Abstract
Regardless of the type, extracorporeal membrane oxygenation (ECMO) requires the use of large intravascular cannulas and results in multiple abnormalities including non-physiologic blood flow, hemodynamic perturbation, rapid changes in blood oxygen and carbon dioxide levels, coagulation abnormalities, and a significant systemic inflammatory response. Among other sequelae, neurologic complications are an important source of mortality and long-term morbidity. The frequency of neurologic complications varies and is likely underreported due to the high mortality rate. Neurologic complications in patients supported by ECMO include ischemic and hemorrhagic stroke, hypoxic brain injury, intracranial hemorrhage, and brain death. In addition to the disease process that necessitates ECMO, cannulation strategies and physiologic disturbances influence neurologic outcomes in this high-risk population. For example, the overall documented rate of neurologic complications in the venovenous ECMO population is lower, but a higher rate of intracranial hemorrhage exists. Meanwhile, in the venoarterial ECMO population, ischemia and global hypoperfusion seem to compose a higher percentage of neurologic complications. In what follows, the literature is reviewed to discuss the pathophysiology, incidence, risk factors, and outcomes related to short-term neurologic complications in patients supported by ECMO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Neurologic complications increase morbidity and mortality during extracorporeal membrane oxygenation (ECMO) support. |
During ECMO support, most encountered neurologic outcomes include ischemic and hemorrhagic stroke, seizures, hypoxic brain injury, intracranial hemorrhage, and brain death. |
Pulmonary pathologies, ECMO circuitry, cardiac insufficiency, and critical illness all contribute to neurologic complications during ECMO support. |
Hypoxemia, hypercapnia, and inflammatory-mediated endothelial dysfunction during respiratory failure and ARDS in patients receiving ECMO may contribute to cerebral microbleeds. |
Introduction
Extracorporeal membrane oxygenation (ECMO) is a highly specialized therapy with rapidly increasing use for appropriately selected patients with cardiac, pulmonary, or combined cardiopulmonary failure that does not respond to less invasive treatments [1,2,3]. The two most common ECMO modalities are venovenous ECMO (VV-ECMO) and venoarterial ECMO (VA-ECMO). Complications during ECMO are common and include hematologic, renal, mechanical, and neurologic events [4]. In particular, neurologic complications are known to increase mortality in the ECMO population, making improved understanding of them an important priority to improve care in this critically ill population [5,6,7]. Reviews of neurologic complications in patients supported by ECMO have provided important insights into their incidence and techniques of monitoring; however, they lack pathophysiologic foundation and contemporary data, warranting an update on this important topic [7].
Neurologic complications and their definitions are documented in the Extracorporeal Life Support Organization (ELSO) registry, the largest database of patients supported by ECMO worldwide [4]. While the types of complications documented in the ELSO registry evolve with time, currently they include brain death, clinically and electro-encephalographically determined seizures, diffuse central nervous system ischemia on imaging, central nervous system infarction, central nervous system hemorrhage, intraventricular central nervous system hemorrhage, and “neurosurgical intervention performed” [8]. Additional neurological outcomes are located in special addendum forms such as the cerebral performance score (CPC) for patients undergoing extracorporeal cardiopulmonary resuscitation (eCPR, which is VA-ECMO cannulation in the setting of chest compressions or the inability to achieve sustained return of spontaneous circulation for at least 20 min) [9]. While these definitions are not universally adopted in the literature, they are very useful for studies involving the ELSO database as they can be searched.
In what follows, the literature is reviewed to discuss the pathophysiology, risk factors, and outcomes related to neurologic complications occurring during ECMO support, which will be referred to as ‘short-term’. The neurologic complications and their pathophysiology of the two most common forms of ECMO, VV-ECMO and VA-ECMO, have similarities and differences and thus will be discussed together and separately as appropriate. Similarly, risk factors and management changing literature will also be discussed. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Pathophysiology
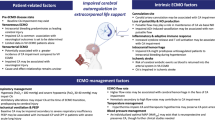
There are multiple mechanisms for neurologic complications in patients supported on ECMO, regardless of configuration (Fig. 1). Hypoperfusion plays an important role in the instance of eCPR and ECMO cannulation for cardiogenic shock (both would involve the use of VA-ECMO) [5, 6, 10,11,12]. ECMO circuit complications such as thrombosis and air entrainment can result in neurologic complications stemming from embolic phenomena, hypoperfusion, and cardiac arrest in circuit-dependent patients [4, 13]. Rapid changes in carbon dioxide levels, hypoxemia, and hyperoxemia may also precipitate neurologic injury due to their impacts on cerebral vasoconstriction, tissue hypoxia, and reperfusion injury [14,15,16,17,18]. Interestingly, compared to patients supported by VA-ECMO, patients undergoing VV-ECMO support have a lower reported overall incidence of neurological complications but higher absolute incidence of intracranial hemorrhage [5, 6, 10,11,12]. The higher observed rate of intracranial hemorrhage in the VV-ECMO population may be related to cerebral microbleeds (CMBs), rapid variation in partial pressure of carbon dioxide (PaCO2), and hypoxia in combination with systemic changes related to the acute respiratory distress syndrome (ARDS), the most common indication for VV-ECMO cannulation [5, 6, 10,11,12, 19, 20]. The COVID-19 pandemic resulted in significant increases in VV-ECMO utilization, and with it a relatively high incidence of intracranial hemorrhage and neurologic complications which may have been exacerbated by anticoagulation use to address the thrombotic risks of the disease, CMBs, and hemorrhagic conversion of ischemic strokes [21,22,23,24,25].
