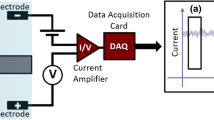
Abstract
Solid-state nanopores/nanochannels, with their high stability, tunable geometry, and controllable surface chemistry, have recently become an important tool for constructing biosensors. Compared with traditional biosensors, biosensors constructed with solid-state nanopores/nanochannels exhibit significant advantages of high sensitivity, high specificity, and high spatiotemporal resolution in the detection single entities (such as single molecules, single particles, and single cells) due to their unique nanoconfined space-induced target enrichment effect. Generally, the solid-state nanopore/nanochannel modification method is the inner wall modification, and the detection principles are the resistive pulse method and the steady-state ion current method. During the detection process, solid-state nanopore/nanochannel is easily blocked by single entities, and interfering substances easily enter the solid-state nanopore/nanochannel to generate interference signals, resulting in inaccurate measurement results. In addition, the problem of low flux in the detection process of solid-state nanopore/nanochannel, these defects limit the application of solid-state nanopore/nanochannel. In this review, we introduce the preparation and functionalization of solid-state nanopore/nanochannel, the research progress in the field of single entities sensing, and the novel sensing strategies on solving the above problems in solid-state nanopore/nanochannel single-entity sensing. At the same time, the challenges and prospects of solid-state nanopore/nanochannel for single-entity electrochemical sensing are also discussed.
Graphical Abstract







Similar content being viewed by others
Data availability
Data is available when requested.
References
Bayat P, Rambaud C, Priem B, Bourderioux M, Bilong M, Poyer S, Pastoriza Gallego M, Oukhaled A, Mathé J, Daniel R (2022) Comprehensive structural assignment of glycosaminoglycan oligo-and polysaccharides by protein nanopore. Nat Commun 13:5113. https://doi.org/10.1038/s41467-022-32800-4
Fu J, Wu L, Hu G, Li F, Ge Q, Lu Z, Tu J (2022) Solid-state nanopore analysis on conformation change of DNA polymerase I induced by DNA substrate. Analyst 147:3087–3095. https://doi.org/10.1039/D2AN00567K
Li J, Li M, Li X, Wu X, Ying Y, Long Y (2022) Full width at half maximum of nanopore current blockage controlled by a single-biomolecule interface. Langmuir 38:1188–1193. https://doi.org/10.1021/acs.langmuir.1c02900
Wang H, Tang H, Li Y (2021) Intrinsic electrocatalytic activity of single MoS2 quantum dot collision on Ag ultramicroelectrodes. J Phys Chem C 125:3337–3345. https://doi.org/10.1021/acs.jpcc.0c09644
Zhao H, Ma J, Zuo X, Li F (2021) Electrochemical analysis for multiscale single entities on the confined interface. Chin J Chem 39:1745–1752. https://doi.org/10.1002/cjoc.202000722
Huang W, Chen Y, Wu L, Long M, Lin Z, Su Q, Zheng F, Wu S, Li H, Yu G (2022) 3D co-doped Ni-based conductive MOFs modified electrochemical sensor for highly sensitive detection of l-tryptophan. Talanta 247:123596. https://doi.org/10.1016/j.talanta.2022.123596
Song J, Lin X, Jiang N, Huang M (2022) Carbon-doped WO3 electrochemical aptasensor based on Box-Behnken strategy for highly-sensitive detection of tetracycline. Food Chem 367:130564. https://doi.org/10.1016/j.foodchem.2021.130564
