Abstract
The gallbladder is an important component of the hepatobiliary system whose primary function is to aid in digestion of foodstuffs, excretion of drugs and facilitate removal of waste products from the body. The gall bladder is principally a storage organ for bile, chemically modified salts and acids of cholesterol which are synthesized in the liver and which function as surfactants to solubilize fatty substances of limited aqueous solubility. Any disruption in the amount or activity of bile surfactant can lead to an accumulation of insoluble molecular clumps (e.g., gallstones) that deposit in and obstruct fluid movement within the gallbladder, and which can lead to pathological congestion and tissue damage. The natural host defense response to any event that causes tissue damage is to stimulate inflammation, which non-specifically but aggressively reacts to the stimuli so to remove the cause and repair damaged tissues. The C-reactive protein (CRP) is a primarily hepatically produced serum protein whose blood levels increase within 6–10 h of any tissue-damaging event. The extent with which it increases correlates with the level of tissue damage and associated inflammation. CRP levels are reported to be of value in diagnosing acute cholecystitis severity, in predicting the outcome and prognosis of cancer-associated gallbladder resection, and in helping identify cystic structures during emergency laparoscopic cholecystectomies. As an understanding of distinctive CRP structural isoforms is evolving, its role not only as a biomarker but as regulator of both physiologic and pathophysiologic processes of inflammation may be relevant in the understanding of and treatment approaches for gallbladder-associated disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gallbladder is an important organ of the human digestive tract. Located beneath the liver, its primary function is to store bile salts and acids which serve as surfactants of dietary fats and oils during the process of digestion. Bile salts and acids, such as cholic acid and chenodeoxycholic acid and their conjugated glycine and taurine analogs, are synthesized from cholesterol in the liver. During digestion, these molecules are transported from the gallbladder through the common bile duct past the pancreas, where they empty into the duodenum and assist in the solubilization of non-polar molecules that enter into the gastrointestinal tract (Di Ciaula et al. 2017). Certain lipophilic and high molecular weight drugs (e.g., mycophenolic acid, warfarin, and digoxin) and/or their metabolites are known to be excreted by forming complexes with bile. Such bile conjugates significantly impact drug systemic exposure, pharmacological effects, and toxicity (Ghibellini et al. 2006).
There are many diseases known to involve the gallbladder, the most common being caused by the formation of gallstones—insoluble fatty deposits containing cholesterol, bile pigments, bilirubin, calcium salts, and phospholipids (Ibrahim et al. 2018). These fatty deposits clump into waxy masses of varying size, which could lead to congestion of the organ and the bile duct. As the size and extent of waxy deposits grow, organ tissues can get stretched and damaged and lead to perforation in extreme cases, triggering a host defense response that is geared at removing the deposits and repairing the damaged tissues. Most fundamentally, these natural host defense responses involve inflammation, which can be acute or chronic, and which can cause pain and abdominal discomfort. Inflammation that affects the gallbladder itself is a condition known as cholecystitis. Inflammation that involves the bile duct is known as cholangitis. Because of the proximity to the pancreas and liver, gallbladder congestion may also cause pancreatitis and lead to hepatic obstruction and jaundice. Damaged gallbladder tissues have also been reported to be sites of infections and abscesses, cancers and necrosis (Stinton and Shaffer 2012), and patients with a medical history of gallstone disease may have increased risk of developing cardiovascular disease (Fairfield et al. 2019; Fan et al. 2017). Gallbladder congestion can also prevent secretion of bile into the duodenum and adversely affect absorption of certain vitamins and fats (Di Ciaula et al. 2017; Thompson 1971).
Acute cholecystitis refers to the rapid onset of gallbladder inflammation. In response to trauma caused by gallstone blockage, prostaglandins I2 and E2 are synthesized from arachidonic acid released from membrane phospholipids by phospholipase A2 (PLA2) and metabolized by the sequential actions of respective synthases such as cyclooxygenase (COX). These lipid hormones are locally acting potent stimulators of inflammatory processes (Ricciotti and FitzGerald 2011). As inflammation is non-specific to its cause, mediators such as histamine and reactive oxygen species are released into affected tissues, leading to neural and muscular damage (Pozo et al. 2004). In about 20% of acute cholecystitis cases confounding bacterial infection occur, most commonly involving Escherichia coli, Klebsiella, Streptococcus, Clostridium, Staphylococcus faecalis, Clostridium perfringens, and Helicobacter Pylori (Cen et al. 2018; Gouma and Obertop 1992; Juvonen et al. 1992). Until the infection is controlled and removed, the growth and expansion of both the bacterial colonies and the amassing host defense cells associated with the inflammatory response can lead to pronounced swelling secondary to the trauma affecting the gallbladder wall.
Chronic cholecystitis is gallbladder inflammation that persists because of multiple recurring episodes of gallstone deposits, unresolved removal of fatty deposits, and unrepaired tissue damage. Generally, chronic inflammation involves a weak or unamplified inflammatory response, leading to production and release of lower levels of inflammatory mediators that get released into affected tissues. Chronic cholecystitis can be more difficult to diagnose than acute cholecystitis, as the level of pain and discomfort caused by the inflammatory response may be vague enough or asymptomatic enough so the patient and healthcare provides ignore or misdiagnose the disease. Chronic cholecystitis can be complicated by many concomitant issues, such as empyema, hydrops, gangrene, fistulas, or limey bile. Calcium may also deposit and harden in the walls of the gallbladder, causing extensive scarring (Elwood 2008; Greenberger and Paumgartner 2018; Lee and Boll 2018).
Reported prevalence of gallstone disease in the adult United States population is 10%–15% (Ibrahim et al. 2018) and 5%–25% of adults in the Western world (Gurusamy and Davidson 2014). Prevalence varies worldwide between different ethnic groups due to variable genetic and environmental factors (Di Ciaula and Portincasa 2018; Hernandez-Nazara et al. 2006; Stinton and Shaffer 2012).
While both acute and chronic inflammations are fundamentally linked to the pathophysiology of gallbladder diseases, little focus has been given to the role of inflammatory markers in diagnosing and assessing the extent of tissue-damaging processes occurring as part of these diseases. This report will summarize how the prototypic acute phase reactant—C-reactive protein (CRP)—may be a useful marker for disease activity. It will also introduce novel concepts involving heretofore unappreciated structural isoforms of CRP and the distinct biofunction(s) of each as a regulator of inflammatory processes.
Inflammation and the Gallbladder
Acute gallbladder inflammation (cholecystitis) is triggered by three main mechanisms: (1) obstruction of the organ and/or the cystic duct by the formation of gallstones, and the subsequent tissue damage that occurs concurrent with their formation; (2) release of “lysolecithin”, a fatty acyl chain from the C2 position of glycerol phospholipids with the associated formation of lysophosphatidyl choline (LysoPC) from membrane lipids, and (3) ascending bacterial infection of the biliary fluid most notably caused by gallstones obstruction (Schuld and Glanemann 2015).
The formation of gallstones occurs when (1) Bile becomes supersaturated with cholesterol; (2) Cholesterol molecules nucleate, changing their organization in a way that causes them to crystalize and grow into insoluble stones; (3) Gallbladder motor function becomes abnormal, reducing emptying of bile into the bile duct and duodenum (leading to bile stasis); or (4) Gastrointestinal hypomobility which reduces enterohepatic cycling of bile salts. The risk of forming insoluble gallstones is directly related to the concentration of cholesterol found in the gallbladder and is inversely related to the concentration of the more amphipathic biliary salt conjugates and to phospholipids. Cholesterol has been implicated in both formation of gallstones and in gallbladder hypomotility (Hernandez-Nazara et al. 2006).
At local sites of tissue damage, one of the first biochemical responses in the hydrolysis of a fatty acyl chain from the C2 carbon of a diacyl glycerol phospholipid. This hydrolysis is mediated by the enzyme phospholipase A2 (PLA2), an enzyme that, while not known as an acute phase reactant, is tightly associated with the onset of early and robust inflammatory responses. There are several isoforms of PLA2 which differ in distribution and function. The isoform cytosolic PLA2 is induced by inflammatory cytokines such as TNF-α and is known to strongly regulate inflammation (Leslie 2015; Yarla et al. 2016). This, in part, is mediated by the release of arachidonic acid from membrane lipids, which enters into eicosanoid pathways using cycooxygenase enzymes (COX) or lipoxygenase enzymes, generating potent prostaglandins or leukotriene lipid inflammatory mediators. After the acyl chain is cleaved from the diacyl glycerol lipid, the remaining monoacyl glycerol lipid (known as a Lysolipid) has a significant change to its hydrophilic–lipophilic balance (HLB), effectively emulsifying and/or solubilizing the membrane bilayer where it is produced (Pichot et al. 2013). The disruption in the bilayer results in a change in the thickness and the curvature of the bilayer, and results in a lateral redistribution of intramembrane components including cholesterol, which can associate into cholesterol-rich microdomains known as lipid rafts (Cheng and Smith 2019; Marquardt et al. 2016). Lysophosphatidyl choline (i.e., LysoPC) expressed at activated membrane sites becomes a beacon for platelet and neutrophil accumulation and activation. COX inhibitors such as aspirin and non-steroidal anti-inflammatory drugs (NSAIDS), lessen the production of prostaglandins which reduces the inflammation and pain associated with a stimulated inflammatory response.
Since PLA2 is also a key enzyme in the activation and progression of an inflammation, inhibiting or otherwise controlling this enzyme is also being evaluated as an effective mechanism for controlling the inflammatory response (Caprio et al. 2018). Indeed, the purported anti-inflammatory activity associated with drinking red wine may involve the inhibition of PLA2 activity by resveratrol, a key compound found in red wine and various vegetables (Fei et al. 2018). Changes in membrane thickness, curvature, and cholesterol content can affect the insertion of peptides into a membrane, which in turn, can alter cellular responses involving signaling mechanisms (Haque and Lentz 2004; Karabadzhak et al. 2018; Lähdesmäki et al. 2010).
