Abstract
Manganese(III) porphyrin complexes with various metal-containing/non-metal bridges reported during the past two decades, including their structural characteristics and magnetic properties, are summarized. As the porphyrin ligands usually adopt a planar chelate form, it is possible that the porphyrin-based complexes, being a coordination-acceptor building block, have two coordination labile sites in trans positions. In particular, the coordination labile sites in an octahedral field face the direction of the Jahn–Teller elongated axis occupying the dz2 orbital. As a result of this characteristic orbital arrangement, the activity and magnetic-electronic properties of the manganese complexes can be tuned by modulating the porphyrin ligand, which is equatorially located around the manganese ion and coupled with the dx2−y2 orbital. The high-spin Mn(III) porphyrin complexes (S = 2) display strong magnetic uniaxial anisotropy with the Jahn–Teller axis as the magnetic easy axis. So far, various manganese(III) porphyrin magnetism systems, including multinuclear clusters, one-dimensional chains, and two- or three-dimensional networks, have been designed and structurally and magnetically characterized. This review shows that the manganese(III) porphyrin complexes have potential as versatile sources for the design of unique magnetic materials as well as other molecular functional materials with various structures.

Adapted from Ref. [29]

Adapted from Ref. [31]

Adapted from Ref. [32]

Reproduced with slight modification and permission from Ref. [36]. Copyright 2009 Elsevier B.V.

Reproduced with permission from Ref. [37]. Copyright 2009 ACS

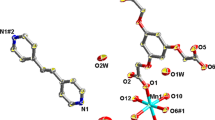
Reproduced with slight modification and permission from Ref. [39]. Copyright 2012 Elsevier B.V




Adapted from Ref. [64]. Copyright 2000 Wiley

Adapted from Ref. [66]. Copyright 2001 RSC

Adapted from Ref. [68]. Copyright 2012 ACS

Adapted from Ref. [74]. Copyright 2008 ACS

Reproduced with slight modification and permission from Ref. [74]. Copyright 2008 ACS

Reproduced with permission from Ref. [74]. Copyright 2008 ACS

Adapted from Ref. [75]

Reproduced with slight modification and permission from Ref. [76]. Copyright 2005 ACS

