Abstract
Introduction
Bronchial artery embolisation (BAE) is an established treatment method for massive haemoptysis. The aim of this study is to evaluate the impact of BAE on in-hospital outcomes and long-term survival in patients with massive haemoptysis.
Methods
Retrospective review of all cases of acute massive haemoptysis treated by BAE between April 2000 and April 2012 with at least a 5 year follow up of each patient. Targeted BAE was performed in cases with lateralising symptoms, bronchoscopic sites of bleeding or angiographic unilateral abnormal vasculature. In the absence of lateralising symptoms or signs, bilateral BAE was performed.
Results
96 BAEs were performed in 68 patients. The majority (64 cases, 67%) underwent unilateral procedures. 83 (86.5%) procedures resulted in immediate/short term control of haemoptysis which lasted for longer than a month. The mean duration of haemoptysis free period after embolisation was 96 months. There were three major complications (cardio-pulmonary arrest, paraparesis and stroke). 38 (56%) patients were still alive at least 5 years following their BAE. Benign causes were associated with significantly longer haemoptysis free periods, mean survival 108 months compared to 32 months in patients with an underlying malignant cause (p = 0.005). An episode of haemoptysis within a month of the initial embolisation was associated reduced overall survival (p = 0.033).
Conclusion
BAE is effective in controlling massive haemoptysis. Long-term survival depends on the underlying pulmonary pathology. Strategies are required to avoid incomplete initial embolisation, which is associated with ongoing haemoptysis and high mortality despite further BAE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bronchial artery embolisation resulted in immediate/short term control of haemoptysis in 86.7% of cases. |
Underlying pathology is an independent predictor of haemoptysis free survival. |
Early recurrence of haemoptysis following embolisation is an independent predictor of overall survival. |
Introduction
Massive haemoptysis is defined as life-threatening bleeding which is primarily due to aspiration and asphyxiation and rarely due to exsanguination [1]. The literature variably describes this as a volume of haemoptysis anywhere between 200 and 1000 ml in 24 h [2]. However, given the difficulties in quantifying the volume of haemoptysis, the functional impact with respiratory distress or hypoxia is probably more important in an individual patient [2]. There is a varied aetiology but the most common causes include bronchiectasis, malignancy, tuberculosis (TB), mycetoma, necrotising pneumonia and cryptogenic haemoptysis [2,3,4].
Massive haemoptysis is associated with a high mortality, with conservative management having a mortality rate of between 50 and 100% [5]. Surgery was once considered the first line treatment for all causes of massive haemoptysis but following improvements in bronchoscopy and interventional radiology it is often reserved for situations where other methods have failed [6, 7]. The mortality for surgery in emergency cases is reported to be as high as 34% [6, 7]. Bronchoscopy can be used to lateralise the side of the haemorrhage, help secure the airway and administer localised treatment; with a number of different endoscopic treatments described in the literature. In each case, the identification of a bleeding point is required and after the initial haemostasis is achieved a more definite treatment is often needed [2, 7]. Bronchial artery embolisation (BAE) is an established minimally invasive method of controlling haemoptysis which was first described following its use in Hanoi after the bombings of 1972 where it was used to control blast contusions [8]. Signs of abnormal bronchial circulation on angiography include hypertrophied vessels, vascular blush, neovascularity, aneurysms and pulmonary arterio-venous shunting (Fig. 1) [9]. Contrast extravasation is the only absolute sign of the site of bronchial haemorrhage but is rarely seen in practice [10].
Angiographic runs demonstrating a hypertrophied right bronchial artery (black arrow) (a), a torturous hypertrophied left intercostal artery (black arrowhead) (b), right broncho-intercostal trunk with abnormal vascularity (white arrow) (c) and a hypertrophied left 4th/5th common intercostal trunk with associated pulmonary blush (asterisk) and pulmonary venous shunting (white arrowhead) (d)
The literature has generally focussed on the short-term outcomes of primary BAE, with only a limited number of studies having followed up patients for at least 5 years in cohorts of greater than 50 patients following successful BAE [11,12,13,14]. The aim of this retrospective study is to add to the current literature by providing the efficacy of BAE in ceasing haemoptysis, its effects on in-hospital survival and medium and long-term overall and haemoptysis-free survival in a large cohort of patients at a single UK centre.