Diagnosis of Gallbladder Pathologies
Symptoms of acute cholecystitis typically include biliary pain, nausea, and vomiting. Biliary pain can manifest as right upper quadrant pain and radiate to the right scapula or shoulder. The right upper quadrant of patients experiencing an acute cholecystitis attack will be tender and sore upon palpation. Abdominal stiffness and chills may also arise with attack. The symptoms typically subside after 2–3 days and resolve after a week but can lead to increasing pain, perforation or abscesses.
Cholecystitis may also present with abdominal soreness or tenderness, fever, and leukocytosis. Jaundice, dark urine, or light-colored stools can be observed when the common bile duct becomes obstructed, leading to incomplete conjugation of the heme breakdown product—bilirubin in the liver and the resultant release of excess bilirubin into the blood. In some cases, involving excessive liver involvement, aminotransferase enzymes (e.g., aspartate amino transferase (AST)) may be released into the serum, which is easily monitored as part of a routine metabolic panel test.
An ultrasound of the right upper quadrant is another diagnostic tool that may be useful. An ultrasound could show signs of wall thickening, fluid buildup, dilatation of the bile duct, or contraction of the gallbladder while fasting. Performing this test with fasting helps minimize confounding interpretations related to active gallbladder function and movement of bile contents from the gallbladder, through the common duct and into the duodenum. If symptoms and other diagnostic tests are inconclusive, a radionuclide (HIDA) scan may be ordered. The HIDA scan shows the radioactivity of the injected radionuclide within the bile duct but does not allow visualization of the gallbladder. The radionuclide may also settle into the gallbladder and not be released upon the patient consuming a fatty cream. This test can be used to show both gallbladder duct obstruction and poor gallbladder function (Elwood 2008; Greenberger and Paumgartner 2018; Lee and Boll 2018).
Cholecystitis is a progressive disease that requires continued monitoring and treatments based on its persistence and severity. The most commonly used diagnostic tests to assess acute cholecystitis include liver function tests to evaluate liver damage, and leukocyte counts to assess the status of the inflammatory response (higher leukocyte counts are reflective of an active inflammatory response). Another blood marker reflective of the presence and extent of inflammation is C-reactive protein (CRP), widely known as a non-specific serum-based marker. Even though the exact role of CRP as a regulator of inflammation remains unknown, its inclusion as a diagnostic marker to assess aspects of gallbladder disease was recommended by the 2007 Tokyo guideline criteria (Hirota et al. 2007) and was reinforced in 2018 (Kiriyama et al. 2018). A retrospective study reported a strong correlation between CRP levels and the grade of acute cholecystitis, suggesting CRP may also be a useful marker to diagnose not only the presence of, but the staging of disease (Gurbulak et al. 2015).
As advances in understanding CRP structure–function relationships have occurred, its presence, concentration, and short-term changes in blood levels are added increased value to healthcare personnel in understanding and treating gallbladder disease. The simplicity, relatively safety of gathering blood samples, and the economic value of ordering CRP tests should be included in routine monitoring of disease activity and the responses to treatments used on afflicted patients. Indeed, simple CRP measurements are preferred options to imaging methods or more invasive procedures.
CRP as the Prototypic Diagnostic Marker of the Acute Phase Response and Ensuing Inflammation
Inflammation is the natural host defense response to any event that causes damage to body tissues (Fig. 1). The most immediate response to tissue damage is a survival response, which is activated to prevent life-threatening bleeding and to mobilize and localize leukocytes to respond to any threats introduced into the exposed tissues. The earliest phases of inflammation, occurring within seconds to minutes of the inciting cause, must not only be activated, but amplified to address the threat to life and homeostasis. Initially, these responses involve the bioactivities of certain proteins found in blood known as the acute phase reactants (APRs). APRs include blood clotting factors such as fibrinogen, opsonins such as complement protein C3, anti-proteases such as alpha-1 anti-trypsin, transport proteins such as haptoglobin, and wound healing factors such as fibroblast growth factor (Kushner et al. 2006). Because these proteins are rapidly consumed during the acute process, their quick replacement is critical, thus describing why such proteins are categorized as key reactants of the “acute phase”. By monitoring their temporal changes in blood concentration as a function of the onset and duration of tissue insult, most APRs are seen to increase modestly (i.e., changing by percentage levels) or to small multiples of their normal levels (e.g., two-folds to four-fold increases). Two proteins are known, however, to increase up to several 100-fold (i.e., up to 500-fold) as part of the acute phase response. One of these proteins is the serum amyloid A protein (SAA), an apolipoprotein expressed on high density lipoprotein particles (Sack 2018). Because of its lipid association, isolating and quantifying SAA from serum has proven to be challenging but reports have appeared describing it can be used to differentiate passive and active movement of blood proteins into extravascular tissues (Okino et al. 2006). The second protein that can rapidly increase several 100-fold in response to tissue damage is C-reactive protein (CRP). CRP is a highly soluble, easy to quantify blood protein with a relative short half-life of 19 h (Vigushin et al. 1993). Its blood levels are related to its hepatic synthesis rate, persisting as long as cytokine signals (i.e., interleukin 6) continue to be produced by damaged tissues (MacIntyre et al. 1983; Sproston and Ashworth 2018). Because its blood levels correlate with any disease or trauma that involves tissue damage, CRP is widely known as the prototypic acute phase reactant. Because tissue damage always involves the stimulation of inflammation processes, the change in CRP levels in blood is commonly interpreted to be a key diagnostic marker for the presence and extent of inflammation.
Baseline levels of CRP vary from 1 to 3 μg/mL in most individuals. Blood levels are found to rise approximately 6–10 h after the inciting stimuli occurs (Pepys and Hirschfield 2003). Any blood level above 10 μg/mL is considered diagnostic of an ongoing inflammatory condition (FDA guideline 2005). The extent and duration of increased blood levels can provide useful diagnostic information in the care and treatment of affected patients. While blood CRP levels correlate with the extent of tissue-damaging inflammation, surprisingly there is no selective localization of CRP from blood into to tissue sites known to be involved with prominent inflammation (Vigushin et al. 1993). CRP reported fractional catabolic rate is independent of its plasma concentration indicating that the change in blood levels during an acute inflammatory response reflects on an increase in the synthesis rate of CRP rather than its consumption. CRP plasma levels above 100 μg/mL (i.e., ~ a 33 to 100-fold increase over baseline) are generally associated with severe tissue damage and robust inflammation. CRP levels that persist at and above this elevated level are considered a poor prognostic indicator and should be an index guiding decisions to use more aggressive medical interventions.
In recent years, significant focus has been placed on the diagnostic and prognostic significance of plasma CRP levels between baseline and 10 μg/mL (i.e., below that value defined by the Food and Drug Administration of the United States (FDA) as diagnostically significant). These values are measured using what is known as a “high sensitivity CRP” assay (i.e., hsCRP). While many studies implicate elevated hsCRP levels as an index of “micro-inflammation”, and some studies suggest higher baseline hsCRP levels in different individuals may be predictive of disease risk (Antonelli and Kushner 2017), “high sensitivity” simply refers to the sensitivity of assays used to detect the very same molecule CRP, no studies are conclusive and the FDA makes no recommendation to the value or significance of hsCRP data (FDA Guideline 2005, updated on 3/13/18). Various reports have appeared suggesting higher baseline hsCRP levels in different individuals may be more related to genetic polymorphisms (i.e., single base polymorphisms (SNPs)) in promoter regions of the gene coding for CRP (Su et al. 2014).
CRP as a Diagnostic Marker of Gallbladder Disease
Use of CRP as a diagnostic marker for inflammatory gallbladder diseases has been relative uncommon (Hirota et al. 2007; Strasberg 2008). Some early studies did report that CRP measurements were more sensitive than erythrocyte sedimentation rates or white cell counts to support a cholecystitis diagnosis. Furthermore, when used in combination with ultrasonographic exams, CRP levels helped increase diagnostic accuracy of acute cholecystitis from 79% to 97% (Juvonen et al. 1992). While CRP was reported to be a useful marker for acute disease, it did not appear to be of benefit in diagnosing chronic disease (Beliaev et al. 2015).
When gallbladder disease presents with cancerous or gangrenous co-morbidities, CRP levels are found to be markedly elevated (Beliaev et al. 2015; Cui et al. 2018; Koshiol et al. 2016; Mok et al. 2014). CRP levels were described as a superior marker than leukocyte count as an index of disease activity (Beliaev et al. 2015; Shabanzadeh et al. 2016).
In efforts to normalize understanding of and treatment options for hepatobiliary diseases, Tokyo Guidelines were developed (2007) and updated (2018) by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. The guidelines recommend that CRP levels >30 μg/mL be used to support a diagnostic of acute cholecystitis. This value further refines the FDA guideline in setting an index for diagnosing an acute episode of disease. Such guidelines support any CRP level >10 μg/mL as indicative of an activated inflammatory response in a patient, especially if elevated CRP levels are noted in conjunction with either a markedly decreased or increased leukocyte count (FDA guideline 2005). Hence, CRP levels of >30 μg/mL along with ultrasonographic findings consistent with acute cholecystitis, have a high sensitivity, specificity, and positive predictive value of 97%, 76%, and 95%, respectively, for aggressive, acute disease (Hirota et al. 2007).
