination 97:365–372. https://doi.org/10.1016/0011-9164(94)00100-6
Marrouche A, Biyoune MG, Mabrouk A, Bachar A, Atbir A, Boukbir L, Mançour-Billah S, Bari H, Bellajrou R, Aydda A, El Hadek M (2019) Evaluation of remineralization performance of osmosis water on the calcite bed at a laboratory scale. Desalin Water Treat 139:64–69. https://doi.org/10.5004/dwt.2019.23125
Ahmed M, Shayya WH, Hoey D, Al-Handaly J (2002) Brine disposal from inland desalination plants research needs assessment. Water Int 27:194–201. https://doi.org/10.1080/02508060208686992
El-Naas MH, Al-Marzouqi AH, Chaalal O (2010) A combined approach for the management of desalination reject brine and capture of CO2. Desalination 251:70–74. https://doi.org/10.1016/j.desal.2009.09.141
Doyle IMP, Dale G, Choi H, City B (2012) UnIted States Patent. 2, (2012). https://doi.org/10.1126/science.Liquids
Carson P (1994) Hazardous Chemicals Handbook, Chapter 14. Hazard. Chem. Handb
El-Naas MH, Mohammad AF, Suleiman MI, Al Musharfy M, Al-Marzouqi AH (2017) A new process for the capture of CO2 and reduction of water salinity. Desalination 411:69–75. https://doi.org/10.1016/j.desal.2017.02.005
Baena-Moreno FM, Vega F, Pastor-Pérez L, Reina TR, Navarrete B, Zhang Z (2020) Novel process for carbon capture and utilization and saline wastes valorization. J Nat Gas Sci Eng 73. https://doi.org/10.1016/j.jngse.2019.103071
Ahmad M, Garudachari B, Al-Wazzan Y, Kumar R, Thomas JP (2019) Mineral extraction from seawater reverse osmosis brine of gulf seawater. Desalin Water Treat 144:45–56. https://doi.org/10.5004/dwt.2019.23679
Davister A (1998) Phosphogypsum a waste (more or less harmful) or a resource. IFA Tech. Conf. Marrakech, Morocco
Vignes JL, Essaddam H, Daligand D (1997) Une vie de plâtre. Bull l’union des physiciens 790:145–164
El Zrelli R, Rabaoui L, Daghbouj N, Abda H, Castet S, Josse C, van Beek P, Souhaut M, Michel S, Bejaoui N, Courjault-Radé P (2018) Characterization of phosphate rock and phosphogypsum from Gabes phosphate fertilizer factories (SE Tunisia): high mining potential and implications for environmental protection. Environ Sci Pollut Res 25:14690–14702. https://doi.org/10.1007/s11356-018-1648-4
Kuryatnyk T, Angulski da Luz C, Ambroise J, Pera J (2008) Valorization of phosphogypsum as hydraulic binder. J Hazard Mater 160:681–687. https://doi.org/10.1016/j.jhazmat.2008.03.014
Luther SM, Dudas MJ, Rutherford PM (1993) Radioactivity and chemical characteristics of Alberta phosphogypsum. Water Air Soil Pollut 69:277–290. https://doi.org/10.1007/BF00478164
Szajerski P (2020) Distribution of uranium and thorium chains radionuclides in different fractions of phosphogypsum grains. Environ Sci Pollut Res 27:15856–15868. https://doi.org/10.1007/s11356-020-08090-y
Macías F, Pérez-López R, Cánovas CR, Carrero S, Cruz-Hernandez P (2017) Environmental Assessment and Management of Phosphogypsum According to European and United States of America Regulations. Procedia Earth Planet Sci 17:666–669. https://doi.org/10.1016/j.proeps.2016.12.178
Romero-Hermida I, Santos A, Pérez-López R, García-Tenorio R, Esquivias L, Morales-Flórez V (2017) New method for carbon dioxide mineralization based on phosphogypsum and aluminium-rich industrial wastes resulting in valuable carbonated by-products. J CO2 Util 18:15–22. https://doi.org/10.1016/j.jcou.2017.01.002
Contreras M, Pérez-López R, Gázquez MJ, Morales-Flórez V, Santos A, Esquivias L, Bolívar JP (2014) Fractionation and fluxes of metals and radionuclides during the recycling process of phosphogypsum wastes applied to mineral CO2 sequestration. Waste Manag 45:412–419. https://doi.org/10.1016/j.wasman.2015.06.046