Methods
A retrospective analysis of all BAE procedures performed for massive haemoptysis at a single tertiary centre between April 2000 and April 2012 was performed. Formal ethics approval or patient consent was not required as this was a retrospective data review and a waiver is granted at our institution in this circumstance.
All patients were identified from the hospital radiology information system and procedural data. Patient demographics, pre-procedural imaging, procedural and follow-up details were collected from patient case notes, electronic records and clinic letters. Where necessary, additional follow-up data was sought from the patient’s general practitioner and/or referring hospital. Follow-up was performed up to April 2017 or death, if earlier.
Basic Demographics and Clinical Work Up
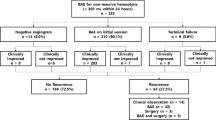
68 patients (41 male and 27 female) with massive haemoptysis underwent a total of 96 embolisation procedures. Mean age was 53 years (range 18–83 years). The spread of the aetiologies is shown in Table 1. Chest radiography, CT scan and bronchoscopy were performed in 32, 50 and 30 patients respectively prior to BAE. Chest radiography was occasionally useful in lateralising the pathology but in most of the cases it had limited value. Bronchoscopy and CT, along with the patient’s symptoms, were the most useful methods of lateralising the bleeding.
Procedure
All patients were referred by respiratory physicians or cardiologists if they had frank haemoptysis which resulted in respiratory or rarely, haemodynamic compromise. Respiratory function, clotting parameters and haemodynamic status were optimised prior to intervention. Patients with confirmed or suspected inter-current pulmonary infection received antibiotic therapy. Oral tranexamic acid (1 g, 3 times a day) was commenced for the period between presentation and BAE. When possible, patient symptoms (unilateral bubbling sensation or chest pain at the time of fresh haemoptysis) and/or pre-interventional investigations including chest radiographs, computerised tomography (CT) scans and bronchoscopy were used to lateralise bleeding site. Anaesthetic input was sought when clinically appropriate. Informed consent was obtained including specific discussion of the risk of paraplegia/paraparesis, stroke, failure to control bleeding and risk of recurrence.
Angiography was performed via 4–5 French common femoral sheaths in all cases. The bronchial arterial supply was identified on either an initial flush aortogram or a recent pre-procedural CT, prior to selective bronchial artery catheterisation. Depending on the patient’s anatomy an Amplatz left coronary 1, Uni Select (USL)or Cobra 1 or 2 selective catheter (Cordis Corporation, Warren, NJ) was used, with a Progreat microcatheter (Terumo Medical Corporation, Somerset, NJ) being required in the majority of cases. BAE was performed when angiography demonstrated abnormal hypertrophied bronchial arteries, neo-vascularity, dense parenchymal blush, bronchopulmonary shunting, false aneurysms or active extravasation. Before embolisation of a vessel, magnified angiography was performed to identify any spinal arterial supply from that vessel.
When the source/side of bleeding was obscure, all bronchial arteries were catheterised, including any ectopic arteries identified on CT scanning. If these were normal or if further target vessels were suspected, a thorough search was made for alternative/additional supplying vessels. This included looking specifically at intercostal, internal mammary (IM), inferior phrenic arteries and subclavian artery branches including the thyro-cervical trunk (Fig. 2).
Flush aortogram showing normal bronchial arteries (arrow) and intercostal arteries (a). Angiographic runs following selective catherisation on the same patient demonstrating normal common origin with normal right and left bronchial arteries (b) and normal right and left subclavian and internal mammary arteries (c and d)
Embolisation was performed using 300–500 µm polyvinyl alcohol (PVA) (Cook Incorporated, Bloomington, IN) particles; 500–700 µm or larger PVA particles were used to close large bronchial artery to pulmonary artery or vein fistulae. If a spinal supply was identified from a target vessel or in cases where the distal circulation needed protection, protective coil embolisation was performed. If only a focal abnormality was identified in a vessel this was super-selectively catheterised and embolised. Procedures were performed by nine experienced consultant vascular interventionists. Procedures were defined as successful if immediate clinical control of haemoptysis was achieved which required no further medical or endovascular interventions (sustained for at least 30 days).