Adapted from Ref. [77]. Copyright 2010 RSC

Reproduced with permission from Ref. [77]. Copyright 2010 RSC

Reproduced with permission from Ref. [77]. Copyright 2010 RSC

Reproduced with permission from Ref. [78]. Copyright 2014 RSC

Reproduced with permission from Ref. [78]. Copyright 2014 RSC

Adapted from Ref. [79]. Copyright 1998 Wiley

Reproduced with permission from Ref. [81]. Copyright 1999 Wiley

Reproduced with permission from Ref. [82]. Copyright 2006 RSC

Reproduced with slight modification and permission from Ref. [83]. Copyright 2009 ACS
Similar content being viewed by others
Abbreviations
- Bipymh:
-
N,N′-Bis(4-pyridylmethylidyne)hydrazine
- bpb2− :
-
1,2-Bis(pyridine-2-carboxamido)benzoate
- bpmb2− :
-
4-Methyl-1,2-bis(pyridine-2-carboxamido)benzoate
- DMe-DCNQI:
-
2,5-Dimethyl-N,N′-dicyanoquinone diimine
- DMeO-DCNQI:
-
2,5-Dimethoxy-N,N′-dicyanoquinone diimine
- DMTCNQ:
-
7,7′,8,8′-Tetracyano-2,5-dimethyl-p-quinodimethanide
- dpa− :
-
Di(α-pyridyl)amido
- DPyBFPP:
-
5,15-Dipyridyl-10,20-bis(pentafluorophenyl)porphinate
- HBCD:
-
Hexacyanobutadiene
- H2TP′P:
-
meso-Tetrakis[3,5-di-tert-butyl-4-hydroxyphenyl)porphyrin
- Im− :
-
Imidazolate
- meta-F:
-
Tetra(meta-fluorophenyl)porphyrin-tetracyanoethenide
- OEP:
-
Octaethylporphinate
- ortho-F:
-
Tetra(ortho-fluorophenyl)porphyrin-tetracyanoethenide
- Pc:
-
Phthalocyaninate
- QCl4 :
-
Tetrachloro-1,4-benzoquinone
- TBrPP:
-
meso-Tetrakis(4-bromophenyl) porphinate
- TClPP:
-
meso-Tetra(4-chlorophenyl)porphinate
- TCNE:
-
Tetracyanoethylene
- [μ4-σ-(TCNQ)2]2− :
-
μ4-σ-Dimerized 7,7,8,8-tetracyano-p-quinodimethane dianion
- TCQMI:
-
N,7,7-Tricyanoquinomethanimine
- TDMeAPP:
-
meso-Tetra(4-dimethylaminophenyl)porphinate
- TMeOPP:
-
meso-Tetra(4-methoxylphenyl)porphinate
- TMesP:
-
meso-Tetrakis(2,4,6-trimethylphenyl)porphyrinate
- TNPP:
-
meso-Tetra(4-nitrylphenyl)porphinate
- TOHPP:
-
Tetra(4-hydroxyphenyl)porphinate
- tpa:
-
Tris(2-pyridylmethyl)amine
- TPP:
-
Tetraphenylporphinate
- TPyP:
-
Tetra(4-pyridyl)porphyrin
- TTMPP:
-
meso-Tetrakis(2,4,6-trimethylphenyl)porphyrinate
References
Lee YA, Kim JO, Cho TS, Song R, Kim SK (2003) Binding of meso-tetrakis(N-methylpyridium-4-yl)porphyrin to triplex oligonucleotides: evidence for the porphyrin stacking in the major groove. J Am Chem Soc 125:8106–8107
Kojima T, Harada R, Nakanishi T, Kaneko K, Fukuzumi S (2007) Porphyrin nanotubes based on self-assembly of Mo(V)-dodecaphenylporphyrin complexes and inclusion of Mo–Oxo clusters: synthesis and characterization by X-ray crystallography and transmission electron microscopy. Chem Mater 19:51–58
Imahori H, Arimura M, Hanada T, Nishimura Y, Yamazaki I, Sakata Y, Fukuzumi S (2001) Photoactive three-dimensional monolayers: porphyrin-alkanethiolate-stabilized gold clusters. J Am Chem Soc 123:335–336
Bearinger JP, Stone G, Christian AT, Dugan L, Hiddessen AL, Wu K, Jen J, Wu L, Hamilton J, Stockton C, Hubbell JA (2008) Porphyrin-based photocatalytic lithography. Langmuir 24:5179–5184
Mojzeš P, Kruglik SG, Baumruk V, Turpin PY (2003) Interactions of electronically excited copper(II)-porphyrin with DNA: resonance raman evidence for the exciplex formation with adenine and cytosine residues. J Phys Chem B 107:7532–7535
Bian YZ, Jiang JZ, Tao Y, Choi MTM, Li RJ, Anthony Ng CH, Zhu PH, Pan N, Sun X, Arnold DP, Zhou ZY, Li HW, Mak TCW, Ng DKP (2003) Tuning the valence of the cerium center in (Na)phthalocyaninate and porphyrinate cerium double-deckers by changing the nature of the tetrapyrrole ligands. J Am Chem Soc 125:12257–12267