Cárdenas-Escudero C, Morales-Flórez V, Pérez-López R, Santos A, Esquivias L (2011) Procedure to use phosphogypsum industrial waste for mineral CO2 sequestration. J Hazard Mater 196:431–435. https://doi.org/10.1016/j.jhazmat.2011.09.039
Fuleihan NF (2012) Phosphogypsum Disposal-The Pros & Cons of Wet Versus Dry Stacking. Procedia Eng 46:195–205
Kacimi L, Simon-Masseron A, Ghomari A, Derriche Z (2006) Reduction of clinkerization temperature by using phosphogypsum. J Hazard Mater 137:129–137. https://doi.org/10.1016/j.jhazmat.2005.12.053
Alcordo IS, Rechcigl JE (1993) Phosphogypsum in Agriculture: A Review. Adv Agron 49:55–118. https://doi.org/10.1016/S0065-2113(08)60793-2
Al Attar L, Al-Oudat M, Kanakri S, Budeir Y, Khalily H, Al Hamwi A (2011) Radiological impacts of phosphogypsum. J Environ Manage 92:2151–2158. https://doi.org/10.1016/j.jenvman.2011.03.041
Ueda S, Hatakeyama Y, Yamaguchi K, Nakamura I, Okura T (2006) Recovery of CaO and CaS from CaSO4 using waste plastics. JOURNAL-MINING Mater Process Inst JAPAN 122:456
Xue S, Li M, Jiang J, Millar GJ, Li C, Kong X (2019) Phosphogypsum stabilization of bauxite residue: Conversion of its alkaline characteristics. J Environ Sci (China) 77:1–10. https://doi.org/10.1016/j.jes.2018.05.016
Gu H, Guo T, Dai Y, Wang N (2018) Non-hazardous Treatment for Barium Slag Using Phosphogypsum. Waste and Biomass Valorization. https://doi.org/10.1007/s12649-018-0308-8
Boot-Handford ME, Abanades JC, Anthony EJ, Blunt MJ, Brandani S, Mac Dowell N, Fernández JR, Ferrari MC, Gross R, Hallett JP, Haszeldine RS, Heptonstall P, Lyngfelt A, Makuch Z, Mangano E, Porter RTJ, Pourkashanian M, Rochelle GT, Shah N, Yao JG, Fennell PS (2014) Carbon capture and storage update. Energy Environ Sci 7:130–189. https://doi.org/10.1039/c3ee42350f
Li H, Tang Z, Xing X, Guo D, Cui L, Mao X (2018) zhong: Study of CO2 capture by seawater and its reinforcement. Energy 164:1135–1144. https://doi.org/10.1016/j.energy.2018.09.066
Yu H, Gao XL, Su BW, Gao CJ (2012) A new method to remove the calcium and magnesium from the sea water with CO2. Adv Mater Res 361–363:990–995. https://doi.org/10.4028/www.scientific.net/AMR.361-363.990
Lee M gyu, Jang YN, Ryu K won, Kim W, Bang JH (2012) Mineral carbonation of flue gas desulfurization gypsum for CO2 sequestration. Energy. 47:370–377. https://doi.org/10.1016/j.energy.2012.09.009
Bouargane B, Marrouche A, Issiouy SE, Mabrouk A, Atbir A, Bachar A, Bellajrou R, Boukbir L, Bakiz B (2019) Recovery of Ca(OH)2, CaCO3, and Na2SO4 from Moroccan phosphogypsum waste. J Mater Cycles Waste Manag. https://doi.org/10.1007/s10163-019-00910-9
Altiner M (2018) Effect of alkaline types on the production of calcium carbonate particles from gypsum waste for fixation of CO2 by mineral carbonation. Int J Coal Prep Util 39:1–19. https://doi.org/10.1080/19392699.2018.1452739
Bouargane B, Biyoune MG, Mabrouk A, Bachar A, Bakiz B, Ait Ahsaine H, Mançour Billah S, Atbir A (2020) Experimental investigation of the effects of synthesis parameters on the Precipitation of calcium carbonate and portlandite from moroccan phosphogypsum and pure gypsum using carbonation route. Waste Biomass Valorization. https://doi.org/10.1007/s12649-019-00923-3
Haounati R, Ouachtak H, El Haouti R, Akhouairi S, Largo F, Akbal F, Benlhachemi A, Jada A, Addi AA (2021) Elaboration and properties of a new SDS/CTAB@Montmorillonite organoclay composite as a superb adsorbent for the removal of malachite green from aqueous solutions. Sep Purif Technol 255:117335. https://doi.org/10.1016/j.seppur.2020.117335