Summary of contributors to neurologic complications during extracorporeal membrane oxygenation support. Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved. ARDS acute respiratory distress syndrome, LV left ventricular, PaCO2, arterial partial pressure of carbon dioxide
The high prevalence of ARDS during the COVID-19 pandemic has increased awareness of the multi-system organ sequelae of this disease [26, 27]. In particular, before the pandemic, researchers postulated the effect of hypoxemia on the development of CMBs in ARDS [19, 28]. Although the mechanism is not entirely understood, prolonged periods of hypoxemia may be associated with CMBs and worsened neurologic outcomes in these patients. This phenomenon was also noted in patients with COVID-19 and ARDS [29]. Interestingly, CMBs are also found in patients without hypoxemia.
Retrospective literature indirectly supports a role for ARDS in neurologic complications independent of acute swings in PaCO2. A large database review has associated pre- to 24-h post-VV-ECMO initiation reductions in PaCO2 of greater than 50% with increased risk of seizure, intracranial hemorrhage, brain death, overall neurologic complications, and overall mortality [15]. It is notable that the asthma population had the largest percentage of patients with wide swings in PaCO2 (179 of 331 with available data or 54.1%) [15]. For comparison, the viral pneumonia population had 195 of 1776 (11.0%) with pre- to post-VV-ECMO PaCO2 reductions of greater than 50%, and the bacterial pneumonia population had 172 of 1345 or 12.8% [15]. The incidence of intracranial hemorrhage in two asthma reviews compared to ARDS was 0% and 4.41% versus 3.5% (p = 0.12 and p = 0.401, respectively) [15, 30, 31]. We believe that the similar or lower incidence of neurologic complications in the asthma population despite a marked difference in PaCO2 variability suggests that ARDS likely plays a role in CMB formation and contributes to intracranial hemorrhage in the VV-ECMO population. Additionally, it may be that a two-hit hypothesis involving pre-existing and developing CMBs explains the seemingly higher incidence of this neurologic complication in the VV-ECMO population [5, 6, 10,11,12].
Risk Factors
Cerebral Perfusion Pressure
An important contributor to neurologic complications is the disruption of normal intracranial physiology and regulatory mechanisms [32]. The brain maintains autoregulation by keeping cerebral perfusion pressure (CPP) and cerebral blood flow (CBF) constant over a wide range of mean arterial pressure (MAP). Specifically, below this range, CPP and CBF both decrease, increasing the risk of ischemia and sequelae, while elevations above will increase the CPP and CBF, increasing risk of edema and intracranial hemorrhage [33]. Cerebral autoregulation can only occur when the blood–brain barrier is intact. Critical illness, stroke, infection of the meninges or brain, and other pathophysiologic states encountered in patients supported by ECMO disrupt cerebral autoregulation.
Blood oxygen levels in the normal brain are also tightly regulated, and CPP and CBF are maintained over a much wider range of oxygen partial pressure. Experimental studies have shown that blood oxygen partial pressures greater than 50 mmHg can maintain a constant CPP and CBF [33, 34]. However, when oxygen partial pressure drops below 50 mmHg, the rate of change of CPP and CBF is logarithmic [34, 35]. This cut-off is of clinical importance in the VV-ECMO population because an arterial partial pressure of oxygen below 50 mmHg for over 3 h is an indication for VV-ECMO initiation [36]. In patients with this degree of hypoxemia and lack of cerebral autoregulation, a sudden increase in CPP and CBF could cause hemorrhage. Cerebral autoregulation does not appear to be effected by transient hyperoxemia, although studies specifically assessing this in patients supported by ECMO are lacking [37].
The role of PaCO2 in CPP and CBF is the least autoregulated. PaCO2 must be maintained within a specific range to regulate CPP and CBF [38]. In critical illness such as ARDS, maintenance of strict control of arterial CO2 in the neurovasculature is crucial given the loss of cerebral autoregulation, but often difficult given the need to perform lung protective ventilation strategies. It is well known that hyperventilating or inducing hypocapnia can lead to global cerebral ischemia though this is rare in ARDS due to the ubiquitous use of low tidal volume ventilation [39, 40]. Much more common is hypercapnia which causes increased CBF and CPP, in turn potentially leading to cerebral edema and hemorrhage. With PaCO2 being the least autoregulated of the three components of CPP and CBF, we believe it is an important contributing factor in the development of neurologic complications in critical illness.