Kazazić S, Klasinc L, McGlynn SP, Srzić D, Vicente MGH (2004) Gas-phase metallation reactions of porphyrins with metal monocations. J Phys Chem A 108:10997–11000
Okamoto K, Fukuzumi S (2003) Self-promoted electron transfer from cobalt(II) porphyrin to p-fluoranil to produce a dimer radical anion-cobalt(III) porphyrin complex. J Am Chem Soc 125:12416–12417
Fukuzumi S, Ohkubo KEW, Ou ZP, Shao JG, Kadish KM, Hutchison JA, Ghiggino KP, Sintic PJ, Crossley MJ (2003) Metal-centered photoinduced electron transfer reduction of a gold(III) porphyrin cation linked with a zinc porphyrin to produce a long-lived charge-separated state in nonpolar solvents. J Am Chem Soc 125:14984–14985
Hecht S, Ihre H, Fréchet JMJ (1999) Porphyrin core star polymers: synthesis, modification, and implication for site isolation. J Am Chem Soc 121:9239–9240
Suijkerbuijk BMJM, Klein Gebbink G, Robertus JM (2008) Merging porphyrins with organometallics: synthesis and applications. Angew Chem Int Ed 47:7396–7421
Ikeda C, Satake A, Kobuke Y (2003) Proofs of macrocyclization of gable porphyrins as mimics of photosynthetic light-harvesting complexes. Org Lett 5:4935–4938
Li BS, Li J, Fu YQ, Bo ZS (2004) Porphyrins with four monodisperse oligofluorene arms as efficient red light-emitting materials. J Am Chem Soc 126:3430–3431
Zhou XL, Kang SW, Kumar S, Kulkarni RR, Cheng SZD, Li Q (2008) Self-assembly of porphyrin and fullerene supramolecular complex into highly ordered nanostructure by simple thermal annealing. Chem Mater 20:3551–3553
Jiang JZ, Dennis KPN (2009) A decade journey in the chemistry of sandwich-type tetrapyrrolato-rare earth complexes. Acc Chem Res 42:79–88
Wong WY, Harvey PD (2010) Recent progress on the photonic properties of conjugated organometallic polymers built upon the trans-bis(paraethynylbenzene)bis(phosphine)platinum(II) chromophore and related derivatives. Macromol Rapid Commun 31:671–713
Xu LL, Ho CL, Liu L, Wong WY (2018) Molecular/polymeric metallaynes and related molecules: solar cell materials and devices. Coord Chem Rev 373:233–257
Zhu XJ, Wong WK, Wong WY, Yang XP (2011) Design and synthesis of near-infrared emissive lanthanide complexes based on macrocyclic ligands. Eur J Inorg Chem 2011:4651–4674
Cornia A, Mannini M, Sainctavit P, Sessoli R (2011) Chemical strategies and characterization tools for the organization of single molecule magnets on surfaces. Chem Soc Rev 40:3076–3091
Dechambenoit P, Long JR (2011) Microporous magnets. Chem Soc Rev 40:3249–3265
Sanvito S (2011) Molecular spintronics. Chem Soc Rev 40:3336–3355
Sorace L, Benelli C, Gatteschi D (2011) Lanthanides in molecular magnetism: old tools in a new field. Chem Soc Rev 40:3092–3104
Ferrando-Soria J, Serra-Crespo P, Lange MD, Gascon J, Kapteijn F, Julve M, Cano J, Lloret F, Pasán J, Ruiz-Pérez C, Journaux Y, Pardo EJ (2012) Selective gas and vapor sorption and magnetic sensing by an isoreticular mixed-metal–organic framework. J Am Chem Soc 134:15301–15304
Roques N, Mugnaini V, Veciana J (2010) Magnetic and porous molecule-based materials. Top Curr Chem 293:207–258
Nowicka B, Korzeniak T, Stefańczyk O, Pinkowicz D, Chorąży S, Podgajny R, Sieklucka B (2012) The impact of ligands upon topology and functionality of octacyanidometallate-based assemblies. Coord Chem Rev 256:1946–1971
Wang XY, Avendaño C, Dunbar KR (2011) Molecular magnetic materials based on 4d and 5d transition metals. Chem Soc Rev 40:3213–3238
Mitra S (1977) Chemical applications of magnetic anisotropy studies on transition metal complexes. Prog Inorg Chem 22:309–312
Figgis BN (1960) Magnetic properties of transition metal ions in asymmetric ligand fields. Part 1. Cubic field A and E terms. Trans Faraday Soc 56:1553–1558