Data Analysis
Both haemoptysis free and overall survival were defined from the time of BAE and was calculated by the Kaplan–Meier method. Cox regression analysis was used to identify predictors of survival, including age and the causal factor (benign versus malignant causes of haemoptysis). A p value less than 0.05 was considered to indicate statistical significance. Analysis of the data was performed using the IBM SPSS for Windows (version 22).
Results
Findings at Angiography and Procedure Performed
78 out of the 96 procedures had one or more of the previously listed abnormal angiographic findings present. Of these cases, a unilateral procedure was performed in 54 (69%). In those with non-specific findings, 10/18 (55%) had unilateral embolisation based on pre-procedural lateralisation; the other eight had bilateral procedures.
Table 2 documents the distribution of target vessels embolised in the 96 BAE procedures. PVA particles alone were used in 77 cases. In 17 procedures, adjunctive coil embolisation was employed for the indications outlined previously. Two procedures were performed with coils alone, these were patients having repeat procedures where there was concern that the target vessel supplied a spinal artery.
Technical Success
There were four failed procedures undertaken with two patients having two failed attempts at BAE each. This resulted in 100 BAE procedures attempted during the study period, but 96 embolisations performed.
In addition to the technical failures previously mentioned there were six procedures where embolisation was incomplete. Four procedures were terminated due to mediastinal discomfort and patient difficulty with breath-holding, all presumed to be due to post embolisation pain. One procedure was terminated due concerns regarding the iodinated contrast load. One procedure was terminated at the patient’s request. This, combined with the four procedures where embolisation was not performed due to the inability to achieve a safe catheter position, results in a technical success rate of 90%.
Clinical Success
Of the 96 embolisations procedures 83 resulted in immediate haemoptysis control (sustained for at least 30 days post-procedure), producing a short-term clinical success rate of 86.5%.
Repeat procedures were required in a total of 19 patients (27.9%) required more than one BAE with a total of 32 repeat procedures performed. 13 (40.6%) of all the repeat procedures occurred within the first month. Ten re-interventions occurred between 1 and 12 months and nine repeat procedures were performed more than a year after the original BAE.
Complications
Three patients suffered major complications during or immediately after the procedure. One patient suffered a cardio-pulmonary arrest from haemoptysis during the BAE (they were deemed too high risk for open surgery) but they were successfully resuscitated with completion of a clinically successful BAE. One patient developed paraparesis immediately after the procedure (their lower limb power subsequently significantly recovered from grade I to grade IV after 12 months) and one patient who had subclavian branches embolised had a cerebellar infarction which was confirmed on CT. The patient made a full recovery from their stroke within a month.
Two patients suffered minor complications due to trauma during selective catheterisation. The first had a traumatic dissection of their right bronchial artery when catheterised causing unintentional proximal occlusion of the artery. The second patient’s right bronchial artery was ruptured leading to proximal embolisation of the artery being performed to control bleeding Neither of these patients developed significant sequela from these minor complications or had recurrent haemoptysis which required further intervention. There were no complications related to non-target coronary, pulmonary or oesophageal embolisation. No significant puncture site or catheter related complications were noted.
Peri-procedural mortality was zero.
Overall Survival and Haemoptysis Free Survival
Follow-up data was available for 57 (83.8%) of the 68 patients. The 11 patients who have had no recorded follow up had either moved out of the area or the follow up data had been lost in the transfer of paper notes to the electronic format. Eight patients required surgery; four of these were lung resections for lung cancer, one patient had a lung transplant, two patients had lobectomies (one for severe bronchiectasis and one for TB cavitation) and one had a thoracoplasty for an aspergilloma. The patient who underwent thoracoplasty died shortly following surgery; the cause of death was not formally identified.
There were 30 deaths during the follow-up period, the data was available for all patients via a national database. 38 patients were alive from the initial cohort of 68 in April 2017 resulting in an overall survival of 55.7% (survival range 0–181 months). Three patients’ deaths were related to pulmonary haemorrhage; the remaining causes of death were due to pneumonia, post thoracotomy (unknown cause), progression of malignancy and end stage respiratory disease due to cystic fibrosis and severe bronchiectasis. The information detailing the cause of death of eight patients was not available. Patients who were deemed to have a clinically unsuccessful embolisation at their initial procedure (further episode of haemoptysis within a month) had a significantly worse overall survival (37 months CI 8.7–65.9) compared to those who were haemoptysis free for over a month (122 months CI 89.8–135.6) (p = 0.033). This remained significant when adjusting for age and underlying diagnosis (p = 0.049, HR = 2.3).
