Abstract
A small fraction of patients with asthma have severe, persistent disease that is often refractory to standard therapy. To meet this need, a growing emphasis has been placed on the development of alternative, novel therapies and the ability to characterize those patients who are most likely to benefit from these therapies. The eosinophil has been identified as a primary mediator in airway inflammation and as a potential pharmacological target. This narrative review outlines the need for more phenotype-directed therapies in severe asthma, and discusses the supporting evidence for monoclonal antibodies directed against key pro-eosinophilic T-helper 2 (Th2) inflammatory cytokines as additive agents in the treatment of severe asthma with an eosinophilic phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma affects 25.7 million people worldwide, and is associated with a significant healthcare and economic burden to patients and society [1]. Three to ten percent of adults diagnosed with asthma are believed to suffer from severe, refractory disease [2, 3]. A single exacerbation requiring urgent intervention can increase the annual treatment costs by as much as threefold [4], and recurrent exacerbations have been shown to result in a progressive loss of lung function in some patients [5]. Up to 30% of adults with severe asthma will require oral corticosteroids in addition to inhaled corticosteroids to maintain control [6–8].
There is a need to identify those patients who are most at risk for exacerbations and to characterize the potential treatment options that might reduce these risks [9]. It has been suggested that within this group of patients, detailed phenotyping or characterizing based on readily observable traits [10] using clinical symptoms, markers of inflammation, and lung function might be useful [3, 9, 11]. Although there is no widely agreed upon phenotypic classification scheme, most proposed groupings include an early-atopic or allergic group, a delayed-onset group (often involving obese patients with a female predominance), and a late-onset, eosinophilic predominant group [3, 10–12], as shown in Table 1. However, in asthma, each phenotype does not necessarily yield a distinct endotype or subgroup defined by pathogenetic mechanisms of disease at a more cellular level [10]. This consensus has shifted the current research focus towards differentiating the pathobiologic mechanisms of each group so that targeted therapies can be developed.
Recurrent exacerbations are a major contributor to asthma-related morbidity in many patients, and seem to predominate in a subgroup with eosinophilic airway inflammation [9, 13]. Although inhaled corticosteroids are effective at reducing airway eosinophilia, up to 50% of patients with severe asthma will have persistent eosinophilia despite treatment [14, 15], suggesting that selective targeting of airway eosinophilia may have benefit.
The purpose of this narrative review is to illustrate both the importance of eosinophilic inflammation in the airway as a distinct subtype of severe asthma, and to review the biologic therapies that are under investigation to specifically treat this patient population. This article is based on previously conducted studies (see Table 2), and does not involve any new studies of human or animal subjects performed by any of the authors.
Role of Eosinophils
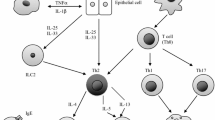
Eosinophils have been shown to promote airway inflammation and remodeling in asthma [16], and elevated peripheral blood eosinophil counts have been identified as an independent risk factor for asthma exacerbations [17–20]. Additionally, a reduction in sputum eosinophil count in those patients with moderate to severe asthma has been shown to reduce exacerbations [21–23] and hospital admissions [21]. T-helper type 2 (Th2) lymphocytes are thought to play a key role in the pathogenesis of asthma [24], with an increased serum immunoglobulin E (IgE) and elevated peripheral blood eosinophil count defining the classic phenotype [25]. Blockade of the key Th2 cytokines interleukins-4, -5, and -13 (IL-4, IL-5, and IL-13) is a plausible target for the development of biologic therapies for patients with asthma who remain uncontrolled on conventional therapies.
Mepolizumab
Interleukin-5 (IL-5) is a pro-inflammatory cytokine released predominantly by helper T cells that stimulates maturation [26] and activation of eosinophils [27], making it a logical target to reduce eosinophil-driven inflammation. Mepolizumab is a humanized monoclonal antibody against IL-5 that prevents the binding of IL-5 to the receptor complex on the surface of the eosinophil [28]. It selectively inhibits eosinophilic inflammation [29–32] and reduces the numbers of eosinophils in the blood and sputum [31, 33–37]. In an early phase-two, double-blinded, randomized, placebo-controlled trial of adults with moderately severe asthma with persistent symptoms despite inhaled corticosteroid therapy [33], Flood-Page and colleagues evaluated the potential benefit of mepolizumab as add-on therapy. While no clinically significant benefit was noted in the treatment arm, the study did demonstrate safety of administration and a nonsignificant trend towards reduced frequency of exacerbations despite a short three-month study period. Additionally, it confirmed mepolizumab’s ability to markedly reduce peripheral blood and sputum eosinophils, raising the important question that perhaps with more careful phenotyping, mepolizumab would be more beneficial.