Miyasaka H, Saitoh A, Abe S (2007) Magnetic assemblies based on Mn(III) salen analogues. Coord Chem Rev 251:2622–2664
Gerritsen HJ, Sabisky ES (1963) Paramagnetic resonance of trivalent manganese in rutile (TiO2). Phys Rev 132:1507–1512
Cheng B, Cukiernik F, Fries PH, Marchon JC, Scheidt WR (1995) A novel dimanganese(III) complex with a single hydroxo bridge. syntheses, structures, and magnetic susceptibilities of (μ-hydroxo)bis((octaethylporphinate)manganese(III)) perchlorate and a monomeric precursor, aquo(octaethylporphinate)manganese(III) perchlorate. Inorg Chem 34:4627–4639
Cheng B, Fries PH, Marchon JC, Scheidt WR (1996) A nearly linear single hydroxo bridge. Synthesis, structure, and magnetic susceptibility of (μ-hydroxo)bis((tetraphenylporphinate)manganese(III)) perchlorate. Inorg Chem 35:1024–1032
Suslick KS, Watson RA, Wilson SR (1991) Structure and photochemistry of manganese porphyrin sulfate complexes. Inorg Chem 30:2311–2317
Donzello MP, Bartolino L, Ercolani C, Rizzoli C (2006) A rare μ-hydroxo-bridged species. Synthesis, structure, and properties of μ-hydroxo(tetraphenylporphyrinatomanganese(III))(phthalocyaninate(azido)chromium(III)), [(TPP)Mn-O(H)-CrPc(N3)]. Inorg Chem 45:6988–6995
Donzello MP, Ercolani C, Russo U, Chiesi-Villa A, Rizzoli C (2001) Metal- and ligand-centered monoelectronic oxidation of μ-nitrido[((tetraphenylporphyrinate)manganese)(phthalocyaninatoiron)], [(TPP)Mn-N-FePc]. X-ray crystal structure of the Fe(IV)-containing species [(THF)(TPP)Mn-N-FePc(H2O)](I5)·2THF. Inorg Chem 40:2963–2967
Zhang DP, Wang HL, Chen Y, Ni ZH, Tian LJ, Jiang JZ (2009) Synthesis, crystal structure and magnetic property of an unusual formate-bridged heterometallic binuclear CrIIIMnIII porphyrin complex. Inorg Chem Commun 12:698–700
Zhang DP, Wang HL, Tian LJ, Kou HZ, Jiang JZ, Ni ZH (2009) Synthesis, crystal structures, and magnetic properties of cyanide-bridged Fe(III)–Mn(III) complexes based on manganese(III)-porphyrin and pyridinecarboxamide dicyanideiron(III) building blocks. Crystal Growth Des 9:3989–3996
Zhang DP, Zhao ZD, Wang P, Ni ZH (2013) Substituent group tuned tri- and binuclear porphyrin-based cyanide-bridged bimetallic complexes: synthesis, crystal structures and magnetic properties. CrystEngComm 15:2504–2511
Li GL, Nie J, Chen H, Ni ZH, Zhao Y, Zhang LF (2012) Syntheses, crystal structures and magnetic properties of two FeIII–MnIII complexes based on manganese(III)-porphyrin and tetracyanideferrite(III) building blocks. Inorg Chem Commun 19:66–69
Huh S, Youm KT, Park YJ, Lough AJ, Ohba M, Jun MJ (2005) Trinuclear MnIII-NC-FeIII-CN-MnIII ferromagnetic system. Bull Korean Chem Soc 26:1031–1032
Kim Y, Choi SK, Park SM, Nam W, Kim SJ (2002) Synthesis and reactivity of rhenium cluster-supported manganese porphyrin complexes. Inorg Chem Commun 5:612–615
Visinescu D, Toma LM, Cano J, Fabelo O, Ruiz-Pérez C, Labrador A, Lloret F, Julve M (2010) Magnetic coupling in discrete cyano-bridged MnIII-FeIII motifs: synthesis, crystal structure, magnetic properties and theoretical study. Dalton Trans 39:5028–5038
Ni WW, Ni ZH, Cui AL, Liang X, Kou HZ (2007) Cyanide-bridged Mn(III)–Fe(III) bimetallic complexes based on the pentacyano(1-methylimidazole)ferrate(III) building block: structure and magnetic characterizations. Inorg Chem 46:22–33
Caneschi A, Gatteschi D, Lalioti N, Sangregorio C, Sessoli R, Venturi G, Vindigni A, Rettori A, Pini MG, Novak MA (2001) Cobalt(II)-nitronyl nitroxide chains as molecular magnetic nanowires. Angew Chem Int Ed 40:1760–1763