Patients with ARDS, the most common indication for VV-ECMO, often develop hypercapnia secondary to ultra-protective lung ventilation maneuvers, prompting the use of neuromuscular blockade, prone ventilation, and occasionally ECMO. It is possible that extended periods of hypercapnia and hypoxemia increase CPP and CBF and, in the setting of loss of cerebral autoregulation, contribute to CMBs. The inflammatory response of ARDS often causes endothelial disruption at the level of the alveolar–capillary membranes [41]. Moreover, ARDS often tends to be a disease of the distal airways (vs. status asthmaticus which affects proximal airways) [41, 42]. The inflammation extends across both the alveoli and perfusing capillaries, leading to leakage and edema which is seen on microscopic analysis of tissue samples and bronchoalveolar lavage fluid. Because of this disruption in the endothelium at the level of the alveolar–capillary membrane, it may be that the endothelial disruption can occur beyond the lungs in other vital organs [43].
In contrast to asthma which is characterized by mast cell degranulation and eosinophilic infiltration, the inflammatory response of ARDS is more cytokine-dependent and results in the releases of multiple factors including tumor necrosis factor-1 and interleukins (e.g., IL-1β, IL-6, IL-8) [43, 44]. These inflammatory markers can be found in organs distant from the lungs, whereas, in asthma, the inflammatory response is typically limited to the pulmonary tissue [44, 45]. Therefore, the systemic nature of ARDS could be a factor in the development of CMBs and intracranial complications. Cytokine-mediated inflammatory responses can change endothelial integrity, and it is conceivable that patients with pre-existing cerebral microvascular disease are especially prone to hemorrhage in the setting of ARDS [19, 28, 29, 43].
Cerebral Microbleeds
A recent review of CMBs by Chen et al. in critically ill patients with respiratory failure or ARDS found a 30% incidence of CMBs, and that the corpus callosum and juxtacortical area were the most commonly involved [46]. This is especially relevant because the usual approach to ECMO cannulation involves systemic heparinization (although it is not required), which increases bleeding risk. Furthermore, ECMO circuits and membrane oxygenators are known to induce von Willebrand factor deficiency for the duration of the ECMO run and a short period after decannulation, which increases the degree of bleeding [47, 48]. These ECMO-associated factors are important considerations for patients that may already have CMBs, as they have the potential to expand them into intracranial hemorrhages and negatively impact neurologic outcomes and mortality [5, 6, 10,11,12].
While there is no “standard of care” regarding the timing of initiation of ECMO, the ELSO guidelines suggest initiation within 7 days of the diagnosis of severe ARDS [36, 49]. If patient factors can be associated with higher risks of CMBs with ARDS, then perhaps certain populations could benefit from earlier ECMO initiation to correct hypoxemia and hypercapnia and reduce central nervous system exposure to physiologic derangement. Unfortunately, no prediction model is available for CMBs.
There is no consensus regarding the optimal imaging modality for CMBs, although they can be detected with magnetic resonance imaging [50, 51]. The presence of CMBs should prompt cannulation teams to avoid the use of anticoagulation during cannulation and to perform post-cannulation head imaging when feasible. When available, pre-existing neuroimaging should be reviewed prior to initiating ECMO and the use of portable head CT may assist in surveillance for evolution to intracranial hemorrhage during ECMO support.
Anticoagulation
Anticoagulation is often used during ECMO to mitigate circuit-related complications and to maintain integrity of the membrane lung [52]. However, ECMO also increases the risk of hemorrhage through mechanical shear forces, uncoiling of von Willebrand multimers, and consumptive and dilutional coagulopathy [53]. Although most ECMO centers worldwide endorse using anticoagulation during VV-ECMO, multiple case series of patients with traumatic brain injury support on ECMO withheld anticoagulation without large increases in circuit sequelae, a strategy that is being further explored in a randomized trial setting (trial no. NCT04273607) [54, 55]. Alternatively, prophylactic dose subcutaneous low molecular weight heparin confers an acceptable thrombosis risk profile with significantly less need for blood transfusion in a cohort study of patients supported by VV-ECMO [56]. The equipoise regarding anticoagulation allows clinicians to judge the risk of bleeding and thrombosis in patients supported by ECMO, and the risk of hemorrhagic neurologic complications should be factored into the goals of anticoagulation.
Alternatives to heparin anticoagulation exist, including bivalirudin, citrate, and prostacyclin, but have not been sufficiently evaluated for neurologic complications during ECMO. Bivalirudin is the most studied non-heparin anticoagulant in the ECMO population. A recent meta-analysis found no difference in bleeding complications and a lower incidence of circuit thrombosis in the bivalirudin group compared to heparin, perhaps due to bivalirudin having a more predictable pharmacokinetic profile and direct engagement with clot-bound thrombin [57]. In vitro studies comparing citrate and heparin anticoagulation with whole fresh blood circulating through ECMO circuits have shown that citrate anticoagulation is less hemolytic compared to heparin anticoagulation [58]. Moreover, heparin anticoagulation revealed higher plasma-free hemoglobin levels with a higher hemolysis index, further raising concern that heparin anticoagulation of ECMO circuits could contribute to adverse neurologic events. A meta-analysis comparing complications of prostacyclin-driven anticoagulation versus either heparin or citrate anticoagulation found fewer bleeding events but more thrombotic events with prostacyclin [59]. This particular meta-analysis focused on prostacyclin anticoagulation on ECMO, but found no difference in mortality. Although interesting, the risk of clotting ECMO circuits should always be considered if prostacyclin is used as an anticoagulant.
