Abstract
Introduction
In mammals, the central circadian clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus, which coordinates the circadian rhythm and controls locomotor activity rhythms. In addition to SCN cells, the peripheral tissues and embryonic fibroblasts also have clock genes, such as Per1/2 and Bmal1, which generate the transcriptional–translational feedback loop to produce an approximately 24-h cycle. Aging adversely affects the circadian clock system and locomotor functions. Oak extract has been reported to improve age-related physiological changes. However, no study has examined the effect of oak extract on the circadian clock system.
Methods
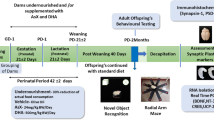
We examined the effects of oak extract and its metabolites (urolithin A [ULT] and ellagic acid [EA]) on clock gene expression rhythms in mouse embryonic fibroblasts (MEFs) and SCN. Furthermore, locomotor activity rhythm was assessed in young and aged mice.
Results
Chronic treatment with EA and ULT delayed the phase of PER2::LUC rhythms in SCN explants, and ULT prolonged the period of PER2::LUC rhythms in MEFs in a dose-dependent manner and increased the amplitude of PER2::LUC rhythms in MEFs, though only at low concentrations. Acute treatment with ULT affected the phase of PER2::LUC rhythms in MEFs depending on the concentration and timing of the treatment. In addition, oak extract prolonged the activity time of behavioral rhythms in old mice and tended to increase daily wheel-running revolutions in both young and old mice.
Conclusions
These results suggest that oak extract is a novel modulator of the circadian clock in vitro and in vivo.






Similar content being viewed by others
References
Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–54.
Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–6.
Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93:107–35.
Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–102.
Fustin JM, Doi M, Yamada H, et al. Rhythmic nucleotide synthesis in the liver: temporal segregation of metabolites. Cell Rep. 2012;1:341–9.
Haraguchi A, Fukuzawa M, Iwami S, et al. Night eating model shows time-specific depression-like behavior in the forced swimming test. Sci Rep. 2018;8:1081.
Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31.
Farajnia S, Deboer T, Rohling JH, et al. Aging of the suprachiasmatic clock. Neuroscientist. 2014;20:44–55.
Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51.
Nakamura TJ, Nakamura W, Yamazaki S, et al. Age-related decline in circadian output. J Neurosci. 2011;31:10201–5.
Nakamura TJ, Takasu NN, Nakamura W. The suprachiasmatic nucleus: age-related decline in biological rhythms. J Physiol Sci. 2016;66:367–74.
Tahara Y, Takatsu Y, Shiraishi T, et al. Age-related circadian disorganization caused by sympathetic dysfunction in peripheral clock regulation. NPJ Aging Mechanism Dis. 2017;3:16030.
Turek FW, Penev P, Zhang Y, et al. Effects of age on the circadian system. Neurosci Biobehav Rev. 1995;19:53–8.
Pittendrigh CS, Daan S. Circadian oscillations in rodents: a systematic increase of their frequency with age. Science. 1974;186:548–50.
Scarbrough K, Losee-Olson S, Wallen EP, Turek FW. Aging and photoperiod affect entrainment and quantitative aspects of locomotor behavior in Syrian hamsters. Am J Physiol. 1997;272:R1219–25.
Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol. 1997;273:R1957–64.
Zhang Y, Kornhauser JM, Zee PC, et al. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience. 1996;70:951–61.
Kallio T, Kallio J, Jaakkola M, et al. Urolithins display both antioxidant and pro-oxidant activities depending on assay system and conditions. J Agric Food Chem. 2013;61:10720–9.
Tomás-Barberán FA, González-Sarrías A, García-Villalba R, et al. Urolithins, the rescue of “old” metabolites to understand a “new” concept: metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol Nutr Food Res. 2017; 61.
Cerdá B, Llorach R, Cerón JJ, et al. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr. 2003;42:18–28.