Clérac R, Miyasaka H, Yamashita M, Coulon C (2002) Evidence for single-chain magnet behavior in a MnIII–NiII chain designed with high spin magnetic units: a route to high temperature metastable magnets. J Am Chem Soc 124:12837–12844
Glauber RJ (1963) Time-dependent statistics of the Ising model. J Math Phys 4:294–307
Miller JS, Calabrese JC, McLean RS, Epstein AJ (1992) Meso-(tetraphenylporphinate)manganese(III)-tetracyanoethenide, [MnIIITPP]•⊕[TCNE]• ⊖. A new structure-type linear-chain magnet with a Tc of 18K. Adv Mater 4:498–501
Miller JS, Vazquez C, Calabrese JC, McLean RS, Epstein AJ (1994) Cooperative magnetic behavior of α- and β-manganese(III) phthalocyanine tetracyanoethenide (1:1), [MnIIIPc] •⊕[TCNE] •⊖. Adv Mater 6:217–221
Miller JS, Vazquez C, Jones NL, Mclean RS, Epstein AJ (1995) Magnetic behaviour of octaethylporphyrinatomanganese(III) tetracyanoethenide, [MnOEP][TCNE], and hexacyanobutadienide, [MnOEP][C4(CN)6]: the importance of a uniform chain for stabilizing strong effective ferromagnetic coupling. J Mater Chem 5:707–711
Böhm A, Vazquez C, McLean RS, Calabrese JC, Kalm SE, Manson JL, Epstein AJ, Miller JS (1996) Weak effect on Tc with increased interchain distances. Structure and magnetic properties of (meso-tetrakis(3,5-di-tert-butyl-4-hydroxyphenyl)porphinate)manganese(III) tetracyanoethenide, [MnIIITP′P]+[TCNE]•−. Inorg Chem 35:3083–3088
Sugiura KI, Mikami S, Tanaka T, Sawada M, Manson JL, Miller JS, Sakata Y (1997) Magnetic ordering of 5,10,15,20-tetrakis[4′-(trifluoromethyl)phenyl]-porphyrinatomanganese(III) tetracyanoethenide with a 6.0 K Tc. Chem Lett 26:1071–1072
Sugiura KI, Arif AM, Rittenberg DK, Schweizer J, Öhrstrom L, Epstein AJ, Miller JS (1997) The importance of magnetic coupling through atoms with large spin densities-structure and magnetic properties of meso-tetrakis-(4′-tert-butylphenyl)porphinatomanganese(III) hexacyanobutadienide, [MnIIITtBuPP]+[C4(CN)6]−. Chem Eur J 3:138–142
Sugiura KI, Mikami S, Tanaka T, Sawada M, Sakata Y (1998) Cheminform abstract: isolation and molecular structure of 1,1,2,4,5,5-hexacyano-1,4-pentadienyl anion (III). An unusual reaction of tetracyanoethylene with p-xylene mediated by a porphyrinatomanganese(II). Chem Lett 27:103–104
Brandon EJ, Rittenberg DK, Arif AM, Miller JS (1998) Ferrimagnetic behavior of multiple phases and solvates of (meso-tetrakis(4-chlorophenyl)porphinate)manganese(III) tetracyanoethenide, [MnTClPP]+[TCNE]•−. Enhancement of magnetic coupling by thermal annealing. Inorg Chem 37:3376–3384
Brandon EJ, Arif AM, Burkhart BM, Miller JS (1998) Structure and magnetic properties of antiferromagnetic manganese(III) tetrakis(4-methoxyphenyl)porphyrin tetracyanoethenide, [MnTOMePP][TCNE]·2PhMe, and manganese(III) tetrakis(2-fluorophenyl)porphyrin tetracyanoethenide, [MnTFPP][TCNE]·2PhMe. Inorg Chem 37:2792–2798
Yates ML, Arif AM, Manson JL, Kalm BA, Burkhart BM, Miller JS (1998) Structure and magnetic properties of (meso-tetraphenylporphinate)manganese(III) pentacyanopropenide, [MnIIITPP]+[C3(CN)5]−. An unusual asymmetric bridge-bonding mode for μ-[C3(CN)5]. Inorg Chem 37:840–841
Miller JS, Epstein AJ (1998) Tetracyanoethylene-based organic magnets. Chem Commun (13):1319–1325
Sugiura KI, Mikami S, Johnson MT, Miller JS, Iwasaki K, Umishita K, Hino S, Sakata Y (1999) First isolation and characterization of an electron transfer salt of a tetracyanoquinodimethane with porphyrinatomanganese(II) having novel cis-μ-coordination manner: a molecule-based magnet with a 2.3 K Tc. Chem Lett 31:925–926