Ma H, Yu R, Ying Y, Long Y (2022) Electrochemically confined effects on single enzyme detection with nanopipettes. J Electroanal Chem 908:116086. https://doi.org/10.1016/j.jelechem.2022.116086
Liu R, Wang D (2022) Pressure-regulated single-entity electrochemistry inside carbon nanopipettes. ACS Sens 7:1138–1144. https://doi.org/10.1021/acssensors.2c00143
Wang J, Zhou Y, Jiang L (2021) Bio-inspired track-etched polymeric nanochannels: steady-state biosensors for detection of analytes. ACS Nano 15:18974–19013. https://doi.org/10.1021/acsnano.1c08582
Zhang R, Liu X, Zeng Q, Shen H, Wang L (2022) Studies on the morphology effect on catalytic ability of a single MnO2 catalyst particle with a solid nanopipette. ACS Sens 7:338–344. https://doi.org/10.1021/acssensors.1c02729
Lu J, Jiang Y, Yu P, Jiang W, Mao L (2022) Light-controlled ionic/molecular transport through solid-state nanopores and nanochannels. Chem Asian J 17:e202200158. https://doi.org/10.1002/asia.202200158
Ryuzaki S, Yasui T, Tsutsui M, Yokota K, Komoto Y, Paisrisarn P, Kaji N, Ito D, Tamada K, Ochiya T, Taniguchi M, Baba Y, Kawai T (2021) Rapid discrimination of extracellular vesicles by shape distribution analysis. Anal Chem 93:7037–7044. https://doi.org/10.1021/acs.analchem.1c00258
Chung NX, Gatty HK, Lu X, Zhang M, Linnros J (2021) Optimized electrochemical breakdown etching using temporal voltage variation for formation of nanopores in a silicon membrane. Sens Actuators B 331:129323. https://doi.org/10.1016/j.snb.2020.129323
Wei G, Hu R, Li Q, Lu W, Liang H, Nan H, Lu J, Li J, Zhao Q (2022) Oligonucleotide discrimination enabled by tannic acid-coordinated film-coated solid-state nanopores. Langmuir 38:6443–6453. https://doi.org/10.1021/acs.langmuir.2c00638
Cayon VM, Laucirica G, Toum TY, Cortez ML, Perez-Mitta G, Shen J, Hess C, Toimil-Molares ME, Trautmann C, Marmisolle WA, Azzaroni O (2021) Borate-driven ionic rectifiers based on sugar-bearing single nanochannels. Nanoscale 13:11232–11241. https://doi.org/10.1039/d0nr07733j
Zhang Y, Ma D, Gu Z, Zhan L, Sha J (2021) Fast fabrication of solid-state nanopores for DNA molecule analysis. Nanomaterials 11:2450. https://doi.org/10.3390/nano11092450
Kishimoto S, Leong IW, Murayama S, Nakada T, Komoto Y, Tsutsui M, Taniguchi M (2022) 3D designing of resist membrane pores via direct electron beam lithography. Sens Actuators B 357:131380. https://doi.org/10.1016/j.snb.2022.131380
Ritt CL, de Souza JP, Barsukov MG, Yosinski S, Bazant MZ, Reed MA, Elimelech M (2022) Thermodynamics of charge regulation during ion transport through silica nanochannels. ACS Nano 16:15249–15260. https://doi.org/10.1021/acsnano.2c06633
Zhang Y, Chen X, Wang C, Chang H, Guan X (2022) Nanoparticle-assisted detection of nucleic acids in a polymeric nanopore with a large pore size. Biosens Bioelectron 196:113697. https://doi.org/10.1016/j.bios.2021.113697
Dutt S, Apel P, Lizunov N, Notthoff C, Wen Q, Trautmann C, Mota-Santiago P, Kirby N, Kluth P (2021) Shape of nanopores in track-etched polycarbonate membranes. J Membr Sci 638:119681. https://doi.org/10.1016/j.memsci.2021.119681