CRP levels are also useful in assessing recurring or exacerbating disease, and possibly even fatal outcomes. Extremely high CRP levels ( >100 μg/mL) that persistent with or without various treatments, are poor prognostic indicators. When the acute phase host defense response successfully controls the treat and tissues begin to heal, acute phase proteins should return to normal blood levels within days. As the threat is controlled and tissues heal, cytokine signals for CRP hepatic synthesis are no longer produced, and the synthesis and secretion of CRP into the blood slow. Because of its 19-h plasma half-life, measured blood levels should return to baseline over 3–4 days (Vigushin et al. 1993). CRP levels that remain persistently high for more than 4 days can be used to help diagnose treatment failures and significant, unresolved tissue damage. Seriously compromised tissues where disease persists will involve a prolonged, non-specific, robust inflammatory response which will add to and worsen tissue damage initially caused by the deposited gallstones. If left untreated, organ function will continue to decrease, threatening survival.
Additional criteria used to assess the severity of acute cholecystitis include the CRP/albumin ratio (CRP/Alb), neutrophil/lymphocyte ratio (NLR), the Glasgow prognosis score (GPS), and modified Glasgow prognosis score (mGPS). Each of these independently predict a severity grade (e.g., moderate or severe), which can be used in combination with clinical, laboratory, and imaging findings to assess disease activity. The GPS score in part includes a CRP-based value which, in accordance with the Tokyo Guidelines, indicates CRP does play a complementary role in assessing the severity of acute cholecystitis (Sato et al. 2018). The GPS score has also been used to predict outcomes of gallbladder resection performed as a treatment for gallbladder cancer. GPS scores that reflect low albumin and elevated CRP levels, used in conjunction with advanced tumor stage and positive lymph node metastasis, was predictive of cancer recurrence and overall survival (Shiba et al. 2015).
For both acute and chronic cholecystitis, surgical intervention is the current preferred therapy when indicated by symptoms or complications. The most common intervention is cholecystectomy, or complete removal of the gallbladder from the body. This is often accompanied, either before or after surgery, by a course of antibiotics against Gram-negative organisms that may have been present in the inflamed gallbladder. Emergency cholecystectomy is preferred in patients with potentially severe complications, such as empyema, gangrene, or perforation. Cholecystectomies are otherwise an elective surgery, usually performed laparoscopically in a preferred time frame of 48–72 h after diagnosis. The delay of elective cholecystectomy from the time of diagnosis has not been shown to increase risk of complications from either surgery or acute cholecystitis. Cholecystectomies are relatively safe procedures and provide symptom relief in 75%–90% of patients (Elwood 2008; Greenberger and Paumgartner 2018; Lee and Boll 2018; Okamoto et al. 2018).
CRP levels have also been used to assess a critical view of safety (CVS) during laparoscopic cholecystectomy in patients presenting with acute cholecystitis. The CVS is described as a method to identify cystic structures during the surgery, enhancing patient safety through correct identification of all structures critical to the operation. A scoring system based on the three preoperative factors, including CRP levels, was found to be an effective evaluation of the likelihood that CVS can be achieved, and patient safety can be enhanced during emergency cholecystectomy. Preoperative CRP levels of >55 μg/mL, along with gallstone impaction and greater than 72 h between symptom onset and time at which surgery is performed, correlate with an inability to achieve CVS. Thus, elevated CRP levels may be indicative of poor patient prognosis (Onoe et al. 2017).
Table 1 summarizes published reports of CRP levels in various gallbladder diseases. These reports underscore and emphasize that the disease/complication severity grade and inflammation seen with gallbladder diseases correlate with extremely high CRP values (>100 μg/mL).
Because of its intimate connection to the presence of any inflammatory response, no matter what was the primary cause that stimulated the inflammation, CRP testing has been used (in conjunction with other tests) by physicians to help make diagnostic decisions regarding infections, heart disease, bowel disease, rheumatoid arthritis, systemic lupus erythematosus and other diseases. Most basically, medical community consensus is that the CRP levels should not be used as a singular diagnostic tool, but rather as a supportive index with other diagnostic findings, to help provide guidance in the care and treatment of patients. The medical community has primarily defined CRP as a non-specific biomarker of inflammation. More correctly, CRP should be viewed as a clinical biomarker for the presence of and severity of tissue damage that accompanies any disease or significant trauma to tissue structures and functions. Since, inflammation is a natural response to tissue damage, CRP levels do represent an index of the extent of inflammation. Extremely high levels are prognostic of severely damaged, seriously compromised tissues, which are more difficult to control, repair, and return to healthful homeostasis. An inability to control and repair such tissues can explain why persistently elevated CRP levels often correlate to worse outcomes (Wu et al. 2015).
Insights into the Biological Function(S) of CRP
For more than a half-century since CRP was first identified as a protein “not normally found in blood” but occurring during “acute infections” (Abernathy and Avery 1941; Macleod and Avery 1941a, b), scientists have attempted to describe a biofunction for CRP related to its occurrence during host defense responses involving inflammation. Most generally, CRP has been studied as a protein that could enhance phagocytosis, accelerate chemotaxis, and promote the activation of platelets, all reactions occurring during the earliest phases of the acute inflammatory response. As CRP levels were found to elevate as a function of the presence and extent of inflammation associated with any disease (i.e., it was not selectively produced in response to infectious diseases), it has been viewed as a factor somehow involved in general reactions and pathologies associated with inflammation, including both acute and chronic inflammatory responses.
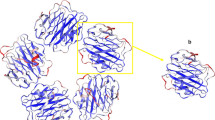
CRP was initially discovered as a protein that precipitated with a polysaccharide cell wall fraction (fraction “C”) isolated from Gram positive Streptococcus pneumonia organisms by (Tillet and Francis 1930). The protein reactive with the “C” polysaccharide fraction (hence, the “C-substance”-reactive protein) demonstrated calcium-dependent binding for the phosphocholine moiety expressed in the gram-positive bacterial cell wall teichoic acid (Gotschlich et al. 1982). CRP was later shown to be a non-glycosylated, non-covalently associated cyclic pentameric protein with each subunit being 23 kDa and being arranged in a discoid orientation surrounding a central void (Shrive et al. 1996; Srinivasan et al. 1994) (see Fig. 2A for a space-filling depiction). In detailed protein structural studies, CRP was categorized as a member of the pentraxin protein family, which includes the serum amyloid P protein (SAP) and the long pentraxin 3 (PTX3), neural pentraxin I (NPI) and II (NPII) (Hsu and Perin 1995; Omeis et al. 1996).
A Visual orientation of PC binding sites, cholesterol binding sequences and residues contributing to stabilization of the pCRP isoform on the PC binding face of the discoid pentameric molecule (PDB code: 1B09). B Similarly visualized residues as in A depicted on an isolated CRP subunit. This depiction is included to show the special relationship between PC binding and cholesterol binding sites on an isolated pCRP subunit. It does not depict the orientation of residues on the mCRP isoform which changes structure when it binds cholesterol and enters into a lipid milieu. To date, structural coordinates for the mCRP isoform have not been established. C pCRP orients “flat” on the PC binding surface, asymmetrically juxtaposing its PC binding face to the surface expressing PC groups, and allowing its opposite face (described as the CRP helical or effector face) to be accessible to cells and factors that will contribute to effector responses activated in response to CRP binding
CRP homologues have been widely found in evolution extending back to the horseshoe crab where the protein limulin shows 30% sequence homology to human CRP and shares at least one antigenic epitope (Pathak and Agrawal 2019; Ying et al. 1992). While human CRP is an acute phase reactant, defined as any protein whose blood level changes rapidly in association with a stimulate host defense response, it is a constitutive protein in some lower species, being found, for example, at about 500 µg/mL in the rat which is about 50–100 times higher than the concentration in humans (Padilla et al. 2003).
Each subunit of CRP contains 206 amino acids and includes one intrachain disulfide bond connecting the only 2 cysteine residues in the CRP subunit primary sequence. In its pentameric conformation (i.e., pentameric CRP or pCRP), subunits are held together by strong apolar and electrostatic forces which help the pentamer assume a tightly packed protein conformation that, while being freely soluble in aqueous solutions, packs so tightly and it resists proteolysis with commonly used proteases (Kinoshita et al. 1989).
Each subunit has a shallow, calcium-dependent binding pocket for ligands presenting phosphocholine (PC) ligands (such as the C-polysaccharide cell wall as discussed above). Each PC binding site is expressed on the same “face” of the discoid protein such that, when CRP binds to surfaces expressing many PC ligands, the CRP pentamer (pCRP) orients “flat” on the PC binding surface, asymmetrically juxtaposing its PC binding face to the surface expressing PC groups, and allowing its opposite face (described as the CRP helical or effector face) to be accessible to cells and factors that will contribute to effector responses activated in response to CRP binding (See Fig. 2B, C). In this way, CRP is an opsonin—making a target surface and triggering a host defense response against the target. CRPs immobilized on PC ligands are known to activate the classical complement pathway and to stimulate phagocytosis (Summarized in Table 2; Deveraj et al. 2003; Du Clos et al. 1988, 1991; Jewell et al. 1993; Mihlan et al. 2009; Mold et al. 1999; Salonen et al. 1984; Singh et al. 2005; Swanson et al. 1989; Tseng and Mortensen 1988; Wu et al. 2015).
While widely recognized as the most prototypic protein representative of an ongoing acute phase response, its definitive role as a regulator of inflammation was not clearly established for decades after its discovery. Many directly conflicting conclusions described CRP as having both pro-inflammatory and anti-inflammatory activities and as being both anti-thrombotic and pro-thrombotic (Wu et al. 2015). Without truly understanding its biofunction, CRP was only perceived as a diagnostic marker of limited use in helping devise treatment strategies or in understanding disease pathologies.