Rittenberg DK, Miller JS (1999) Observation of magnetic ordering as high as 28 K for meso-tetrakis(4-halophenyl)porphinatomanganese(III) tetracyanoethenide, [MnTXPP][TCNE] (X = F, Br, I). Inorg Chem 38:4838–4848
Rittenberg DK, Sugiura KI, Sakata Y, Mikami S, Epstein AJ, Miller JS (2000) Large coercivity and high remanent magnetization organic-based magnets. Adv Mater 12:126–130
Rittenberg DK, Arif AM, Miller JS (2000) Effect of thermal annealing on the ferrimagnetic behavior and ordering of the [MnTXPP]+[TCNE]˙−·solv (X = F, Cl, Br, I; solv = PhMe, CH2Cl2) family of magnets. J Chem Soc Dalton Trans (21):3939–3948
Johnson MT, Arif AM, Miller JS (2000) Structure and magnetic properties of tetrakis(3,4,5-trimethoxy-phenyl)porphinatomanganese(III) 2,3,5,6-tetrafluoro-7,7,8,8-tetracyanoquinodimethenide: the first example of an isolated [TCNQF4]•. Eur J Inorg Chem 2000:1781–1787
Miller JS (2000) Organometallic- and organic-based magnets: new chemistry and new materials for the new millennium. Inorg Chem 39:4392–4408
Rittenberg DK, Sugiura KI, Arif AM, Sakata Y, Incarvito CD, Rheingold AL, Miller JS (2000) Products from the reaction of meso-tetrakis(4-halophenyl)porphinate-manganese(II) and hexacyanobutadiene (HCBD): formation of π-[HCBD] 2−2 dimers, μ-[HCBD]−, σ-[HCBD]−, and [C4(CN)5O]−. Chem Eur J 6:1811–1819
Sugiura KI, Mikami S, Johnson MT, Miller JS, Iwasaki K, Umishita K, Hino S, Sakata Y (2000) Structure and magnetic properties of meso-tetrakis(2,4,6-trimethylphenyl)porphyrinatomanganese(III) 7,7,8,8-tetracyano-2,5-dimethyl-p-quinodimethanide with a 2.3 K Tc. The first example of cis coordination of a tetracyano-p-quinodimethanide. J Mater Chem 10:959–964
Sugiura KI, Mikami S, Johnson MT, Raebiger JW, Miller JS, Iwasaki K, Okada Y, Hino S, Sakata Y (2001) Ferrimagnetic ordering of one-dimensional N,N′-dicyanoquinone diimine (DCNQI) electron transfer salts with porphyrinatomanganese(II). J Mater Chem 11:2152–2158
Arthur JL, Moore CE, Rheingold AL, Miller JS (2011) Stabilization of magnetic ordering observed for the bridging NCN group. Inorg Chem 50:2735–2737
Tomkowicz Z, Rams M, Bałanda M, Foro S, Nojiri H, Krupskaya Y, Kataev V, Büchner B, Nayak SK, Yakhmi JV, Haase W (2012) Slow magnetic relaxations in manganese(III) tetra(meta-fluorophenyl)porphyrin-tetracyanoethenide. Comparison with the relative single chain magnet ortho compound. Inorg Chem 51:9983–9994
Brandon EJ, Rogers RD, Burkhart BM, Miller JS (1998) The structure and ferrimagnetic behavior of meso-tetraphenyl-porphinatomanganese(III) tetrachloro-1,4-benzoquinonide, [MnIIITPP]+[QCl4]−·PhMe: evidence of a quinoidal structure for [QCl4]−. Chem Eur J 4:1938–1943
Landrum JT, Hatano K, Scheidt WR, Reed CA (1980) Imidazolate complexes of iron and manganese tetraphenylporphyrins. J Am Chem Soc 102:6729–6735
Turner P, Gunter MJ, Hambley TW, White AH, Skelton BW (1992) Two unusual fonnate-bridged manganese(III) tetraphenylporphyrin complexes. Inorg Chem 31:2295–2297
Kumar RK, Balasubramanian S, Goldberg I (1998) Crystal engineering with tetraarylporphyrins, an exceptionally versatile building block for the design of multidimensional supramolecular structures. Chem Commun (14):1435–1436
Diskin-Posner Y, Patra GK, Goldberg I (2001) Supramolecular assembly of metalloporphyrins in crystals by axial coordination through amine ligands. J Chem Soc Dalton Trans (19):2775–2782
Bernot K, Luzon J, Sessoli R, Vindigni A, Thion J, Richeter S, Leclercq D, Larionova J, van der Lee A (2008) The canted antiferromagnetic approach to single-chain magnets. J Am Chem Soc 130:1619–1627