Jordão JBR, Porto HKP, Lopes FM, et al. Protective effects of ellagic acid on cardiovascular injuries caused by hypertension in rats. Planta Med. 2017;83:830–6.
Ryu D, Mouchiroud L, Andreux PA, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22:879–88.
Yoo S-H, Yamazaki S, Lowrey PL, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–46.
Fukuda T, Haraguchi A, Kuwahara M, et al. l-Ornithine affects peripheral clock gene expression in mice. Sci Rep. 2016;6:34665.
Sugiyama M, Nishijima I, Miyazaki S, Nakamura TJ. Secretin receptor-deficient mice exhibit altered circadian rhythm in wheel-running activity. Neurosci Lett. 2020;722:134814.
Hashimoto A, Fujiki S, Nakamura W, Nakamura TJ. Effects of testosterone on circadian rhythmicity in old mice. J Physiol Sci. 2019;69:791–8.
Satou R, Shibukawa Y, Kimura M, Sugihara N. Light conditions affect rhythmic expression of aquaporin 5 and anoctamin 1 in rat submandibular glands. Heliyon. 2019;5: e02792.
Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–64.
Mizoro Y, Yamaguchi Y, Kitazawa R, et al. Activation of AMPA receptors in the suprachiasmatic nucleus phase-shifts the mouse circadian clock in vivo and in vitro. PLoS ONE. 2010;5: e10951.
Shigeyoshi Y, Taguchi K, Yamamoto S, et al. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–53.
Furuta S, Kuwahara R, Hiraki E, et al. Hericium erinaceus extracts alter behavioral rhythm in mice. Biomed Res. 2016;37:227–32.
Kang I, Kim Y, Tomás-Barberán FA, et al. Urolithin A, C, and D, but not iso-urolithin A and urolithin B, attenuate triglyceride accumulation in human cultures of adipocytes and hepatocytes. Mol Nutr Food Res. 2016;60:1129–38.
Lamia KA, Sachdeva UM, DiTacchio L, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–40.
Velagapudi R, Lepiarz I, El-Bakoush A, et al. Induction of autophagy and activation of SIRT-1 deacetylation mechanisms mediate neuroprotection by the pomegranate metabolite urolithin A in BV2 microglia and differentiated 3D human neural progenitor cells. Mol Nutr Food Res. 2019;63: e1801237.
Asher G, Gatfield D, Stratmann M, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–28.
Tamai TK, Nakane Y, Ota W, et al. Identification of circadian clock modulators from existing drugs. EMBO Mol Med. 2018;10: e8724.
Oike H, Kobori M, Suzuki T, Ishida N. Caffeine lengthens circadian rhythms in mice. Biochem Biophys Res Commun. 2011;410:654–8.
Belcaro G, Saggino A, Cornelli U, et al. Improvement in mood, oxidative stress, fatigue, and insomnia following supplementary management with Robuvit®. J Neurosurg Sci. 2018;62:423–7.
Acknowledgements
This work was supported by JSPS KAKENHI [grant number 17H04022 (T.J.N.)]. We would like to thank Editage (www.editage.com) for English language editing.
Funding
Japan Society for the Promotion of Science, 17H04022, Takahiro Nakamura.
Author information
Authors and Affiliations
Contributions
AH, TJN, and SS conceived and designed the study; AH, YD, RS, YT, and TJN performed the experiments; AH, YD, RS, YT, and TJN analyzed the data; AH, TJN, and SS wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest concerning this article.
Ethical approval
Appropriate approvals for the use of animals in the described experiments have been obtained from the Institutional Animal Care and Use Committee at the School of Agriculture, Meiji University (approval number: IACUC16-0012).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haraguchi, A., Du, Y., Shiraishi, R. et al. Oak extracts modulate circadian rhythms of clock gene expression in vitro and wheel-running activity in mice. Sleep Biol. Rhythms 20, 255–266 (2022). https://doi.org/10.1007/s41105-021-00365-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-021-00365-2