Discovery of a Modified, Monomeric Isoform of CRP
Clarity in the biofunction of CRP was introduced in the 1990s with the discovery that the pentameric CRP structure could be induced to dissociate into monomeric subunits under physiologically relevant conditions. When the subunits are separated, biochemical energies are rapidly and irreversibly redistributed such that the globular subunits become modified into a unique structural isoform, described as a modified, monomeric CRP (i.e., mCRP). mCRP derives from pCRP by a non-proteolytic structural change that occurs when pCRP binds to PC groups on plasma membrane phosphatidylcholine lipids. This binding only occurs to exposed PC groups expressed at localized activated tissue sites when the enzyme phospholipase A2 cleaves the C2 fatty acid off a diacyl lipid, producing monoacyl phosphatidylcholine molecule (i.e., LysoPC) (Caprio et al. 2018). Figure 3 depicts how membrane surfaces and expression of phosphocholine groups are altered after PLA2 cleaves a fatty acyl chain off a diacyl lipid. Because each pCRP subunit has a PC binding site, pCRP can bind to a membrane surface, but only after the membrane has been “activated” by PLA2 which causes the PC ligands to become extended to be able to fit into the CRP binding pocket. Since all pCRP PC binding sites are located on the same face of the discoid pentamer, multivalent binding energy enables pCRP to be held next to the perturbed membrane surface, drawn close to the apolar zone of the activated membrane. Apolar biochemical forces are now available to weaken the hydrophobic forces holding the CRP pentamer together and keeping the subunit structures tightly compacted. The pCRP molecule begins to “swell”, forming an intermediate isoform that has been described as both a membrane-mCRP (mCRPm) (Ji et al. 2007) and a pCRP* (i.e., pCRP-star) protein (Braig et al. 2017). When sufficient biochemical energy is achieved to effectively separate subunits, pCRP undergoes a full structural conversion into mCRP, which now expresses its unique structural, binding, immunogenic and functional properties (Wu et al. 2015). Indeed, electron micrographs directly comparing pCRP and mCRP show that mCRP no longer appears as a pentameric disc, but as elongated short, fat fibrils that can self-aggregate into large multimers (Potempa et al. 2015). Formation of mCRP from pCRP does not involve a proteolytic cleavage; each protein shares the exact same primary protein sequence. The proteins are conformationally distinctive isoforms which have distinctive antigenicity. While the pCRP antigen is primarily detected and quantified in blood, various studies have reported the mCRP antigen is naturally expressed not in body fluids, but in various tissues, including those involving active inflammatory reactions. By recognizing and carefully controlling the presence and concentrations of each of pCRP and mCRP in experimental systems, it has become clear that mCRP is a potent pro-inflammatory stimulant while pCRP has weak anti-inflammatory activities (summarized in Table 2; Braig et al. 2017; McFadyen et al. 2018; Potempa et al. 2015; Wu et al. 2015).
mCRP Binds Cholesterol
One significant, unique binding ligand now recognized for CRP is cholesterol. The binding site involves a 13-amino acid peptide that is oriented near the PC binding sites of pCRP, extending toward the central void and the inter-subunit contact regions of the pentamer (Fig. 2A). This peptide extends into a hydrophobic cleft of the globular subunit structure, reaching down into the sole intrachain disulfide bond known to be present in the CRP subunit (Ji et al. 2009; Li et al. 2016). mCRP binding for cholesterol is maximized when the intrachain disulfide bond is reduced, suggesting this mCRP bioactivity may be physiologically regulated by a reduction reaction as might be mediated by thioredoxin or disulfide isomerase (Wang et al. 2011). In membranes, mCRP can directly insert into cholesterol-rich lipid rafts (Ji et al. 2007, 2009; Wang et al. 2011). As lipid raft microdomains are implicated in various receptor medicated cell activation processes involving signaling pathways and nuclear transcription factors (Cheng and Smith 2019), a mechanism by which CRP may regulate inflammation is becoming apparent. Indeed, mCRP has been shown to stimulate intracellular signaling pathways that lead to transcription of pro-inflammatory genes that control the production of the neutrophil chemotactic factor and Interleukin 8 (IL-8), factors known to amplify the acute inflammatory response (Boras et al. 2014, 2017; Khreiss et al. 2002, 2004a, b, 2005). mCRP binding to lipids is also relevant to lipoproteins metabolism as mCRP has been shown to affects the uptake of low-density lipoproteins (LDLs) by endothelial cells and macrophages (Ji et al. 2006; Schwedler et al. 2009).
Possible Functional Relationship of CRP to Gallbladder Disease
As the gallbladder is the key storage organ for bile salts and acids, and since bile compounds are synthesized from cholesterol, any compound that binds cholesterol may affect the synthesis and bioactivities of bile. Since gallbladder disease is known to involve significant inflammation in reactions known to be mediated by CRP, interrelationships between CRP, mCRP and cholesterol may be relevant to a better understanding of gallbladder diseases.
While pCRP is known to be synthesized by hepatocytes (Macintyre et al. 1983, 1985, 1992), many tissues have been reported to express the human CRP gene and to express a CRP primary protein transcript. As the updates are summarized in the human protein atlas (Thul et al. 2018) which describes more than 80% of the human protein-coding genes in 27 organs, the gallbladder is identified as the second ranked organ expressing the CRP gene (Fagerberg et al. 2014, updated on 2-Jul.-2019).
In a biodistribution study in normal mice, a small amount of intravenously injected radiolabeled mCRP selective accumulated to the gallbladder (Motie et al. 1998). While initially discounted as a relevant event, in light of the newly recognized binding affinity of mCRP for cholesterol and cholesterol analogues, the physiological significance of CRP expression or accumulation in such organs may have important physiological significance.
Of interest, various studies have looked at the effect of statin drugs on CRP levels. Most commonly, studies have looked at how statin treatment, CRP levels and inflammation correlate with disease progression. Since statin drugs target the synthesis of cholesterol, and since the mCRP isoform binds cholesterol and amplifies the acute inflammatory response, studies of statin drug effects and CRP should include an interpretation not only of pCRP blood levels, but of its conversion into mCRP and its interaction with cholesterol. Studies indicate only 20%–64% of patients taking statins achieve reasonable low-density lipoprotein cholesterol (LDL-C) thresholds (Schleyer et al. 2019). The question remains regarding the efficiency of a common current strategy in most medical treatments to restore the biological system to its homeostasis by lowering the elevated of marker(s). While statin therapy in cardiovascular disease is associated with reduction in LDL cholesterol levels, it does not appear to affect CRP levels, including high sensitive CRP levels (hsCRP). Diagnosticians are cautioned not to overinterpret a 50% reduction in hsCRP levels (i.e., from 4 to 2 µg/mL) in assessing disease severity or progression. Further studies evaluating the interrelationships of CRP levels, hsCRP levels, cholesterol levels and LDL levels in relation to diseases and associated inflammation levels are warranted.
Recommended Treatments for Gallbladder Diseases
Once confirmation of an acute cholecystitis is obtained, treatment is promptly initiated. Treatment paths differ by operative risk and severity grade. However, a typical course includes withholding oral intake, administering intravenous (IV) fluids and antibiotics, and pain management. The definitive therapy for acute cholecystitis is either early laporascopic cholecystectomy or delayed laparoscopic cholecystectomy, potentially with urgent or early gallbladder drainage prior to surgical removal.
Stable patients with no evidence of perforation or gangrene and no organ dysfunction and mild inflammatory changes are classified as mild (grade I) severity (Yokoe et al. 2018). In addition to supportive care, these patients are typically treated with oral antibiotic therapy with coverage against microorganisms in the Enterobacteriaceae family, with or without additional anaerobic organism coverage (Gomi et al. 2018). Early laparoscopic cholecystectomy is the preferred treatment course in patients with mild disease unless elevated surgical risk precludes this strategy.
Patients with acute cholecystitis accompanied with any one of the following: elevated white blood cell count (>18,000 µL), palpable tender mass in the right upper abdominal quadrant, duration of complaints >72 h, and marked local inflammation (gangrenous cholecystitis, pericholecystic abscess, hepatic abscess, biliary peritonitis, emphysematous cholecystitis), are classified as moderate (grade II) severity (Yokoe et al. 2018). Patients in this grade severity are treated with IV antibiotics in addition to supportive care. Early cholecystectomy is preferred, but these patients may need to their procedure delayed due to operative risk, and in this case gallbladder drainage may be used to facilitate medical stabilization prior to a delayed cholecystectomy (Okamoto et al. 2018).
Patients with organ dysfunction in at least any one of the following: hypotension requiring treatment with dopamine ≥ 5 µg/(kg·min), or any dose of norepinephrine, decreased level of consciousness, PaO2/FiO2 ratio < 300, oliguria, creatinine > 2.0 mg/dL, INR > 1.5, or platelet count < 100,000 cells/µL, and/or severe local inflammation are classified as severe (grade III) severity (Yokoe et al. 2018). Patients in this category are treated with IV antibiotics as well as are referred for urgent management of severe local inflammation by early cholecystectomy or percutaneous gallbladder drainage (i.e., percutaneous cholecystostomy tube) followed where indicated by delayed cholecystectomy at least 6 weeks later, when the patient's general condition has improved (Okamoto et al. 2018).
Discussion
Inflammation is the innate immune response to potentially harmful stimuli such as pathogens, injury, cancers and metabolic stress. The ultimate and essential function the naturally stimulated host defense inflammatory response is to rapidly react to and control any threats to survival and to restore the optimal homeostatic state.
When the acutely activated inflammatory response incompletely or inefficiently coordinates host defenses and restores healthful homeostasis, a muted response can persist, leading to a chronic condition that can be deleterious to the individual. Chronic inflammation can damage rather than protect and repair tissues by continuing to produce and secrete non-specific oxidizers (e.g., peroxide and peroxynitrite) and degrative enzymes (e.g., proteases, hydrolases) which indiscriminately continue to attack both disease-associated and normal tissues involved in the host defense response. Various vasoactive substances continue to be generated, as are cytokine mediators and neuropathic substances which cause continued tissue damage and pain. Chronic inflammation can lead to continued and progressive tissue damage which can, over time, contribute to the exacerbation of diseases that can become life threatening. While acute inflammation is vital to survival and an aggressive host defense against threats to health, chronic inflammation is pathological and problematic to good health (Antonelli and Kushner 2017; Chen et al. 2018).