25/57 (43.8%) patients had a further episode of hemoptysis following primary BAE during the follow up period. The mean haemoptysis free period after embolisation was 96 months (95% CI 76–117; Kaplan–Meier) (Fig. 3). Significantly longer mean haemoptysis free survival was achieved when the BAE was performed for benign causes (108 months; 95% CI 86–130) than for malignant causes (32 months; 95% CI 0–66.3) (p = 0.005) (Fig. 4). The haemoptysis free survival following a bilateral procedure versus a unilateral procedure tendered towards significance (p = 0.056), 119 months; 95% CI 86.9–151.3 and 51 months; 95% CI 16.2–85.7 respectively. Underlying diagnosis was the only independent factor for haemoptysis free survival when analysed with patient age and side of the procedure, with patients with an underlying malignancy having an HR of 2.6 (p = 0.013).
Discussion
The first published BAE appeared in the literature in 1973 [8]. Over the next 4 decades several investigators have demonstrated its efficacy in the management of de novo haemoptysis and recurrent haemoptysis with high BAE technical success rates being reported; between 77 and 98% and a 30-day clinical success rate of between 64 and 94.4% [10, 11, 13,14,15,16,17,18,19,20,21,22,23,24,25]. As far as the authors are aware this is the largest UK study to publish its long-term outcomes on BAE with our technical success rate and 30-day clinical success rate being comparable at 90% and 86.5% respectively.
BAE tackles the symptomatic consequence of a disease process rather than the underlying pathology and therefore it is likely that the numbers requiring repeat intervention or surgery will increase with longer follow-up. Hayakawa noted two peaks of re-bleeding. An early peak within the first month of BAE is typically due to incomplete embolisation [25]. In our study there were 13 early re-interventions; four were technical failures and six were due to the procedure being stopped due to patient discomfort or concerns regarding iodinated contrast load. The majority of the procedures were performed under local anaesthesia without anaesthetic support. Only one of the repeat procedures, performed due to the previous BAE being terminated due to discomfort, required the aid of anaesthetic input.
There is a reported second re-bleeding peak at 1 to 2 years after the initial intervention [25]. This was seen in nine patients in our study, including six of the patients who had an early re-intervention. This late recurrence is thought to be due to recruitment of new arterial supply by the progressive inflammatory process or progression of the underlying pathology [25]. A recent study by Maleux et al. concluded that repeat embolisation was both safe and efficacious especially in patients with bronchiectasis [26].
There are few studies which provide at least a 5 year follow up for patients, with overall survival for our cohort being 55.8% (survival range 0–181 months). Syha et al. found a similar overall survival at 5 years (59%) which remained stable at 10 years following BAE [12]. Their results also demonstrated that disease free survival is significantly longer with benign compared to malignant causes of haemoptysis. However, long term haemoptysis free survival in the benign group can be affected by conditions such as cystic fibrosis which is associated with poor long-term survival and relatively high recurrence of haemoptysis [12, 27]. Yoo et al. found a 5-year haemoptysis free recurrence rate of 43.2% following primary BAE which is again comparable to our results of 43.8% [11]. Kato et al. found a 5-year haemoptysis recurrence rate of 37.5% following primary BAE, however, the differences in outcomes may reflect the different aetiologies studied, as their paper focused on BAE in benign causes of haemoptysis [13].
A significant finding in this study was the limited need for surgical intervention during follow-up despite the progressive nature of the underlying pathology in most patients. The literature shows little data regarding the need for further surgical interventions after BAE. Only eight of the 68 patients who were discharged from the hospital following the initial and early interventions subsequently required surgery (11.7%).
Successful and safe BAE requires a thorough knowledge of the variations in the systemic arterial blood supply to the lungs and recognition of the possibility of spinal arterial supply arising from the bronchial circulation. Cauldwell has described four classic bronchial artery branching patterns [28]. Others have further defined several variations and aberrant vessels [29,30,31]. Recognition of these variations allows a systematic search of possible culprit vessels and minimises the chance of spinal cord infarction. The prevalence rate of spinal cord ischaemia as a complication of BAE is reported to be 0.6–4.4% [32]. In our series, there was one case of paraplegia in 96 embolisation procedures. High quality fluoroscopy, including intermittent use of higher radiation doses, and patient co-operation is vital to identify the opening of spinal collaterals during embolisation. When there is any doubt, additional angiographic runs should be performed but with care to not reflux recently injected particles. There was also one case of a cerebellar infarct during subclavian artery branch embolisation which is reported to occur in 0.6–2% [32, 33].