Xiong Y, Li M, Lu W, Wang D, Tang M, Liu Y, Na B, Qin H, Qing G (2021) Discerning tyrosine phosphorylation from multiple phosphorylations using a nanofluidic logic platform. Anal Chem 93:16113–16122. https://doi.org/10.1021/acs.analchem.1c03889
Gong J, Zhang T, Chen P, Yan F, Liu J (2022) Bipolar silica nanochannel array for dual-mode electrochemiluminescence and electrochemical immunosensing platform. Sens Actuators B 368:132086. https://doi.org/10.1016/j.snb.2022.132086
Xia X, Li H, Zhou G, Ge L, Li F (2020) In situ growth of nano-gold on anodized aluminum oxide with tandem nanozyme activities towards sensitive electrochemical nanochannel sensing. Analyst 145:6617–6624. https://doi.org/10.1016/j.snb.2022.132086
Fei W, Xue M, Qiu H, Guo W (2019) Heterogeneous graphene oxide membrane for rectified ion transport. Nanoscale 11:1313–1318. https://doi.org/10.1039/c8nr07557c
Jia P, Wang L, Zhang Y, Yang Y, Jin X, Zhou M, Quan D, Jia M, Cao L, Long R, Jiang L, Guo W (2021) Harnessing ionic power from equilibrium electrolyte solution via photoinduced active ion transport through van-der-waals-like heterostructures. Adv Mater 33:2007529. https://doi.org/10.1002/adma.202007529
Qin S, Liu D, Wang G, Portehault D, Garvey CJ, Gogotsi Y, Lei W, Chen Y (2017) High and stable ionic conductivity in 2D nanofluidic ion channels between boron nitride layers. J Am Chem Soc 139:6314–6320. https://doi.org/10.1021/jacs.6b11100
Lao J, Zhou K, Pan S, Luo J, Gao J, Dong A, Jiang L (2022) Spontaneous and selective potassium transport through a suspended tailor-cut Ti3C2Tx MXene film. ACS Nano 16:9142–9149. https://doi.org/10.1021/acsnano.2c01304
Yang M, Ma C, Ding S, Zhu Y, Shi G, Zhu A (2019) Rational design of stimuli-responsive polymers modified nanopores for selective and sensitive determination of salivary glucose. Anal Chem 91:14029–14035. https://doi.org/10.1021/acs.analchem.9b03646
Chen X, Zhao X, Ma R, Hu Y, Cui C, Mi Z, Dou R, Pan D, Shan X, Wang L, Fan C, Lu X (2022) Ionic current fluctuation and orientation of tetrahedral DNA nanostructures in a solid-state nanopore. Small 18:2107237. https://doi.org/10.1002/smll.202107237
Kant K, Priest C, Shapter JG, Losic D (2014) The influence of nanopore dimensions on the electrochemical properties of nanopore arrays studied by impedance spectroscopy. Sensors 14:21316–21328. https://doi.org/10.3390/s141121316
Wang C, Jin D, Yu Y, Tang L, Sun Y, Sun Z, Zhang G (2020) A dual antibody-modified nanochannel biosensor for capture and identification of exosomes. Sens Actuators B 314:128056. https://doi.org/10.1016/j.snb.2020.128056
Pardehkhorram R, Andrieu-Brunsen A (2022) Pushing the limits of nanopore transport performance by polymer functionalization. Chem Commun 58:5188–5204. https://doi.org/10.1039/d2cc01164f
Xiao K, Xie G, Li P, Liu Q, Hou GL, Zhang Z, Ma J, Tian Y, Wen LP, Jiang L (2014) A biomimetic multi-stimuli-response ionic gate using a hydroxypyrene derivation-functionalized asymmetric single nanochannel. Adv Mater 26:6560–6565. https://doi.org/10.1002/adma.201402247
Xiao Z, Huang C, Jiang S, Kong X, Teng Y, Niu B, Zhu C, Xin W, Chen X, Wen L, Wei Y, Deng X (2021) Ultra-sensitive and selective electrochemical bio-fluid biopsy for oral cancer screening. Small Methods 5:2001205. https://doi.org/10.1002/smtd.202001205