CRP is widely understood as a blood diagnostic marker whose blood levels change rapidly correlate with active inflammatory processes. While its quantified presence correlates with inflammation, its role contributing to or regulating such processes had remained unknown even with decades of study. With the recent discovery that CRP can change structure into a distinctive isoform with distinctive solubility, antigenicity and binding activities, the biofunction of CRP as a modulator of inflammation is emerging. The widely recognized, serum-soluble pentameric CRP (pCRP) can bind structures presenting phosphorylcholine moieties, activate the classis complement pathway and act as an opsonin for leukocyte phagocytosis. Its overall biofunction, however, is now recognized being anti-inflammatory. When pCRP is converted into mCRP, however, it expresses cholesterol binding activity and can insert into lipid rafts where it activates many pathways that amplify the acute inflammatory response. mCRP is known to promote chemotaxis and recruitment of circulating leukocytes to areas of inflammation and to delay apoptosis. mCRP increases IL-8 and MCP-1 production and to affect the uptake of oxidized LDL. Most notably, mCRP is strongly pro-inflammatory. When it is produced at local sites of tissue damage, it, rather than the pCRP molecule, is the prototypic acute phase reactant, amplifying acute inflammatory responses (Sproston and Ashworth 2018; Wu et al. 2015). Acute inflammation can be healthful and protective and may be a preferred response to threats to tissue integrity and function. As the understanding of how and where mCRP is produced in tissues involved in both health and disease, the true role of CRP in reactions of inflammation will finally be established.
The gallbladder is an important component of the hepatobiliary system whose primary function is to aid in digestion of foodstuffs and facilitate removal of waste products from the body into the intestine. The gallbladder is principally a storage organ for bile—chemically modified salts and acids of cholesterol which are synthesized in the liver and which function as surfactants to solubilize lipids and other fatty substances that are introduced into the body. However, since cholesterol has an important role in cellular function, it can also be directly synthesized by each cell in the body (Huff and Jialal 2019). The biochemical nature of fatty substances is to avoid or minimize contact with water. To minimize the energy needed to maintain an interface with the aqueous phase, fatty substances self-associate into aggregates with a non-aqueous core that can form waxy, molecular clumps of various size. Bile is a natural detergent molecule that functions by sitting on the surface of fatty aggregates, lowering the energy barriers needed to maintain an interface with water and limiting the size of any apolar clumps. By limiting particle size and establishing an aqueous interface, bile facilitates the movement of bile-coated lipids through the digestive system.
The structure of the hepatobiliary system (i.e., the hepatobiliary tree) includes the liver as a source of bile, the cystic duct which carries bile from the liver to the gallbladder, the gallbladder itself which is the primary storage and concentrating organ for bile, the common biliary duct that carries bile through the pancreas (where digestive enzymes can be introduced into processed foodstuffs), and the duodenum (i.e., the first part of the small intestine) where compounds moving through this tree are deposited. As the hepatobiliary system is designed to help process poorly- or non-soluble lipidic masses, any defect in the biochemistry of how bile is formed, stored, transported, or how it successfully functions to disaggregate fatty clumps, can lead to unhealthful accumulation of these clumps which can congest the proper flow of materials through the hepatobiliary tree, and lead to disease. Non-solubilized fatty deposits can grow into and form gallstones, a hallmark structures of gallbladder diseases. Gallstones can obstruct any part of the biliary tree and in turn introduce structural stress into the system anatomy, leading to damaged tissues. When any tissue become damaged, no matter what the cause, the body reacts to the introduced threat to health and homeostasis by activating natural host defense responses which includes inflammation.
The initial host defense response to tissue damage includes immediate and aggressive activation of inflammation that stimulates vasoactive and cellular responses focused on controlling the threat, attacking and removing any foreign material, and restoring healthful tissue structure and function. Inflammation is non-specific to the inciting cause initiating the response; its destructive responses (e.g., generation of reactive oxygen species and degradative enzymes) will affect near-by normal tissues. Inflammation that persists over long times (i.e., chronic inflammation) can adversely compromise normal tissue structures and functions and contribute to many symptoms and pathologies associated with continued, prolonged disease.
Gallbladder disease that involves inflammation is known as Cholecystitis. In diagnosing diseases of the gallbladder, consideration should be given to whether disease is caused by obstructions, whether disease involves tissue damage caused by obstruction or some other factor, the extent of any tissue damage, and whether there are confounding contributions of infections or cancers in affected tissues. In severe cases of cholecystitis, a cholecystectomy, i.e., the surgical removal of the gallbladder and other involved tissues, remains the mainstay of treatment.
In gallbladder diseases, significantly elevated CRP levels correlate with diagnosis of acute cholecystitis and may be of value in differentiating chronic cholecystitis and gallbladder cancer. Using well defined elevated CRP levels in conjunction with ultrasound exams and white blood cell counts, CRP can be used to enhance the diagnosis of acute cholecystitis. CRP levels have been used to predict the outcome and prognosis of gallbladder resection in gallbladder cancer, and to predict acute cholecystitis severity. Also, the short-term changes of CRP levels in blood (i.e., 6–10 h), is suggesting the needs of more frequent testing/a profile testing especially throughout the complication evaluation to better understanding the CRP turnover during its 19 h half-life. For example, to achieve 95% confidence (0.95 probability) 14 samples per period concluded to be required to characterize the CRP levels. (Dorraki et al. 2018).
As an understanding of CRP structural isoforms is evolving, CRP should now be evaluated not only as a marker for gallbladder disease, but as a modulator of natural defense mechanisms that are activated in response to disease pathologies. The functional interrelationships of CRP and its different isoforms as natural mediators of gallbladder disease pathophysiology is entering a new era.
References
Abernethy TJ, Avery OT (1941) The occurrence during acute infections of a protein not normally present in the blood: I. Distribution of the reactive protein in patients’ sera and the effect of calcium on the flocculation reaction with C polysaccharide of pneumococcus. J Exp Med 73(2):173–182
Akimoto S, Banshodani M, Nishihara M, Nambu J, Kawaguchi Y, Shimamoto F, Dohi K, Sugino K, Ohdan H (2016) Acute Cholecystitis with significantly elevated levels of serum carbohydrate antigen 19–9. Case Rep Gastroenterol 10:410–416. https://doi.org/10.1159/000448068
Antonelli M, Kushner I (2017) It's time to redefine inflammation. FASEB J 31(5):1787–1791. https://doi.org/10.1096/fj.201601326R
Ay S, Tanrikulu CS (2019) Diagnostic utility of neutrophil lymphocyte ratio in acute complicated cholecystitis. Ann Med Res 26(2):135–138. https://doi.org/10.5455/annalsmedres.2018.09.198
Beliaev AM, Marshall RJ, Booth M (2015) C-reactive protein has a better discriminative power than white cell count in the diagnosis of acute cholecystitis. J Surg Res 198(2015):66–72
Boras E, Slevin M, Alexander MY, Gilmore W, Ashworth J, Krupinski J, Potempa LA, Al Abdulkareem I, Elobeid A, Matou-Nasri S (2014) Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signaling pathway. Cytokine 69(2):165–179. https://doi.org/10.1016/j.cyto.2014.05.027
Boras E, Slevin M, Gilmore W, Potempa LA, Matou-Nasri S (2017) Common angiogenic signalling pathways induced by monomeric-CRP and FGF-2 through MAP and PI3K. Eur J Exp Biol 7:18–29