Neurologic Adverse Events during ECMO
VV-ECMO Subgroups
The most common indication for VV-ECMO is ARDS with one study of patients in Germany finding that in the year 2018, 1703 of 2768 patients (61.5%) receiving VV-ECMO carried the diagnosis [20]. The high percentage of patients supported by VV-ECMO with ARDS likely means that studies evaluating neurologic outcomes are more reflective of the interaction with VV-ECMO and the ARDS population.
The height of the COVID-19 pandemic was a time of high utilization of VV-ECMO [4, 21, 22, 36]. A systematic review and meta-analysis of 1322 patients found 78 (5.9%) patients were diagnosed with intracranial hemorrhage, 15 (1.1%) were diagnosed with ischemic stroke, and 4 (0.3%) were diagnosed with hypoxic ischemic brain injury [23]. Patients with neurologic injury suffered significantly higher mortality: 92% versus 36% for those without neurologic complications [23]. The VV-ECMO cohort of an initial large retrospective review of the ELSO database in 2020 by Barbaro et al., included in the aforementioned meta-analysis, found similar results noting 44 of 738 patients with intracranial hemorrhage (6.0%), 5 of 738 (0.7%) with ischemic stroke, and seizures in 5 of 738 patients (0.7%) [21]. A subsequent ELSO database analysis in 2021, also by Barbaro et al., of patients with COVID-19 receiving ECMO grouped centers that began cannulating patients at various time points [22]. The groups contained 1157, 2767, and 782 patients, respectively, with intracranial hemorrhage rates of 68 (6%), 192 (7%), and 42 (5%) and ischemic stroke rates of 7 (1%), 53 (2%), and 8 (1%) [22].
Another important patient population supported by VV-ECMO is those with refractory status asthmaticus. A contemporary database review of patients predominantly supported by VV-ECMO from 2010 to 2020 found no instances of cerebral hemorrhage, brain death, or other neurologic complications [30]. Another study of patients with status asthmaticus treated with predominantly VV-ECMO over a longer time span (1992 to 2016) identified a 4.8% incidence of neurological complications, although the complications were not separated by time period [31].
VA-ECMO
Neurologic complications in the VA-ECMO population have important similarities and differences from the VV-ECMO population. By definition, VA-ECMO requires access to both the venous and arterial systems for hemodynamic and respiratory support which is accompanied by additional risks including embolization and ischemia. The difference in neurologic complications between VV-ECMO and VA-ECMO is related to multiple factors including vascular access and clinical differences in the patient populations. Important similarities include acute swings in carbon dioxide, insufficient tissue oxygen delivery in the pre-cannulation period, and the use of anticoagulation [14,15,16]. Important differences include hemodynamic instability, low flow state due to peri-arrest or cardiac arrest (i.e., eCPR), embolic phenomenon to the arterial system through the arterial cannula, and north–south syndrome [13, 60,61,62,63].
The most robust literature on neurologic outcomes in the VA-ECMO population is derived from the ELSO database [5, 10, 12]. First, Lorusso et al. in 2016 found a 15.1% (682 of 4522 patients) incidence of neurologic complications as defined by brain death (7.9%), infarction (3.6%), seizures (1.8%), and hemorrhage (1.8%) [5]. A subsequent study by Cho et al. in 2020 examined stroke risk in 10,342 patients supported by VA-ECMO and found that 401 and 229 (3.9% and 2.2%) experienced ischemic and hemorrhagic strokes, respectively [10]. A study by Hwang et al. published in 2023 from the ELSO database found that between the years 2012 and 2021, 946 of 20,297 patients (or 4.7%) suffered a stroke (either hemorrhagic or ischemic) [12]. Of these 946 patients, 659 (3.2%) and 287 (1.4%) suffered ischemic and hemorrhagic strokes [12].
Neurologic outcomes in the eCPR population should be thought of separately given the significant degree of low-flow or no-flow pre-cannulation state experienced by this patient population [8]. In a 2020 addendum, ELSO added the CPC for patients undergoing eCPR which will add to the understanding of neurologic outcomes in this patient population [9]. CPC is a scale from 1 to 5 with 1 representing a good outcome and 5 representing brain death [64, 65]. The 2022 ELSO registry report, by Tonna et al., noted that of 900 patients undergoing eCPR from the time period 2020–2022, 11.9% had a good neurologic outcome (CPC 1), 3.1% had moderate cerebral disability (CPC 2), and 9.9% had a poor neurologic outcome (CPC 3–5) [4]. Of note, mortality in this cohort was approximately 65% [4]. In the eCPR population, one of the most important modifiable factors for positive outcome, with respect both to survival and neurological complications, is time from conventional CPR initiation to VA-ECMO circuit initiation [66,67,68]. Additionally, epinephrine administration is ubiquitous during cardiac arrest due to studies demonstrating improved survival; however, survivors experience more neurologic dysfunction [69]. While low doses of epinephrine have been shown to improve macrovascular CBF, doses in excess of 3 mg have been shown to compromise microvascular CBF in pigs receiving eCPR [70, 71]. While such effects of excessive epinephrine necessitate confirmation in humans, they may compound other neurologic predispositions and should be carefully considered when evaluating eCPR candidacy [72].