Hu J, Jiang W, Chen Q, Liu R, Lou X, Xia F (2021) Solid-state nanochannel with multiple signal outputs for furin detection based on the biocompatible condensation reaction. Anal Chem 93:14036–14041. https://doi.org/10.1021/acs.analchem.1c03727
Ishizaki Y, Yamamoto S, Miyashita T, Mitsuishi M (2021) pH-responsive ultrathin nanoporous SiO2 films for selective ion permeation. Langmuir 37:5627–5634. https://doi.org/10.1021/acs.langmuir.1c00486
Li X, Zhai T, Gao P, Cheng H, Hou R, Lou X, Xia F (2018) Role of outer surface probes for regulating ion gating of nanochannels. Nat Commun 9:40. https://doi.org/10.1038/s41467-017-02447-7
Harrell CC, Kohli P, Siwy Z, Martin CR (2004) DNA-nanotube artificial ion channels. J Am Chem Soc 126:15646–15647. https://doi.org/10.1021/ja044948v
Acar ET, Buchsbaum SF, Combs C, Fornasiero F, Siwy ZS (2019) Biomimetic potassium-selective nanopores. Sci Adv 5:2568. https://doi.org/10.1126/sciadv.aav2568
Tang H, Wang H, Zhao D, Cao M, Zhu Y, Li Y (2022) Nanopore-based single-entity electrochemistry for the label-free monitoring of single-molecule glycoprotein-boronate affinity interaction and its sensing application. Anal Chem 94:5715–5722. https://doi.org/10.1021/acs.analchem.2c00860
Qin H, Ding X, Cheng S, Qin S, Han X, Sun Y, Liu Y (2022) An H2S-regulated artificial nanochannel fabricated by a supramolecular coordination strategy. J Phys Chem Lett 13:9232–9237. https://doi.org/10.1021/acs.jpclett.2c02233
Ma Q, Si Z, Li Y, Wang D, Wu X, Gao P, Xia F (2019) Functional solid-state nanochannels for biochemical sensing. Trends Anal Chem 115:174–186. https://doi.org/10.1016/j.trac.2019.04.014
Lenart WR, Kong W, Oltjen WC, Hore MJ (2019) Translocation of soft phytoglycogen nanoparticles through solid-state nanochannels. J Mater Chem B 7:6428–6437. https://doi.org/10.1039/c9tb01048c
Yi W, Li X, He X, Yue F, Wang T (2022) Glass nanopipette sensing of single entities. J Electroanal Chem 909:116106. https://doi.org/10.1016/j.jelechem.2022.116106
Zhou Y, Wang D, Li C, Hu P, Jin Y (2019) Resistive-pulse sensing and surface charge analysis of a single nanoparticle collision at a conical glass nanopore. Anal Chem 91:7648–7653. https://doi.org/10.1021/acs.analchem.9b00553
Li T, He X, Zhang K, Wang K, Yu P, Mao L (2016) Observing single nanoparticle events at the orifice of a nanopipet. Chem Sci 7:6365–6368. https://doi.org/10.1039/c6sc02241c
Zhang S, Shi W, Li K, Han D, Xu J (2022) Ultrasensitive and label-free detection of multiple DNA methyltransferases by asymmetric nanopore biosensor. Anal Chem 94:4407–4416. https://doi.org/10.1021/acs.analchem.1c05332
Xiao P, Wan Q, Liao T, Tu J, Zhang G, Sun Z (2021) Peptide nucleic acid-functionalized nanochannel biosensor for the highly sensitive detection of tumor exosomal microRNA. Anal Chem 93:10966–10973. https://doi.org/10.1021/acs.analchem.1c01898
Gao P, Wang D, Che C, Ma Q, Wu X, Chen Y, Xu H, Li X, Lin Y, Ding D, Lou X, Xia F (2021) Regional and functional division of functional elements of solid-state nanochannels for enhanced sensitivity and specificity of biosensing in complex matrices. Nat Protoc 16:4201–4226. https://doi.org/10.1038/s41596-021-00574-6
Liu L, Luo C, Zhang J, He X, Shen Y, Yan B, Huang Y, Xia F, Jiang L (2022) Synergistic effect of bio-inspired nanochannels: hydrophilic DNA probes at inner wall and hydrophobic coating at outer surface for highly sensitive detection. Small 18:2201925. https://doi.org/10.1002/smll.202201925