Braig D, Nero TL, Koch H-G, Kaiser B, Wang X, Thiele JR, Morton CJ, Zeller J, Kiefer J, Potempa LA, Mellett NA, Miles LA, Du XJ, Meikle PJ, Huber-Lang M, Stark GB, Parker MW, Peter K, Eisenhardt SU (2017) Transitional changes in the CRP structure lead to the exposure of proinflammatory binding sites. Nat Commun 23(8):14188–14207. https://doi.org/10.1038/ncomms14188
Brent AJ, Hull R, Jeffery KJM, Phillips RR, Atkins B (2006) Acute cholecystitis complicating mumps. Clin Infect Dis 42:302–303. https://doi.org/10.1086/499107
Caprio V, Badimon L, Di Napoli M, Fang W-H, Ferris GR, Guo B, Iemma RS, Liu D, Zeinolabediny Y, Slevin M (2018) pCRP-mCRP dissociation mechanisms as potential targets for the development of small-molecule anti-inflammatory chemotherapeutics. Front Immunol 9:1089–1096. https://doi.org/10.3389/fimmu.2018.01089
Cen L, Pan J, Zhou B, Yu C, Li Y, Chen W, Shen Z (2018) Helicobacter Pylori infection of the gallbladder and the risk of chronic cholecystitis and cholelithiasis: a systematic review and meta-analysis. Helicobacter 23(1):1–10. https://doi.org/10.1111/hel.12457
Chaudhry S, Hussain R, Rajasundaram R, Corless D (2011) Gangrenous cholecystitis in an asymptomatic patient found during an elective laparoscopic cholecystectomy: a case report. J Med Case Rep 5:199. https://doi.org/10.1186/1752-1947-5-199
Chen L, Deng Z, Deng J, Li Y, Wang X, Zhao L (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget (Jan) 9(6):7204–7218. https://doi.org/10.18632/oncotarget.23208
Cheng X, Smith JC (2019) Biological membrane organization and cellular signaling. Chem Rev 119:5849–5880. https://doi.org/10.1021/acs.chemrev.8b00439
Choi YJ, Yoon HY, Jang SA, Hong MJ, Lee WS, Yoo W-H (2014) A case of systemic lupus erythematosus initially presented with acute acalculous cholecystitis. J Rheum Dis 21(3):140–142. https://doi.org/10.4078/jrd.2014.21.3.140
Cui J, Hay K, Hendahewa R (2018) C-reactive protein as a diagnostic marker for gangrenous cholecystitis. J Surg Sci Oper Care 1(1):105–108
del del-Moral-Martínez M, Barrientos-Delgado A, Crespo-Lora V, Cervilla-Sáez-de-Tejada ME, Salmerón-Escobar J (2015) Eosinophilic cholecystitis: an infrequent cause of acute cholecystitis. Rev Esp Enferm Dig 107:45–47
Devaraj S, Xu DY, Jialal I (2003) C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation 107:398–404
Di Ciaula A, Portincasa P (2018) Recent advances in understanding and managing cholesterol gallstones. F1000Research. https://doi.org/10.12688/f1000research.15505.1
Di Ciaula A, Garruti G, Baccetto RL, Molina-Molina E, Bonfrate L, Wang DQ-H, Portincasa P (2017) Bile acid physiology. Ann Hepatol 16 (Suppl. 1):s4–s14. https://doi.org/10.5604/01.3001.0010.5493
Díaz-Flores A, Cárdenas-Lailson E, Cuendis-Velázquez A, Rodríguez-Parra A, Trejo-Ávila M (2017) C-reactive protein as a predictor of difficult laparoscopic cholecystectomy in patients with acute calculous cholecystitis: a multivariate analysis. J Laparoendosc Adv Surg Tech. https://doi.org/10.1089/lap.2017.0139
Dorraki M, Fouladzadeh A, Salamon SJ, Allison A, Coventry BJ, Abbott D (2018) On detection of periodicity in C-reactive protein (CRP) levels. Nat Sci Rep 8:11979. https://doi.org/10.1038/s41598-018-30469-8
Du Clos TW, Zlock LT, Rubin RL (1988) Analysis of the binding of C-reactive protein to histones and chromatin. J Immunol 141(12):4266–4270
Du Clos TW, Zlock LT, Marnell L (1991) Definition of a C-reactive protein binding determinant on histones. J Biol Chem 266(4):2167–2171
Elwood DR (2008) Cholecystitis. Surg Clin N Am 88:1241–1252. https://doi.org/10.1016/j.suc.2008.07.008
Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjöstedt E, Lundberg E, Szigyarto CA, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, Lindskog C, Danielsson F, Mardinoglu A, Sivertsson A, von Feilitzen K, Forsberg M, Zwahlen M, Olsson I, Navani S, Huss M, Nielsen J, Ponten F, Uhlén M (2014) Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 13(2):397–406
Fairfield CJ, Wigmore SJ, Harrison EM (2019) Gallstone disease and the risk of cardiovascular disease. Nat Sci Rep 9:5830–5838. https://doi.org/10.1038/s41598-019-42327-2
Fan LL, Chen BH, Dai ZJ (2017) The relation between gallstone disease and cardiovascular disease. Nat Sci Rep. https://doi.org/10.1038/s41598-017-15430-5
FDA. U.S. Department of Health and Human Services (2005) Guidance for industry and FDA staff. Review criteria for assessment of C-reactive protein (CRP), high sensitivity C-reactive protein (hsCRP) and cardiac C-reactive protein (cCRP) assays. Document issued on September 22, 2005. Accessed 13 Mar 2018
Fei Q, Kent D, Botello-Smith WM, Nur F, Nur S, Alsamarah A, Chatterjee P, Lambros M, Luo Y (2018) Molecular mechanism of resveratrol’s lipid membrane protection. Sci Rep 8:1587–1599. https://doi.org/10.1038/s41598-017-18943-1
Ghibellini G, Leslie EM, Brouwer KLR (2006) Methods to evaluate biliary excretion of drugs in humans: an updated review. Mol Pharm 3(3):198–211. https://doi.org/10.1021/mp060011k
Gomi H, Solomkin JS, Schlossberg D, Okamoto K, Takada T, Strasberg SM, Ukai T, Endo I, Iwashita Y, Hibi T, Pitt HA, Matsunaga N, Takamori Y, Umezawa A, Asai K, Suzuki K, Han HS, Hwang TL, Mori Y, Yoon YS, Huang WS, Belli G, Dervenis C, Yokoe M, Kiriyama S, Itoi T, Jagannath P, Garden OJ, Miura F, de Santibañes E, Shikata S, Noguchi Y, Wada K, Honda G, Supe AN, Yoshida M, Mayumi T, Gouma DJ, Deziel DJ, Liau KH, Chen MF, Liu KH, Su CH, Chan ACW, Yoon DS, Choi IS, Jonas E, Chen XP, Fan ST, Ker CG, Giménez ME, Kitano S, Inomata M, Mukai S, Higuchi R, Hirata K, Inui K, Sumiyama Y, Yamamoto M (2018) Tokyo Guidelines 2018: antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci 25(1):3–16. https://doi.org/10.1002/jhbp.518
Goodier M, Mulira S, Andronikou S (2012) Acalculous cholecystitis presenting in an out-patient with no risk factors. S Afr J Radiol 16(1):8–10
Gotschlich EC, Liu T-Y, Oliveira E (1982) Binding of C-reactive protein to C-carbohydrate and PC-substituted protein. Ann N Y Acad Sci 389:163–171
Gouma DJ, Obertop H (1992) Acute calculous cholecystitis. What is new in diagnosis and therapy? Hepatopancreatobiliary Surg 6(2):69–78
Greenberger NJ, Paumgartner G (2018) Diseases of the gallbladder and bile ducts. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J (eds) Harrison’s principles of internal medicine, 20th edn. McGaw-Hill, New York
Gregory GC, Kuzman M, Sivaraj J, Navarro AP, Cameron IC, Irving G, Gomez D (2019) C-reactive protein is an independent predictor of difficult emergency cholecystectomy. Cureus 11(4):e4573. https://doi.org/10.7759/cureus.4573
Gurbulak EK, Gurbulak B, Akgun IE, Duzkoylu Y, Battal M, Celayir MF, Demir U (2015) Prediction of the grade of acute cholecystitis by plasma level of C-reactive protein. Iran Red Crescent Med J 17(4):e28091. https://doi.org/10.5812/ircmj.17(4)2015.28091
Haque ME, Lentz BR (2004) Roles of curvature and hydrophobic interstice energy in fusion: studies of lipid perturbant effects. Biochemistry 43(12):3507–3517. https://doi.org/10.1021/bi035794j
Hernandez-Nazara A, Curiel-Lopez F, Martinez-Lopez E, Hernandez-Nazara Z, Panduro A (2006) Genetic predisposition of cholesterol gallstone disease. Ann Hepatol 5(3):140–149
Hirota M, Takada T, Kawarada Y, Nimura Y, Miura F, Hirata K, Mayumi T, Yoshida M, Strasberg S, Pitt H, Gadacz TR, de Santibanes E, Gouma DJ, Solomkin JS, Belghiti J, Neuhaus H, Büchler MW, Fan ST, Ker CG, Padbury RT, Liau KH, Hilvano SC, Belli G, Windsor JA, Dervenis C (2007) Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg 14:78–82. https://doi.org/10.1007/s00534-006-1159-4
Hsu YC, Perin MS (1995) Human neuronal pentraxin II (NPTX2): conservation, genomic structure, and chromosomal localization. Genomics 28(2):220–227
Huff T, Jialal I (2019) Physiology, cholesterol. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL). https://www.ncbi.nlm.nih.gov/books/NBK470561/. Accessed 13 Mar 2019
Ibrahim M, Sarvepalli S, Morris-Stiff G, Rizk M, Bhatt A, Walsh M, Hayat U, Garber A, Vargo J, Burke CA (2018) Gallstones: watch and wait, or intervene? Clevel Clin J Med 85(4):323–331. https://doi.org/10.3949/ccjm.85a.17035
Jewell WS, Marnell LL, Rokeach LA, Du Clos TW (1993) C-reactive protein (CRP) binding to the Sm-D protein of snRNPS. Identification of a short polypeptide binding region. Mol Immunol 30(8):701–708. https://doi.org/10.1016/0161-5890(93)90141-w
Ji SR, Wu Y, Potempa LA, Qiu Q, Zhao J (2006) The interactions of low-density lipoprotein with different forms of C-reactive protein: implication of an active role of modified C-reactive protein in the pathogenesis of atherosclerosis. Int J Biochem Cell Biol 38:648–661
Ji SR, Wu Y, Zhu L, Potempa LA, Sheng FL, Wei L, Zhao J (2007) Cell membranes and liposomes dissociate C-reactive protein (CRP) to form a new, biologically active structural intermediate: mCRPm. FASEB J 21:284–294