Comparison of VV-ECMO and VA-ECMO
Neurologic complications in the VV-ECMO and VA-ECMO populations are different in frequency and distribution (Table 1). Large analyses of neurologic sequelae in patients undergoing VV-ECMO and VA-ECMO support were performed by Lorusso et al. and utilize the ELSO database [5, 6]. An analysis published in 2016 by Lorusso et al. examining patients supported by VA-ECMO in the ELSO database (from 1992 to 2013) found that 682 of 4522 patients, or 15.1%, suffered neurologic complications [5]. A 2017 VV-ECMO analysis by Lorusso et al. of the ELSO database evaluated neurologic outcomes in patients that underwent VV-ECMO from 1992 to 2015 and found 356 of 4988 patients, or 7.1%, suffered neurologic complications [6]. In addition to the difference in cannulation strategies, there are important differences in the patients included in these studies. For example, a minor difference includes the inclusion of patients above 16 years old in the VA-ECMO study and 18 years or older in the VV-ECMO study [5, 6]. Additionally, the VA-ECMO study included patients undergoing VA-ECMO cannulation for the purposes of eCPR, respiratory failure (presumably with attendant right ventricular failure requiring high-dose vasoactive medications), and cardiac failure [5]. The distribution of neurologic events in the two studies were markedly different, though difficult to accurately compare given the differing ways in which they were reported. In the VA-ECMO analysis, 80 patients suffered intracranial hemorrhage (approximately 1.8% of all patients), while, in the VV-ECMO analysis, intracranial hemorrhage accounted for 181 of 426 (approximately 42.5% of complications) [5, 6]. In the VA-ECMO analysis, 70 of 682 patients suffered more than one neurologic complication, with 64 suffering two, 5 suffering three, and 1 suffering four [5]. In the VV-ECMO analysis, the authors noted that 66 of 356 patients suffered more than one neurologic complication with 62 suffering two and 4 suffering three [6]. Of particular interest in the VA-ECMO cohort is that the respiratory subgroup has a trend (p = 0.08) toward higher intracranial hemorrhage rates in comparison to the eCPR and cardiac cohorts (3.3% vs. 2.2% and 1.9%, respectively) [5]. Interestingly, this 3.3% rate of intracranial hemorrhage in the patients supported by VA-ECMO with respiratory failure is similar to the VV-ECMO rate of intracranial hemorrhage (181/4988 or 3.6%, assuming one instance of intracranial hemorrhage per patient) [5, 6].
Additional analyses by Cho et al. of large cohorts of patients supported by VV-ECMO and VA-ECMO have noted higher rates of ischemic stroke in the VA-ECMO population (3.9% VA vs. 1.4% VV) and higher rates of hemorrhagic stroke in the VV-ECMO population (3.1% VV vs. 2.2% VA) [10, 11]. Interestingly, in the VV-ECMO population, VV-ECMO for status asthmaticus has an 0.6% (3 of 484) rate of hemorrhagic stroke despite making up 2.5% (387 of 15,206) of the total population [11]. Meanwhile, influenza, bacterial pneumonia, and viral pneumonia, make up 6.1% (931 of 15,206), 4.5% (678 of 15,206), and 3.1% (465 of 15,206) of the total population while comprising higher percentages of hemorrhagic stroke (12% (60 of 484), 6% (30 of 484), 5% (25 of 484), respectively) each of which is either statistically significant or strongly trends toward statistical significance [11]. ARDS, while not statistically significant, makes up 16.7% (2534 of the 15,206) patient cohort and accounts for 19.4% (94 of 484) of the hemorrhagic strokes [11].
Conclusion
There are numerous open questions regarding the modifiable and unmodifiable risk factors for neurologic sequelae in the ECMO population. With regard to neurologic complications, patients supported by VV-ECMO, VA-ECMO, and those cannulated for eCPR have very different risk profiles and complication rates, with VA-ECMO having a higher overall rate of neurologic complications, but likely fewer hemorrhagic complications than VA-ECMO. CMBs, an interesting phenomenon gaining attention in recent years, may play a role in the increased risk of intracranial hemorrhage in the VV-ECMO cohort (and the VA-ECMO cohort with respiratory failure-induced myocardial suppression). It is also important to note that disease state likely has a significant effect on the incidence of neurologic complications: patients supported by ECMO with status asthmaticus seem to have lower rates of neurologic complications compared to those ARDS. It is likely that CMBs in combination with strict attention to PaCO2 and hemodynamic optimization play important roles in the neurologic outcomes of patients supported by VV-ECMO, particularly the ARDS population. Finally, the eCPR population has the highest incidence of neurologic complications due to the exceptionally low-flow state that occurs during VA-ECMO cannulation. Although neurologic complications in these three populations should be studied independently, important information can potentially be inferred by comparing their distribution among these populations. Future directions in the study of neurologic complications in the ECMO population include further separation of patients by the diagnosis indicating ECMO cannulation, and identifying risk factors that potentially prompt higher levels of surveillance to facilitate earlier intervention.