Gao P, Ma Q, Ding D, Wang D, Lou X, Zhai T, Xia F (2018) Distinct functional elements for outer-surface anti-interference and inner-wall ion gating of nanochannels. Nat Commun 9:4557. https://doi.org/10.1038/s41467-018-06873-z
Liu T, Wu X, Xu H, Ma Q, Du Q, Yuan Q, Gao P, Xia F (2021) Revealing ionic signal enhancement with probe grafting density on the outer surface of nanochannels. Anal Chem 93:13054–13062. https://doi.org/10.1021/acs.analchem.1c03010
Mao H, Ma Q, Xu H, Xu L, Du Q, Gao P, Xia F (2021) Exploring the contribution of charged species at the outer surface to the ion current signal of nanopores: a theoretical study. Analyst 146:5089–5094. https://doi.org/10.1039/d1an00826a
King S, Briggs K, Slinger R, Tabard-Cossa V (2022) Screening for group a streptococcal disease via solid-state nanopore detection of PCR amplicons. ACS Sens 7:207–214. https://doi.org/10.1021/acssensors.1c01972
Duleba D, Johnson RP (2022) Sensing with ion current rectifying solid-state nanopores. Curr Opin Electrochem 34:100989. https://doi.org/10.1016/j.coelec.2022.100989
Zhang J, Zhang L, Li Z, Zhang Q, Li Y, Ying Y, Fu Y (2021) Nanoconfinement effect for signal amplification in electrochemical analysis and sensing. Small 17:2101665. https://doi.org/10.1002/smll.202101665
Wu Y, Gooding JJ (2022) The application of single molecule nanopore sensing for quantitative analysis. Chem Soc Rev 51:3862–3885. https://doi.org/10.1039/d1cs00988e
Hu R, Tong X, Zhao Q (2020) Four aspects about solid-state nanopores for protein sensing: fabrication, sensitivity, selectivity, and durability. Adv Healthc Mater 9:2000933. https://doi.org/10.1002/adhm.202000933
Lou X, Song Y, Liu R, Cheng Y, Dai J, Chen Q, Gao P, Zhao Z, Xia F (2019) Enzyme and AIEgens modulated solid-state nanochannels: in situ and noninvasive monitoring of H2O2 released from living cells. Small Methods 4:1900432. https://doi.org/10.1002/smtd.201900432
Taniguchi M, Takei H, Tomiyasu K, Sakamoto O, Naono N (2022) Sensing the performance of artificially intelligent nanopores developed by integrating solid-state nanopores with machine learning methods. J Phys Chem C 126:12197–12209. https://doi.org/10.1021/acs.jpcc.2c02674
Hayashida T, Tsutsui M, Murayama S, Nakada T, Taniguchi M (2021) Dielectric coatings for resistive pulse sensing using solid-state pores. ACS Appl Mater Interfaces 13:10632–10638. https://doi.org/10.1021/acsami.0c22548
Zhou J, Zlotnick A, Jacobson SC (2022) Disassembly of single virus capsids monitored in real time with multicycle resistive-pulse sensing. Anal Chem 94:985–992. https://doi.org/10.1021/acs.analchem.1c03855
Liu Y, Xu C, Chen X, Wang J, Yu P, Mao L (2018) Voltage-driven counting of phospholipid vesicles with nanopipettes by resistive-pulse principle. Electrochem Commun 89:38–42. https://doi.org/10.1016/j.elecom.2018.02.015
Si W, Sha J, Sun Q, He Z, Wu L, Chen C, Yu S, Chen Y (2020) Shape characterization and discrimination of single nanoparticles using solid-state nanopores. Analyst 145:1657–1666. https://doi.org/10.1039/c9an01889a
Wang HW, Lu SM, Chen M, Long YT (2022) Optical-facilitated single-entity electrochemistry. Curr Opin Electrochem 34:100999. https://doi.org/10.1016/j.coelec.2022.100999