Ji S-R, Bai L, Shi J-M, Li H-Y, Potempa LA, Filep JG, Zhao J, Wu Y (2009) Monomeric C-reactive protein activates endothelial cells via lipid raft membrane microdomains. FASEB J 23(6):1806–1816. https://doi.org/10.1096/fj.08-116962
Juvonen T, Kiviniemi H, Niemelä O, Kairaluoma MI (1992) Diagnostic accuracy of ultrasonography and C reactive protein concentration in acute cholecystitis: a prospective clinical study. Eur J Surg 158(6–7):365–369
Karabadzhak AG, Weerakkody D, Deacon J, Andreev OA, Reshetnyak YK, Engelman DM (2018) Bilayer thickness and curvature influence binding and insertion of a pHLIP peptide. Biophys J 114:2107–2115. https://doi.org/10.1016/j.bpj.2018.03.036
Khreiss T, József L, Hossain S, Chan JSD, Potempa LA, Filep JG (2002) Loss of pentameric symmetry of C-reactive protein is associated with delayed apoptosis of human neutrophils. J Biol Chem 277:40775–40781
Khreiss T, József L, Potempa LA, Filep JG (2004a) Conformational rearrangement in C-reactive protein is required for proinflammatory actions on human endothelial cells. Circulation 109:2016–2022
Khreiss T, József L, Potempa LA, Filep JG (2004b) Opposing effects of C-reactive protein isoforms on shear-induced neutrophil-platelet adhesion and neutrophil aggregation in whole blood. Circulation 110:2713–2720
Khreiss T, József L, Potempa LA, Filep JG (2005) Loss of pentameric symmetry in C-reactive protein induces Interleukin-8 secretion through peroxynitrite signaling in human neutrophils. Circ Res 97:690–697
Kinoshita CM, Ying S-C, Hugli TE, Siegel JN, Potempa LA, Jiang H, Houghten RA, Gewurz H (1989) Elucidation of a protease-sensitive site involved in the binding of calcium to C-reactive protein. Biochemistry 28:9840–9848
Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Huang WS, Kim MH, Okamoto K, Belli G, Dervenis C, Chan ACW, Lau WY, Endo I, Gomi H, Yoshida M, Mayumi T, Baron TH, de Santibañes E, Teoh AYB, Hwang TL, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Higuchi R, Kitano S, Inomata M, Deziel DJ, Jonas E, Hirata K, Sumiyama Y, Inui K, Yamamoto M (2018) Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci 25:17–30. https://doi.org/10.1002/jhbp.512
Koshiol J, Castro F, Kemp T, Gao Y-T, Roa JC, Wang B, Nogueira L, Araya JC, Shen MC, Rashid A, Hsing AW, Hildesheim A, Ferreccio C, Pfeiffer RM, Pinto LA (2016) Association of inflammatory and other immune markers with gallbladder cancer: results from two independent case-control studies. Cytokine 83:217–225. https://doi.org/10.1016/j.cyto.2016.05.003
Kushner I, Rzewnicki D, Samols D (2006) What does minor elevation of C-reactive protein signify? Am J Med 119(2):166. https://doi.org/10.1016/j.amjmed.2005.06.057
Lähdesmäki K, Ollila OH, Koivuniemi A, Kovanen PT, Hyvönen MT (2010) Membrane simulations mimicking acidic pH reveal increased thickness and negative curvature in a bilayer consisting of lysophosphatidylcholines and free fatty acids. Biochim Biophys Acta 1798(5):938–946. https://doi.org/10.1016/j.bbamem.2010.01.020
Lee JM, Boll DT (2018) Disease of the Gallbladder And Biliary Tree. In: Hodler J, Kubik-Huch RA, von Schulthess GK (eds) Diseases of the abdomen and pelvis 2018–2021. IDKD Springer Series, Cham, pp 49–56
Lee R, Ha H, Han YS, Kwon HJ, Ryeom H, Chun JM (2017) Percutaneous transhepatic gallbladder drainage followed by elective laparoscopic cholecystectomy for patients with moderate to severe acute cholecystitis. Medicine (Baltimore) 96(44):e8533. https://doi.org/10.1097/MD.0000000000008533
Leslie CC (2015) Cytosolic phospholipase A2: physiological function and role in disease. J Lipid Res 56:1386–1402. https://doi.org/10.1194/jlr.R057588
Li H-Y, Jing Wang J, Meng F, Zhe-Kun Jia Z-K, Su Y, Bai Q-F, Lv LL, Ma FR, Potempa LA, Yan YB, Ji SR, Wu Y (2016) An intrinsically disordered motif mediates diverse actions of monomeric C-reactive protein. J Biol Chem 291(16):8795–8804. https://doi.org/10.1074/jbc.M115.695023
Macintyre SS (1992) Regulated export of a secreted protein from the ER of the hepatocyte. A specific binding site retaining C-reactive protein within the ER is downregulated during the acute phase response. J Cell Biol 118(2):253–265
Macintyre SS, Schultz D, Kushner I (1983) Synthesis and secretion of C-reactive protein by rabbit hepatocyte cultures. Biochem J 210:707–715
Macintyre SS, Kushner I, Samols D (1985) Secretion of C-reactive protein becomes more efficient during the course of the acute phase response. J Biol Chem 260:4169–4173
Macleod CM, Avery OT (1941a) The occurrence during acute infections of a protein not normally present in blood: II. Isolation and properties of the reactive protein. J Exp Med 73(2):183–190
Macleod CM, Avery OT (1941b) The occurrence during acute infections of a protein not normally present in the blood: III. Immunological properties of the C-reactive protein and its differentiation from normal blood proteins. J Exp Med 73(2):191–200
Marquardt D, Heberle FA, Greathouse DV, Koeppe RE II, Standaert RF, van Oosten BJ, Harroun TA, Kinnun JJ, Williams JA, Wassall SR, Katsaras J (2016) Lipid bilayer thickness determines cholesterol’s location in model membranes. Soft Matter 12:9417–9428. https://doi.org/10.1039/c6sm01777k
McFadyen JD, Kiefer J, Braig D, Loseff-Silver J, Potempa LA, Eisenhardt SU, Peter K (2018) Dissociation of C-reactive protein localizes and amplifies inflammation: evidence for a direct biological role of C-reactive protein and its conformational changes. Front Immunol 9:1351–1361. https://doi.org/10.3389/fimmu.2018.01351
Mihlan M, Stippa S, Jozsi M, Zipfel PF (2009) Monomeric CRP contributes to complement control in fluid phase and on cellular surfaces and increases phagocytosis by recruiting factor H. Cell Death Differ 16(12):1530–1640
Mok KWJ, Reddy R, Wood F, Turner P, Ward JB, Pursnani KG, Date RS (2014) Is C-reactive protein a useful adjunct in selecting patients for emergency cholecystectomy by predicting severe/gangrenous cholecystitis? Int J Surg 12:649–653
Mok KWJ, Gog YL, Howell LE, Date RS (2016) Is C-reactive protein the single most useful predictor of difficult laparoscopic cholecystectomy or its conversion? A pilot study. J Minim Access Surg 12(1):26–32. https://doi.org/10.4103/0972-9941.158963
Mold C, Gewurz H, Du Clos TW (1999) Regulation of complement activation by C-reactive protein. Immunopharmacology 42(1–3):23–30. https://doi.org/10.1016/s0162-3109(99)00007-7
Motie M, Schaul KW, Potempa LA (1998) A study of the plasma clearance and biodistribution characteristics of human 125I-mCRP in the mouse. Drug Metab Disposition 26:977–981
Ng JY, Gu J (2018) Conservative management of acalculous cholecystitis in a seven-year-old child. Cureus 10(1):e2092. https://doi.org/10.7759/cureus.2092
Nizri E, Epstein L, Ben-Yehuda A, Greenberg R (2016) Admission CRP level as an indicator for the need of percutaneous cholecystostomy in acute cholecystitis. J Gastrointest Dig Syst 6:2. https://doi.org/10.4172/2161-069X.1000413
Okamoto K, Suzuki K, Takada T, Strasberg SM, Asbun HJ, Endo I, Iwashita Y, Hibi T, Pitt HA, Umezawa A, Asai K, Han HS, Hwang TL, Mori Y, Yoon YS, Huang WS, Belli G, Dervenis C, Yokoe M, Kiriyama S, Itoi T, Jagannath P, Garden OJ, Miura F, Nakamura M, Horiguchi A, Wakabayashi G, Cherqui D, de Santibañes E, Shikata S, Noguchi Y, Ukai T, Higuchi R, Wada K, Honda G, Supe AN, Yoshida M, Mayumi T, Gouma DJ, Deziel DJ, Liau KH, Chen MF, Shibao K, Liu KH, Su CH, Chan ACW, Yoon DS, Choi IS, Jonas E, Chen XP, Fan ST, Ker CG, Giménez ME, Kitano S, Inomata M, Hirata K, Inui K, Sumiyama Y, Yamamoto M (2018) Tokyo Guidelines 2018: flowchart for the management of acute cholecystitis. J Hepatobiliary Pancreat Sci 25(1):55–72. https://doi.org/10.1002/jhbp.516
Okino AM, Bürger C, Cardoso JR, Lavado EL, Lotufo PA, Campa A (2006) The acute-phase proteins serum amyloid A and C reactive protein in transudates and exudates. Mediat Inflamm. https://doi.org/10.1155/MI/2006/47297
Omeis IA, Hsu Y-C, Perin MS (1996) Mouse and human neuronal pentraxin 1 (NPTX1): conservation, genomic structure and chromosomal localization. Genomics 36:543–545
Onoe S, Maeda A, Takayama Y, Fukami Y, Kaneoka Y (2017) A preoperative predictive scoring system to predict the ability to achieve the critical view of safety during laparoscopic cholecystectomy for acute cholecystitis. Int Hepato-Pancreato-Biliary (HPB) 19:406–410. https://doi.org/10.1016/j.hpb.2016.12.013
Padilla ND, Bleeker WK, Lubbers Y, Rigter GM, van Mierlo GJ, Daha MR, Hack CE (2003) Rat C-reactive protein activates the autologous complement system. Immunology 109:564–571. https://doi.org/10.1046/j.1365-2567.2003.01681.x
Pathak A, Agrawal A (2019) Evolution of C-reactive protein. Rev Front Immunol 10:943–955. https://doi.org/10.3389/fimmu.2019.00943