This is a retrospective observational study with its inherent limitations. Massive haemoptysis does not lend itself to randomisation between no treatment, surgery and BAE as the patient’s clinical condition usually dictates further management. The role of BAE in these patients is well accepted. Some further specific information such as procedure time (which usually has an impact on patient compliance and may be a possible reason for incomplete procedure—a cause for early re-intervention and death), contrast load and in some cases the exact cause of death have not been elucidated. Despite the limitations, this study does add to the current literature especially in regard to long term outcomes. Over the follow-up period, the main determinant of long-term survival is the underlying pathology. Not surprisingly patients with malignancy had shorter survival.
Conclusion
Highly selective targeted BAE with a thorough assessment of the bronchial and non-bronchial arterial tree controls massive haemoptysis. Incomplete embolisation is associated with poor prognosis and is usually the result of having to discontinue the procedure due to complication or patient compliance. The most important factors affecting early survival and haemoptysis free survival is the completeness of initial BAE but the primary determinant of longer-term survival is the underlying disease process itself, with patients with benign disease surviving longer.
References
Jean-Baptiste E. Clinical assessment and management of haemoptysis. Crit Care Med. 2000;28:1642–7.
Radchenko C, Alraiyes AH, Shojaee S. A systematic approach to the management of massive hemoptysis. J Thorac Dis. 2017;9(Suppl 10):S1069–86.
Fartoukh M, Khoshnood B, Parrot A, et al. Early prediction of in-hospital mortality of patients with hemoptysis: an approach to defining severe hemoptysis. Respiration. 2012;83(2):106–14.
Mondoni M, Carlucci P, Job S, et al. Observational, multicentre study on the epidemiology of haemoptysis. Eur. Respir. J. 2018;. https://doi.org/10.1183/13993003.01813-2017.
Narjarian KE, Morris CS. Arterial embolisation of the chest. J Thorac Imaging. 1998;13:93–104.
Andréjak C, Parrot A, Bazelly B. Surgical lung resection for severe hemoptysis. Ann Thorac Surg. 2009;88(5):1556–65.
Shojaee DK. Managing massive hemoptysis. Chest. 2020;157(1):77–88.
Remy J, Voisin C, Ribet M, et al. Treatment, by embolisation, of severe or repeated haemoptysis associated with systemic hypervascularisation. Nouv Presse Med. 1973;2:2060–8.
Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolisation for life-threatening haemoptysis: a comprehensive review. Radiographics. 2002;22:1395–409.
Ramakantan R, Bandekar VG, Gandhi MS, Aulakh BG, Deshmukh HL. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology. 1996;200:691–4.
Yoo DH, Yoon CJ, Kang SG, Burke CT, Lee JH, Lee CT. Bronchial and nonbronchial systemic artery embolization in patients with major hemoptysis: safety and efficacy of N-butyl cyanoacrylate. Am J Roentgenol. 2011;196:W199–204.
Syha R, Benz T, Hetzel J, et al. Bronchial artery embolization in hemoptysis: 10-year survival and recurrence-free survival in benign and malignant etiologies—a retrospective study. Rofo. 2016;188:1061–6.
Kato A, Kudo S, Matsumoto K, et al. Bronchial artery embolization for haemoptysis due to benign diseases: immediate and long-term results. Cardiovasc Intervent Radiol. 2000;23:351–7.
Woo S, Yoon CJ, Chung JW, et al. Bronchial artery embolization to control hemoptysis: comparison of N-butyl-2-cyanoacrylate and polyvinyl alcohol particles. Radiology. 2013;269(2):594–602.
Uflacker R, Kaemmerer A, Neves C, Picon PD. Management of massive haemoptysis by bronchial artery embolisation. Radiology. 1983;146:627–34.