Ding S, Liu C, Fu D, Shi G, Zhu A (2021) Coordination of ligand-protected metal nanoclusters and glass nanopipettes: conversion of a liquid-phase fluorometric assay into an enhanced nanopore analysis. Anal Chem 93:1779–1785. https://doi.org/10.1021/acs.analchem.0c04620
Cai S, Sze JY, Ivanov AP, Edel JB (2019) Small molecule electro-optical binding assay using nanopores. Nat Commun 10:1797. https://doi.org/10.1038/s41467-019-09476-4
Cai S, Pataillot-Meakin T, Shibakawa A, Ren R, Bevan CL, Ladame S, Ivanov AP, Edel JB (2021) Single-molecule amplification-free multiplexed detection of circulating microRNA cancer biomarkers from serum. Nat Commun 12:3515. https://doi.org/10.1038/s41467-021-23497-y
Ren R, Sun M, Goel P, Cai S, Kotov NA, Kuang H, Xu C, Ivanov AP, Edel JB (2021) Single-molecule binding assay using nanopores and dimeric NP conjugates. Adv Mater 33:2103067. https://doi.org/10.1002/adma.202103067
Wang L, Ma Y, Wang L (2021) High selectivity sensing of bovine serum albumin: the combination of glass nanopore and molecularly imprinted technology. Biosens Bioelectron 178:113056. https://doi.org/10.1016/j.bios.2021.113056
Tang H, Wang H, Yang C, Zhao D, Qian Y, Li Y (2020) Nanopore-based strategy for selective detection of single carcinoembryonic antigen(CEA) molecules. Anal Chem 92:3042–3049. https://doi.org/10.1021/acs.analchem.9b04185
Wang H, Tang H, Yang C, Li Y (2019) Selective single molecule nanopore sensing of microRNA using PNA functionalized magnetic core-shell Fe3O4-Au nanoparticles. Anal Chem 91:7965–7970. https://doi.org/10.1021/acs.analchem.9b02025
Yi W, Xu C, Xiong T, Gao T, Yu P, He X, Mao L (2020) Label-free analysis of adsorbed protein heterogeneity on individual particles, based on single particle collision events. Electrochem Commun 111:106666. https://doi.org/10.1016/j.elecom.2020.106666
Liu Y, Xu C, Gao T, Chen X, Wang J, Yu P, Mao L (2020) Sizing single particles at the orifice of a nanopipette. ACS Sens 5:2351–2358. https://doi.org/10.1021/acssensors.9b02520
Gao T, Gao X, Xu C, Wang M, Chen M, Wang J, Ma F, Yu P, Mao L (2021) Label-free resistance cytometry at the orifice of a nanopipette. Anal Chem 93:2942–2949. https://doi.org/10.1021/acs.analchem.0c04585
Gao T, Xu C, Chen M, Wang J, Mao L, Yu P (2022) Insights into surface charge of single particles at the orifice of a nanopipette. Anal Chem 94:8187–8193. https://doi.org/10.1021/acs.analchem.1c05579
Soozanipour A, Sohrabi H, Abazar F, Khataee A, Noorbakhsh A, Asadnia M, Taheri Kafrani A, Majidi MR, Razmjou A (2021) Ion selective nanochannels: from critical principles to sensing and biosensing applications. Adv Mater Technol 6:2000765. https://doi.org/10.1002/admt.202000765
Ma T, Janot JM, Balme S (2020) Track-etched nanopore/membrane: from fundamental to applications. Small Methods 4:2000366. https://doi.org/10.1002/smtd.202000366
Ma Q, Liu T, Xu R, Du Q, Gao P, Xia F (2021) Revealing the critical role of probe grafting density in nanometric confinement in ionic signal via an experimental and theoretical study. Anal Chem 93:1984–1990. https://doi.org/10.1021/acs.analchem.0c03090
Wang D, Qi G, Zhou Y, Zhang Y, Wang B, Hu P, Jin Y (2020) Single-cell ATP detection and content analyses in electrostimulusInduced apoptosis by functionalized glass nanopipette. Chem Commun 56:1561–1564. https://doi.org/10.1039/C9CC08889J