Tsao TB, Lee GH, Yeh CY, Peng SM (2003) Supramolecular assembly of linear trinickel complexes incorporating metalloporphyrins: a novel one-dimensional polymer and oligomer. Dalton Trans (8):1465–1471
Dawe LN, Miglioi J, Turnbow L, Taliaferro ML, Shum WW, Bagnato JD, Zakharov LN, Rheingold AL, Arif AM, Fourmigué M, Miller JS (2005) Structure and magnetic properties of (meso-tetraphenylporphinato)manganese(III) bis(dithiolato)nickelates. Inorg Chem 44:7530–7539
Zhang DP, Zhang LF, Chen YT, Wang HL, Ni ZH, Wernsdorfer W, Jiang JZ (2010) Heterobimetallic porphyrin-based single-chain magnet constructed from manganese(III)-porphyrin and trans-dicyanobis(acetylacetonato) ruthenate(III) containing co-crystallized bulk anions and cations. Chem Commun 46:3550–3552
Chen X, Wu SQ, Cui AL, Kou HZ (2014) A cyano-bridged single-chain magnet featuring alternate high- and low-spin manganese(III) porphyrins. Chem Commun 50:2120–2122
Kumar RK, Goldberg I (1998) Supramolecular assembly of heterogeneous multiporphyrin arrays—structures of [{ZnII(tpp)}2(tpyp)] and the coordination polymer [{[MnIII(tpp)]2(tpyp)(ClO4)2}∞]. Angew Chem Int Ed 37:3027–3030
Mikami S, Sugiura KI, Miller JS, Sakata Y (1999) Two-dimensional honeycomb network formed by porphyrinatomanganese(III) and μ4-σ-dimerized 7,7,8,8-tetracyano-p-quinodimethane dianion. Chem Lett 28:413–414
Lin KJ (1999) SMTP-1: the first functionalized metalloporphyrin molecular sieves with large channels. Angew Chem Int Ed 38:2730–2732
George S, Lipstman S, Muniappan S, Goldberg I (2006) Porphyrin network solids: examples of supramolecular isomerism, noncentrosymmetric architectures and competing solvation. CrystEngComm 8:417–424
Barron PM, Son HT, Hu CH, Choe W (2009) Highly tunable heterometallic frameworks constructed from paddle-wheel units and metalloporphyrins. Cryst Growth Des 9:1960–1965
Farha OK, Shultz AM, Sarjeant AA, Nguyen ST, Hupp JT (2011) Active-site-accessible, porphyrinic metal-organic framework materials. J Am Chem Soc 133:5652–5655
Chandrasekhar S, Sadashiva BK, Suresh KA (1977) Liquid crystals of disc-like molecules. Pramana J Phys 9:471–480
Griesar K, Athanassopoulou MA, Bustamante ES, Haase W, Tomkowicz Z, Zaleski AJ, Haase W (1997) A ferrimagnetically coupled liquid crystal. Adv Mater 9:45–48
Hill J, Sugino T, Shimizu Y (1999) Synthesis and characterization of some manganese complexes of 5,10,15,20-tetrakis(4-n-dodecylphenyl)porphyrin, molecular crystals and liquid crystals science and technology. Mol Cryst Liq Cryst 332:119–125
Haase W, Wrobel S, Falk K (2003) Recent results on some columnar paramagnetic metallomesogens. Pramana J Phys 61:189–198
Winter H, Kelemen M, Dormann E, Gompper R, Janner R, Kothrade S, Wagner B (1995) Characterisation of new organic ligand-based magnets: [MNTTP][TCNE] and [MnTTP][TCNQ]. Mol Cryst Liq Cryst 273:111–116
Brandon EJ, Miller JS, Brinckerhoff WB, Zhou P, Epstein AJ (1994) In: 4th international conference on molecule based magnets, Salt Lake City, October 1994
Acknowledgement
This work was supported by the Natural Science Foundation of China (Nos. 21671121, 21773006), National Key Basic Research Program of China (Grant Nos. 2013CB933402 and 2012CB224801), Program for New Century Excellent Talents in University, Fundamental Research Funds for the Central Universities and Beijing Natural Science Foundation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, D., Lan, W., Zhou, Z. et al. Manganese(III) Porphyrin-Based Magnetic Materials. Top Curr Chem (Z) 377, 18 (2019). https://doi.org/10.1007/s41061-019-0244-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-019-0244-5