Pepys MB, Hirschfield GM (2003) C-reactive protein: a critical update. J Clin Invest 111(12):1805–1812. https://doi.org/10.1172/JCI18921
Pichot R, Watson RL, Norton IT (2013) Phospholipids at the interface: current trends and challenges. Int J Mol Sci 14:11767–11794. https://doi.org/10.3390/ijms140611767
Potempa LA, Yao Z-Y, Ji S-R, Filep JG, Wu Y (2015) Solubilization and purification of recombinant modified C-reactive protein from inclusion bodies using reversible anhydride modification. Biophys Rep 1(1):18–33. https://doi.org/10.1007/s41048-015-0003-2
Pozo MJ, Camello PJ, Mawe GM (2004) Chemical mediators of gallbladder dysmotility. Curr Med Chem 11:1801–1812. https://doi.org/10.2174/0929867043364955
Pulkkinen J, Eskelinen M, Kiviniemi V, Kotilainen T, Pöyhönen M, Kilpeläinen L, Käkelä P, Kastarinen H, Paajanen H (2014) Effect of statin use on outcome of symptomatic cholelithiasis: a case–control study. BMC Gastroenterol 14:119–126. https://doi.org/10.1186/1471-230X-14-119
Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31(5):986–1000. https://doi.org/10.1161/ATVBAHA.110.207449
Sack GH (2018) Serum amyloid A—a review. Mol Med 24:46. https://doi.org/10.1186/s10020-018-0047-0
Salonen E-M, Vartio T, Hedman K, Vaheri A (1984) Binding of fibronectin by the acute phase reactant C-reactive protein. J Biol Chem 259:1496–1501
Sato N, Kinoshita A, Imai N, Akasu T, Yokota T, Iwaku A, Koike K, Saruta M (2018) Inflammation-based prognostic scores predict disease severity in patients with acute cholecystitis. Eur J Gastroenterol Hepatol 30:484–489. https://doi.org/10.1097/MEG.0000000000001063
Schleyer T, Hui S, Wang J, Zhang Z, Knapp K, Baker J, Chase M, Boggs R, Simpson RJ Jr (2019) Quantifying unmet need in statin-treated hyperlipidemia patients and the potential benefit of further LDL-C reduction through an EHR-based retrospective cohort study. J Manag Care Spec Pharm 25(5):544–554
Schuld J, Glanemann M (2015) Acute cholecystitis. Viszeralmedizin Gastrointest Med Surg 31:163–165. https://doi.org/10.1159/000431275
Schwedler SB, Hansen-Hagge T, Reichert M, Schmiedeke D, Schneider R, Galle J, Potempa LA, Wanner C, Filep JG (2009) Monomeric C-reactive protein decreases acetylated LDL uptake in human endothelial cells. Clin Chem 55:1728–1731. https://doi.org/10.1373/clinchem.2009.125732
Shabanzadeh DM, Sørensen LT, Jørgensen T (2016) Determinants for gallstone formation - a new data cohort study and a systematic review with meta-analysis. Scand J Gastroenterol 51(10):1239–1248. https://doi.org/10.1080/00365521.2016.1182583
Shiba H, Misawa T, Fujiwara Y, Futagawa Y, Furukawa K, Haruki K, Iwase R, Iida T, Yanaga K (2015) Glasgow prognostic score predicts outcome after surgical resection of gallbladder cancer. World J Surg 39:753–758. https://doi.org/10.1007/s00268-014-2844-0
Shreders A, Michie C (2010) Acalculous cholecystitis in 14-year old boy with no predisposing factors. West Lond Med J 2:11–14
Shrive AK, Cheetham GMT, Holden D, Myles DAA, Turnell WG, Volanakis JE, Pepys MB, Bloomer AC, Greenhough TJ (1996) Three-dimensional structure of human C-reactive protein. Nat Struc Biol 3:346–354
Singh U, Devaraj S, Jialal I (2005) C-reactive protein decreases tissue plasminogen activator activity in human aortic endothelial cells: evidence that C-reactive protein is a procoagulant. Arterioscler Throm Vasc Biol 25:2216–2221
Sproston NR, Ashworth JJ (2018) Role of C-reactive protein at sites of inflammation and infection. Front Immunol 9:754–765. https://doi.org/10.3389/fimmu.2018.00754
Srinivasan N, White HE, Emsley J, Wood SP, Pepys MB, Blundell TL (1994) Comparative analyses of pentraxins: implications for protomer assembly and ligand binding. Structure 2:1017–1027
Stinton LM, Shaffer EA (2012) Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 6(2):172–187. https://doi.org/10.5009/gnl.2012.6.2.172
Strasberg SM (2008) Acute calculous cholecystitis. N Engl J Med 358:2804–2811
Su HX, Zhou HH, Wang MY, Cheng J, Zhang SC, Hui F, Chen XZ, Liu SH, Liu QJ, Zhu ZJ, Hu QR, Wu Y, Ji SR (2014) Mutations of C-reactive protein (CRP) -286 SNP, APC and p53 in colorectal cancer: implication for a CRP-Wnt crosstalk. PLoS ONE 9(7):e102418. https://doi.org/10.1371/journal.pone.0102418
Swanson SJ, McPeek MM, Mortensen RF (1989) Characteristics of the binding of human C-reactive protein (CRP) to laminin. J Cell Biol 40:121–132
Teckchandani N, Garg PK, Hadke NS, Jain SK, Kant R, Mandal AK, Bhalla P (2010) Predictive factors for successful early laparoscopic cholecystectomy in acute cholecystitis: a prospective study. Int J Surg 8:623–627. https://doi.org/10.1016/j.ijsu.2010.05.014
Thompson GR (1971) Absorption of fat-soluble vitamins and sterols. J Clin Pathol 5:85–89
Thul PJ, Lindskog C (2018) The human protein atlas: a spatial map of the human proteome. Protein Sci 27(1):233–244. https://doi.org/10.1002/pro.3307
Tillet WS, Francis T (1930) Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med 52(4):561–571
Tseng J, Mortensen RF (1988) Binding of human C-reactive protein (CRP) to plasma fibronectin occurs via the phosphorylcholine-binding site. Mol Immunol 25:679–686
Tsukada T, Nakano T, Miyata T, Sasaki S, Ohta T (2012) Cholecystomucoclasis: revaluation of safety and validity in aged populations. BMC Gastroenterol 12:113. https://doi.org/10.1186/1471-230X-12-113
Vaishnavi C, Singh S, Kochhar R, Singh G, Singh K (2004) C-reactive protein in patients with gallbladder and biliary tract diseases. Trop Gastroenterol 25(2):73–75
Vigushin DM, Pepys MB, Hawkins PN (1993) Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest 91:1351–1357
Wang M-Y, Ji S-R, Bai C-J, El Kebir D, Li H-Y, Shi J-M, Zhu W, Costantino S, Zhou HH, Potempa LA, Zhao J, Filep JG, Wu Y (2011) A redox switch in C-reactive protein modulates activation of endothelial cells. FASEB J 25:3186–3196. https://doi.org/10.1096/fj.11-182741
Wevers KP, van Westreenen HL, Patijn GA (2013) Laparoscopic cholecystectomy in acute cholecystitis: C-reactive protein level combined with age predicts conversion. Surg Laparosc Endosc Percutan Tech 23(2):163–166. https://doi.org/10.1097/SLE.0b013e31826d7fb0
Wu Y, Potempa LA, Kebir DE, Filep JG (2015) C-reactive protein and inflammation: conformational changes affect function. Biol Chem 396(11):1181–1197. https://doi.org/10.1515/hsz-2015-0149
Yamamoto T, Komori J, Morimoto T, Kobayashi H, Kaihara S, Hosotani R (2017) Prediction of surgical difficulty in laparoscopic cholecystectomy for acute cholecystitis performed within 24 hours after hospital admission. Int Surg 102:145–150. https://doi.org/10.9738/INTSURG-D-16-00014.1
Yarla NS, Bishayee A, Vadlakonda L, Chintala R, Duddukuri GR, Reddanna P, Dowluru KSVGK (2016) Phospholipase A2 isoforms as novel targets for prevention and treatment of inflammatory and oncologic diseases. Curr Drug Targets 17(16):1940–1962. https://doi.org/10.2174/1389450116666150727122501
Ying SC, Marchalonis JJ, Gewurz AT, Siegel JN, Jiang H, Gewurz BE, Gewurz H (1992) Reactivity of anti-human C-reactive protein (CRP) and serum amyloid P component (SAP) monoclonal antibodies with limulin and pentraxins of other species. Immunology 76(2):324–330
Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, Kozaka K, Endo I, Deziel DJ, Miura F, Okamoto K, Hwang TL, Huang WS, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Noguchi Y, Shikata S, Ukai T, Higuchi R, Gabata T, Mori Y, Iwashita Y, Hibi T, Jagannath P, Jonas E, Liau KH, Dervenis C, Gouma DJ, Cherqui D, Belli G, Garden OJ, Giménez ME, de Santibañes E, Suzuki K, Umezawa A, Supe AN, Pitt HA, Singh H, Chan ACW, Lau WY, Teoh AYB, Honda G, Sugioka A, Asai K, Gomi H, Itoi T, Kiriyama S, Yoshida M, Mayumi T, Matsumura N, Tokumura H, Kitano S, Hirata K, Inui K, Sumiyama Y, Yamamoto M (2018) Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci 25(1):41–54. https://doi.org/10.1002/jhbp.515
Yuzbasioglu Y, Ucoz D, Icme F, Ge H, Uzunosmanoglu H, Pekcici R (2017) The role of C-reactive protein in the evaluation of the severity of acute cholecystitis. Acta Medica Mediterranea 33:347–352
Acknowledgements
This work was supported by The Roosevelt University, College of Pharmacy, Faculty Research and Professional Development Award (LAP)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Ibraheem M. Rajab, Daniel Majerczyk, Margaret E. Olson, Jenna M.B. Addams, Mihee L. Choe, Matthew S. Nelson and Lawrence A. Potempa declare that they have no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rajab, I.M., Majerczyk, D., Olson, M.E. et al. C-reactive protein in gallbladder diseases: diagnostic and therapeutic insights. Biophys Rep 6, 49–67 (2020). https://doi.org/10.1007/s41048-020-00108-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41048-020-00108-9