References
Makdisi G, Wang I-W. Extra corporeal membrane oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7(7):E166-176.
Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a Review. JAMA. 2019;322(6):557.
Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ Heart Fail. 2018;11(9):e004905.
Tonna JE, Boonstra PS, MacLaren G, et al. Extracorporeal life support organization registry international report 2022: 100,000 survivors. ASAIO J. 2024;70(2):131–43.
Lorusso R, Barili F, Mauro MD, et al. In-Hospital Neurologic complications in adult patients undergoing venoarterial extracorporeal membrane oxygenation: results from the extracorporeal life support organization registry. Crit Care Med. 2016;44(10):e964–72.
Lorusso R, Gelsomino S, Parise O, et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: findings from the extracorporeal life support organization database. Crit Care Med. 2017;45(8):1389–97.
Zhang H, Xu J, Yang X, et al. Narrative review of neurologic complications in adults on ECMO: prevalence, risks, outcomes, and prevention strategies. Front Med. 2021;8:713333.
ELSO registry data definitions, Pages 120–121. Accessed 2 Jan 2024. https://www.elso.org/portals/0/files/elso%20registry%20data%20definitions%2002_13_23.pdf.
ECLS ECPR Addendum Form, Page 3. Accessed 2 Jan 2024. https://www.elso.org/portals/0/files/pdf/elsoecprform4.0_2020.pdf.
Cho S-M, Canner J, Chiarini G, et al. Modifiable risk factors and mortality from ischemic and hemorrhagic strokes in patients receiving venoarterial extracorporeal membrane oxygenation: results from the extracorporeal life support organization registry. Crit Care Med. 2020;48(10):e897-905.
Cho S-M, Canner J, Caturegli G, et al. Risk factors of ischemic and hemorrhagic strokes during venovenous extracorporeal membrane oxygenation: analysis of data from the extracorporeal life support organization registry. Crit Care Med. 2021;49(1):91–101.
Hwang J, Kalra A, Shou BL, et al. Epidemiology of ischemic stroke and hemorrhagic stroke in venoarterial extracorporeal membrane oxygenation. Crit Care. 2023;27(1):433.
Cho S-M, Ziai W, Geocadin R, Choi CW, Whitman G. Arterial-Sided Oxygenator clot and transcranial doppler ultrasound emboli in venoarterial extracorporeal membrane oxygenation. Ann Thorac Surg. 2019;107(1):326–7.
Diehl A, Burrell AJC, Udy AA, et al. Association between arterial carbon dioxide tension and clinical outcomes in venoarterial extracorporeal membrane oxygenation*. Crit Care Med. 2020;48(7):977–84.
Cavayas YA, Munshi L, Del Sorbo L, Fan E. The early change in Pa CO 2 after extracorporeal membrane oxygenation initiation is associated with neurological complications. Am J Respir Crit Care Med. 2020;201(12):1525–35.
Munshi L, Kiss A, Cypel M, Keshavjee S, Ferguson ND, Fan E. Oxygen thresholds and mortality during extracorporeal life support in adult patients*. Crit Care Med. 2017;45(12):1997–2005.
Al-Kawaz MN, Canner J, Caturegli G, et al. Duration of hyperoxia and neurologic outcomes in patients undergoing extracorporeal membrane oxygenation. Crit Care Med. 2021;49(10):e968–77.
Shou BL, Ong CS, Premraj L, et al. Arterial oxygen and carbon dioxide tension and acute brain injury in extracorporeal cardiopulmonary resuscitation patients: analysis of the extracorporeal life support organization registry. J Heart Lung Transplant. 2023;42(4):503–11.
Gedansky A, Huang M, Hassett CE, et al. Cerebral microbleeds in acute respiratory distress syndrome. J Stroke Cerebrovasc Dis. 2023;32(10):107332.
Friedrichson B, Mutlak H, Zacharowski K, Piekarski F. Insight into ECMO, mortality and ARDS: a nationwide analysis of 45,647 ECMO runs. Crit Care. 2021;25(1):38.
Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. The Lancet. 2020;396(10257):1071–8.
Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international extracorporeal life support organization registry. The Lancet. 2021;398(10307):1230–8.
Kannapadi NV, Jami M, Premraj L, et al. Neurological complications in COVID-19 Patients With ECMO Support: a systematic review and meta-analysis. Heart Lung Circ. 2022;31(2):292–8.
Sholzberg M, Tang GH, Rahhal H, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021. https://doi.org/10.1136/bmj.n2400.
Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern Med. 2021;181(12):1612.
Ziaka M, Exadaktylos A. ARDS associated acute brain injury: from the lung to the brain. Eur J Med Res. 2022;27(1):150.
Bos LDJ, Ware LB. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. The Lancet. 2022;400(10358):1145–56.
Fanou EM, Coutinho JM, Shannon P, et al. Critical illness-associated cerebral microbleeds. Stroke. 2017;48(4):1085–7.
Lersy F, Willaume T, Brisset J-C, et al. Critical illness-associated cerebral microbleeds for patients with severe COVID-19: etiologic hypotheses. J Neurol. 2021;268(8):2676–84.
Zakrajsek JK, Min S-J, Ho PM, et al. Extracorporeal membrane oxygenation for refractory asthma exacerbations with respiratory failure. Chest. 2023;163(1):38–51.
Yeo HJ, Kim D, Jeon D, Kim YS, Rycus P, Cho WH. Extracorporeal membrane oxygenation for life-threatening asthma refractory to mechanical ventilation: analysis of the Extracorporeal Life Support Organization registry. Crit Care. 2017;21(1):297.
Benson JC, Madhavan AA, Cutsforth-Gregory JK, Johnson DR, Carr CM. The Monro-Kellie doctrine: a review and call for revision. AJNR Am J Neuroradiol. 2023;44(1):2–6.
Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin. 2016;34(3):465–77.
Lucas SJE, Tzeng YC, Galvin SD, Thomas KN, Ogoh S, Ainslie PN. Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension. 2010;55(3):698–705.
Megjhani M, Weiss M, Ford J, et al. Optimal cerebral perfusion pressure and brain tissue oxygen in aneurysmal subarachnoid hemorrhage. Stroke. 2023;54(1):189–97.
Tonna JE, Abrams D, Brodie D, et al. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021;67(6):601–10.
Ciliberti P, Cardim D, Giardina A, et al. Effects of short-term hyperoxemia on cerebral autoregulation and tissue oxygenation in acute brain injured patients. Front Physiol. 2023;14:1113386.
Meng L, Gelb AW. Regulation of cerebral autoregulation by carbon dioxide. Anesthesiology. 2015;122(1):196–205.
Curley G, Kavanagh BP, Laffey JG. Hypocapnia and the injured brain: More harm than benefit. Crit Care Med. 2010;38(5):1348–59.
Beitler JR, Ghafouri TB, Jinadasa SP, et al. Favorable neurocognitive outcome with low tidal volume ventilation after cardiac arrest. Am J Respir Crit Care Med. 2017;195(9):1198–206.
Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–72.
Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. The Lancet. 2021;398(10300):622–37.
Zhou H, Fan EK, Fan J. Cell-cell interaction mechanisms in acute lung injury. Shock. 2021;55(2):167–76.
Duchesne M, Okoye I, Lacy P. Epithelial cell alarmin cytokines: Frontline mediators of the asthma inflammatory response. Front Immunol. 2022;13:975914.
Maspero J, Adir Y, Al-Ahmad M, et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022;8(3):00576–2021.
Chen BY, Dang J, Cho S-M, Harnegie MP, Uchino K. Cerebral Microbleeds in Critically Ill Patients with Respiratory Failure or Sepsis: A Scoping Review. Neurocrit Care [Internet] 2024 [cited 2024 Apr 4]; Available from: https://link.springer.com/https://doi.org/10.1007/s12028-024-01961-z
Kalbhenn J, Zieger B. Bleeding during veno-venous ECMO: prevention and treatment. Front Med. 2022;9:879579.
Valladolid C, Yee A, Cruz MA. von Willebrand factor, free hemoglobin and thrombosis in ECMO. Front Med. 2018;5:228.
Li X, Hu M, Zheng R, et al. Delayed initiation of ECMO Is associated with poor outcomes in patients with severe COVID-19: a multicenter retrospective cohort study. Front Med. 2021;8:716086.
Huang M, Gedansky A, Hassett CE, et al. Structural brain injury on brain magnetic resonance imaging in acute respiratory distress syndrome. Neurocrit Care. 2024;40(1):187–95.
Liu J, Kou Z, Tian Y. Diffuse axonal injury after traumatic cerebral microbleeds: an evaluation of imaging techniques. Neural Regen Res. 2014;9(12):1222.
Wieruszewski PM, Ortoleva JP, Cormican DS, Seelhammer TG. Extracorporeal membrane oxygenation in acute respiratory failure. Pulm Ther. 2023;9(1):109–26.
Chlebowski MM, Baltagi S, Carlson M, Levy JH, Spinella PC. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care. 2020;24(1):19.
Protti A, Iapichino GE, Di Nardo M, Panigada M, Gattinoni L. Anticoagulation management and antithrombin supplementation practice during veno-venous extracorporeal membrane oxygenation. Anesthesiology. 2020;132(3):562–70.