Cremaschi P, Nascimbene C, Vitulo P, et al. Therapeutic embolisation of bronchial artery: a successful treatment in 209 cases of relapse haemoptysis. Angiology. 1993;44:295–9.
Mal H, Rullon I, Mellot F, et al. Immediate and long-term results of bronchial artery embolisation for life threatening haemoptysis. Chest. 1999;115:996–1001.
Chun JY, Belli AM. Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur Radiol. 2010;20:558–65.
Shin BS, Jeon GS, Lee SA, Park MH. Bronchial artery embolisation for the management of haemoptysis in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2011;15:1093–8.
Anuradha C, Shyamkumar NK, Vinu M, Babu NR, Christopher DJ. Outcomes of bronchial artery embolization for life-threatening hemoptysis due to tuberculosis and post-tuberculosis sequelae. Diagn Interv Radiol. 2012;18:96–101.
Hwang HG, Lee HS, Choi JS, Seo KH, Kim YH, Na JO. Risk factors influencing rebleeding after bronchial artery embolization on the management of hemoptysis associated with pulmonary tuberculosis. Tuberc Respir Dis. 2013;74:111–9.
Pei R, Zhou Y, Wang G, et al. Outcomes of bronchial artery embolization for life-threatening hemoptysis secondary to tuberculosis. PLoS One. 2014;9:e115956.
Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolisation: experience with 54 patients. Chest. 2002;121:789–95.
Shao H, Wu J, Wu Q, et al. Bronchial artery embolization for hemoptysis: a retrospective observational study of 344 patients. Chin Med J (Engl). 2015;128:58–62.
Hayakawa K, Tanaka F, Torizuka T, et al. Bronchial artery embolisation for haemoptysis: immediate and long-term results. Cardiovasc Intervent Radiol. 1992;15:154–9.
Maleux G, Matton T, Laenen A, Bonne L, Cornelissen S, Dupont L. Safety and efficacy of repeat embolization for recurrent hemoptysis: a 16-year retrospective study including 223 patients. J Vasc Interv Radiol. 2018;29(4):502–9.
Flight WG, Barry PJ, Bright-Thomas RJ, Butterfield S, Ashleigh R, Jones AM. Outcomes following bronchial artery embolisation for haemoptysis in cystic fibrosis. Cardiovasc Intervent Radiol. 2017;40:1164–8.
Cauldwell EW, Siekert RG, Lininger RE, Anson BJ. The bronchial arteries: an anatomic study of 150 human cadavers. Surg Gynecol Obstet. 1948;86:395–412.
Keller FS, Rosch J, Loflin TG, Nath PH, McElvein RB. Nonbronchial systematic collateral arteries: significance of percutaneous embolotherapy for haemoptysis. Radiology. 1987;164:687–92.
McPherson S, Routh WD, Nath H, Keller FS. Anomalous origin of bronchial arteries: potential pitfalls of embolotherapy for haemoptysis. J Vasc Intervent Radiol. 1990;1:86–8.
Sancho C, Escalante E, Dominguez J, et al. Embolization of bronchial arteries of anomalous origin. Cardiovasc Intervent Radiol. 1998;21:300–4.
Panda A, Bhalla A, Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol. 2017;23(4):307–17.
Laborda A, Tejero C, Fredes A, Cebrian L, Guelbenzu S, de Gregorio MA. Posterior circulation stroke after bronchial artery embolization. A rare but serious complication. Cardiovasc Intervent Radiol. 2013;36:860–3.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Russell Frood, Shishir Karthik, Saeed Mirsadraee, Ian Clifton, Karen Flood and Simon J. McPherson have nothing to disclose.
Compliance with Ethics Guidelines
Formal ethics approval or patient consent was not required as this was a retrospective data review and a waiver is granted at our institution in this circumstance.
Data Availability
The datasets generated during and/or analysed during the current study are not publicly available due to the sharing of datasets not being covered by the institutional agreement for retrospective studies. The datasets are available from the corresponding author on reasonable request and with the appropriate institutional approval.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.11891502.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any non-commercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Frood, R., Karthik, S., Mirsadraee, S. et al. Bronchial Artery Embolisation for Massive Haemoptysis: Immediate and Long-Term Outcomes—A Retrospective Study. Pulm Ther 6, 107–117 (2020). https://doi.org/10.1007/s41030-020-00112-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-020-00112-x