Zhao XP, Cao J, Nie XG, Wang SS, Wang C, Xia XH (2017) Label-free monitoring of the thrombin-aptamer recognition reaction using an array of nanochannels coupled with electrochemical detection. Electrochem Commun 81:5–9. https://doi.org/10.1016/j.elecom.2017.05.018
Zhang X, Zhang L, Li J (2019) Peptide-modified nanochannel system for carboxypeptidase B activity detection. Anal Chim Acta 1057:36–43. https://doi.org/10.1016/j.aca.2019.01.018
Shi L, Wang L, Ma X, Jalalah M, Alsareii SA, Gao T, Harraz FA, Li G (2021) Electrochemical trans-channel assay for efficient evaluation of tumor cell invasiveness. ACS Appl Mater Interfaces 13:17268–17275. https://doi.org/10.1021/acsami.1c01236
Zhang K, Xiong T, Wu F, Yue Q, Ji W, Yu P, Mao L (2020) Real-time and in-situ intracellular ATP assay with polyimidazolium brush-modified nanopipette. Sci China Chem 63:1004–1011. https://doi.org/10.1007/s11426-020-9715-x
Hu P, Zhang Y, Wang D, Qi G, Jin Y (2021) Glutathione content detection of single cells under ingested doxorubicin by functionalized glass nanopores. Anal Chem 93:4240–4245. https://doi.org/10.1021/acs.analchem.0c05004
Guo J, Yang L, Xu H, Zhao C, Dai Z, Gao Z, Song Y (2019) Biomineralization-driven ion gate in TiO2 nanochannel arrays for cell H2S sensing. Anal Chem 91:13746–13751. https://doi.org/10.1021/acs.analchem.9b03119
Ran X, Qian H, Yan X (2021) Aptamer self-assembly-functionalized nanochannels for sensitive and precise detection of chloramphenicol. Anal Chem 93:14287–14292. https://doi.org/10.1021/acs.analchem.1c03396
Zhao X, Liu F, Hu W, Younis MR, Wang C, Xia X (2019) Biomimetic nanochannel-ionchannel hybrid for ultrasensitive and label-free detection of microRNA in cells. Anal Chem 91:3582–3589. https://doi.org/10.1021/acs.analchem.8b05536
Wang C, Fu Q, Wang X, Kong D, Sheng Q, Wang Y, Chen Q, Xue J (2015) Atomic layer deposition modified track-etched conical nanochannels for protein sensing. Anal Chem 87:8227–8233. https://doi.org/10.1021/acs.analchem.5b01501
Ma Q, Li Y, Wang R, Xu H, Du Q, Gao P, Xia F (2021) Towards explicit regulating-ion-transport: nanochannels with only function-elements at outer-surface. Nat Commun 12:1573. https://doi.org/10.1038/s41467-021-21507-7
Huang Y, Xue H, Lou X, Xia F (2021) Precise measurement of single molecule and single cell based on nanopores/nanochannels’ charge transfer. Sci Bull 66:1599–1600. https://doi.org/10.1016/j.scib.2021.03.011
Acknowledgements
This work was supported by the National Key Research and Development Project from the Ministry of Science and Technology of China (2021YFE0191500), the National Natural Science Foundation of China (21875203), the Scientific Research Fund of Hunan Provincial Education Department (20A490), the Scientific Research Project of Colleges and Universities of the Guizhou Provincial Education Department (Youth Project) (2022396), and the Science and Technology Planning Project of the Science and Technology Bureau of Qianxinan Prefecture (2022-1-20).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yi, W., Zhang, C., Zhang, Q. et al. Solid-State Nanopore/Nanochannel Sensing of Single Entities. Top Curr Chem (Z) 381, 13 (2023). https://doi.org/10.1007/s41061-023-00425-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-023-00425-w