Largo F, Haounati R, Akhouairi S, Ouachtak H, El Haouti R, El Guerdaoui A, Hafid N, Santos DMF, Akbal F, Kuleyin A, Jada A, Addi AA (2020) Adsorptive removal of both cationic and anionic dyes by using sepiolite clay mineral as adsorbent: Experimental and molecular dynamic simulation studies. J Mol Liq 318 (2020). https://doi.org/10.1016/j.molliq.2020.114247
Marrouche A, Bouargane B, Mabrouk A, Biyoune MG, Bachar A, Atbir A, Boukbir L, Mançour-Billah S, Bellajrou R, El Hadek M (2019) Solubility in the ternary system MgCl2-FeCl2-H2O at 288 K by conductance method. Mediterr J Chem 8, 10. https://doi.org/10.13171/mjc811902523am
Ennaciri Y, Zdah I, Alaoui-belghiti HE, Alaoui-belghiti HE, Bettach M (2019) Characterization and purification of waste phosphogypsum to make it suitable for use in the plaster and the cement industry. Chem Eng Commun, 1–11. https://doi.org/10.1080/00986445.2019.1599865
Tamer N, Azouggaghoualen MD, Atbir A, Mançour-Billah S, Hadek M (2004) El: Evolution des rejets saumatres des stations de dessalement de l’ eau de mer au cours de leur evaporation isotherme. J Thermal Anal Calorim 77::789–801
Bouargane B, Marrouche A, Issiouy SE, Biyoune MG, Mabrouk A, Atbir A, Bachar A, Bellajrou R, Boukbir L, Bakiz B (2019) Recovery of Ca(OH)2, CaCO3, and Na2SO4 from Moroccan phosphogypsum waste. J Mater Cycles Waste Manag 21:1563–1571. https://doi.org/10.1007/s10163-019-00910-9
El Housse M, Abdallah H, Ilham K, Benaazza S, Belattar M. ’bare, Errami M, Mohareb S, Driouiche A (2021) Study of the effect of inorganic inhibitor on the calcium carbonate precipitation in the localized irrigation systems. Nanotechnol Environ Eng. 2:1–9. https://doi.org/10.1007/s41204-021-00107-2
Karmal I, Mohareb S, El housse M, Hafid N, Hadfi A, Belattar M, Ben-Aazza S, Addi AA, Akbour RA, Hamdani M, Driouiche A (2020) Structural and morphological characterization of scale deposits on the reverse osmosis membranes: Case of brackish water demineralization station in Morocco. Groundw Sustain Dev 11:100483. https://doi.org/10.1016/j.gsd.2020.100483
Pérez-Moreno SM, Gázquez MJ, Bolívar JP (2015) CO2 sequestration by indirect carbonation of artificial gypsum generated in the manufacture of titanium dioxide pigments. Chem Eng J 262:737–746. https://doi.org/10.1016/j.cej.2014.10.023
Rahmani O, Junin R, Tyrer M, Mohsin R (2014) Mineral carbonation of red gypsum for CO2 sequestration. Energy Fuels 28:5953–5958. https://doi.org/10.1021/ef501265z
Yadav VS, Prasad M, Khan J, Amritphale SS, Singh M, Raju CB (2010) Sequestration of carbon dioxide (CO2) using red mud. J Hazard Mater 176:1044–1050. https://doi.org/10.1016/j.jhazmat.2009.11.146
Acknowledgements
The authors would like to acknowledge the financial support provided by the University of Ibn Zohr, Faculty of sciences Agadir through the research project (2020). The authors wish to express their gratitude to Dr. Limame Barbouchi from Laayoun High school of technology (ESTL) for proofreading our manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed to the study conception and design (MGB, BB, and AI); material preparation and data collection (MGB, BB, and AI); data analysis (MGB, BB, and AI); commented on previous versions of the manuscript (AM, AA, and LB); and read and approved the final manuscript (AA and SM).
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Biyoune, M.G., Bouargane, B., Idboufrade, A. et al. New procedure for water-salinity reduction using Phosphogypsum waste and carbon dioxide resulting in useful compounds formation. Nanotechnol. Environ. Eng. 6, 33 (2021). https://doi.org/10.1007/s41204-021-00125-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41204-021-00125-0