Muellenbach RM, Kredel M, Kunze E, et al. Prolonged heparin-free extracorporeal membrane oxygenation in multiple injured acute respiratory distress syndrome patients with traumatic brain injury: The. J Trauma Acute Care Surg. 2012;72(5):1444–7.
Krueger K, Schmutz A, Zieger B, Kalbhenn J. Venovenous extracorporeal membrane oxygenation with prophylactic subcutaneous anticoagulation only: an observational study in more than 60 patients: thoughts and progress. Artif Organs. 2017;41(2):186–92.
Wieruszewski PM, Macielak SA, Nei SD, et al. Heparin versus bivalirudin for anticoagulation in adult extracorporeal membrane oxygenation: a systematic review and meta-analysis. ASAIO J. 2023;69(2):137–44.
Chan C, Ki K, Zhang M, et al. Extracorporeal membrane oxygenation-induced hemolysis: an in vitro study to appraise causative factors. Membranes. 2021;11(5):313.
Aldairi N, Al Ali AS, Alabdulqader M, Al Jeraisy M, Cyrus J, Karam O. Efficacy of Prostacyclin Anticoagulation in Critically Ill Patients Requiring Extracorporeal Support: A Systematic Review and Meta-Analysis. Cureus [Internet] 2023 [cited 2024 Feb 16];Available from: https://www.cureus.com/articles/158863-efficacy-of-prostacyclin-anticoagulation-in-critically-ill-patients-requiring-extracorporeal-support-a-systematic-review-and-meta-analysis
Kalra A, Kang JK, Wilcox C, et al. Impact of Pulse Pressure on Acute Brain Injury in Venoarterial ECMO Patients with Cardiogenic Shock During the First 24 Hours of ECMO Cannulation: Analysis of the Extracorporeal Life Support Organization Registry [Internet]. In Review; 2023 [cited 2024 Feb 16]. Available from: https://www.researchsquare.com/article/rs-3646443/v1
Gravesteijn BY, Schluep M, Disli M, et al. Neurological outcome after extracorporeal cardiopulmonary resuscitation for in-hospital cardiac arrest: a systematic review and meta-analysis. Crit Care. 2020;24(1):505.
Wang L, Li C, Hao X, et al. Percutaneous cannulation is associated with lower rate of severe neurological complication in femoro-femoral ECPR: results from the Extracorporeal Life Support Organization Registry. Ann Intensive Care. 2023;13(1):77.
Khanduja S, Kim J, Kang JK, et al. Hypoxic-Ischemic brain injury in ECMO: pathophysiology, neuromonitoring, and therapeutic opportunities. Cells. 2023;12(11):1546.
Brain Resuscitation Clinical Trial I Study Group. Randomized clinical study of thiopental loading in comatose survivors of cardiac arrest. N Engl J Med. 1986;314(7):397–403.
Sandroni C, Cariou A, Cavallaro F, et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation. 2014;85(12):1779–89.
Yannopoulos D, Bartos J, Raveendran G, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. The Lancet. 2020;396(10265):1807–16.
Richardson ASC, Tonna JE, Nanjayya V, et al. Extracorporeal cardiopulmonary resuscitation in adults. Interim guideline consensus statement from the extracorporeal life support organization. ASAIO J. 2021;67(3):221–8.
Tran A, Rochwerg B, Fan E, et al. Prognostic factors associated with favourable functional outcome among adult patients requiring extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: a systematic review and meta-analysis. Resuscitation. 2023;193:110004.
Perkins GD, Ji C, Deakin CD, et al. A Randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med. 2018;379(8):711–21.
Ristagno G, Tang W, Huang L, et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med. 2009;37(4):1408–15.
Mavroudis CD, Ko TS, Morgan RW, et al. Epinephrine’s effects on cerebrovascular and systemic hemodynamics during cardiopulmonary resuscitation. Crit Care. 2020;24(1):583.
Garcia SI, Seelhammer TG, Saddoughi SA, Finch AS, Park JG, Wieruszewski PM. Cumulative epinephrine dose during cardiac arrest and neurologic outcome after extracorporeal cardiopulmonary resuscitation. Am J Emerg Med. 2024;80:61–6.
Medical Writing/Editorial Assistance
No medical writing or editorial assistance (including AI) was used to write this manuscript.
Funding
No funding or sponsorship was received for writing or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors (Dominic V. Pisano, Jamel P. Ortoleva, and Patrick M. Wieruszewski) contributed to the article conception and design, drafting, revising, and editing of the manuscript, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Dominic V. Pisano has nothing to disclose. Jamel P. Ortoleva has previously received honoraria from La Jolla Pharmaceutical Company. Patrick M. Wieruszewski has served as a consultant for La Jolla Pharmaceutical Company and Viatris.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pisano, D.V., Ortoleva, J.P. & Wieruszewski, P.M. Short-Term Neurologic Complications in Patients Undergoing Extracorporeal Membrane Oxygenation Support: A Review on Pathophysiology, Incidence, Risk Factors, and Outcomes. Pulm Ther (2024). https://doi.org/10.1007/s41030-024-00265-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41030-024-00265-z