Subsequent clinical studies of mepolizumab began to better elucidate the relevant phenotype in which this agent might be effective. In 2009, Haldar et al. compared mepolizumab versus placebo in the treatment of refractory eosinophilic asthma [34]. The study population included 61 adult patients who met the American Thoracic Society definition of refractory asthma [38], evidence of eosinophilic inflammation in sputum samples defined by a sputum eosinophil count of 3% or greater at least once in the previous two years despite high-dose (either inhaled or systemic) corticosteroid therapy, and recurrent exacerbations. Over the 50-week trial period, there was a significant reduction in total number and rate of exacerbations [relative risk, 0.57; 95% confidence interval (CI), 0.32–0.92; p = 0.02], as well as a modest improvement in the Asthma Quality of Life Questionnaire (AQLQ) score (mean improvement in AQLQ 0.55 (minimal clinically important difference 0.5 [39])). However, there was no effect on asthma symptoms or forced expiratory volume in one second (FEV1), suggesting that these markers of disease may be improved through other mechanisms. The only serious adverse events reported were hospitalizations for severe asthma (10% mepolizumab vs. 34% placebo).
A follow-up report by the same authors 12 months after trial completion included the majority (56 of 61) of subjects included in the original study [40]. After cessation of mepolizumab, participants experienced significant increases in peripheral blood and sputum eosinophil counts as soon as three months after trial completion, followed by an increase in exacerbation frequency and worsening asthma control as noted by the Juniper Asthma Control Questionnaire (JACQ). The increase in both peripheral blood and sputum eosinophils preceding the increase in rate of exacerbations suggests a relationship between eosinophilic inflammation and asthma control.
In a similar cohort of patients, Nair et al. showed an association between intravenous mepolizumab administration and overall reduction in daily prednisone dose [35] in a smaller, proof of concept study. Twenty adult patients who required daily treatment with oral prednisone and high-dose inhaled glucocorticoids (600–2000 μg of fluticasone or equivalent) to control symptoms and had persistent sputum eosinophilia with a least 3% of cells in an induced sputum sample were randomized to either mepolizumab 750 mg or placebo IV every four weeks for 26 weeks. Administration of mepolizumab resulted in fewer exacerbations, a longer time to first exacerbation, and a significant reduction in prednisone requirements. Subjects treated with mepolizumab were able to reduce their dose of prednisone by a mean (±SD) of 83.8 ± 33.4% of the maximum possible dose compared to 47.7 ± 40.5% in the placebo group (p = 0.04), but there was no difference in the perhaps more clinically meaningful final mean dose of prednisone between the groups. There were no serious adverse events in the study.
In the first of two large international multicenter, randomized, double-blinded, placebo-controlled trials, the Dose Ranging Efficacy And safety with Mepolizumab in severe asthma (DREAM) trial [36], Pavord et al. enrolled 621 participants with severe asthma with recurrent exacerbations and evidence of eosinophilic inflammation defined as a sputum eosinophil count ≥3%, peripheral blood eosinophil count ≥300 cells/μL, exhaled nitric oxide concentration (FeNo) of at least 50 ppb, or “prompt deterioration of asthma control after a 25% or less reduction in regular maintenance inhaled or oral corticosteroids.” Participants were randomized to one of three doses of mepolizumab (75, 250, or 750 mg) or placebo IV every four weeks for 13 infusions. The investigators found that, over the course of 52 weeks, intravenous mepolizumab reduced peripheral blood and, to a lesser degree, sputum eosinophil counts, and reduced the rate of clinically significant exacerbations at a similar rate regardless of dose when compared to placebo. Similar to previous studies [33–35], there was no difference between the groups in asthma control questionnaire (ACQ) scores or FEV1, suggesting that strategies for managing exacerbations and these other aspects of control might be considered differently in this population. A subgroup analysis indicated that the efficacy of mepolizumab in reducing exacerbation rates increased with higher baseline eosinophil counts and number of exacerbations within the preceding year but not baseline FeNo, suggesting that careful phenotyping in patient selection for use of this agent will be important in future studies. The broad definition of eosinophilic inflammation for the entry criteria for this study may have limited its ability to show significant change, but the idea of noninvasive, clinically available markers is appealing.
The second large randomized, double-blind, placebo-controlled trial, the Mepolizumab as Adjunctive Therapy in Patients with Severe Asthma (MENSA) study [37], evaluated a similar population of refractory asthma with recurrent exacerbations, but included the subcutaneous (SQ) route of administration as an intervention arm, and tightened the definition of eosinophilic inflammation to include only those subjects with peripheral blood eosinophil counts of at least 150 cells/μL at screening or 300 cells/μL at some point in the previous year. Participants were randomized to mepolizumab administered 75 mg IV or 100 mg SQ or placebo every four weeks for 32 weeks. Both routes of administration had similar efficacies. When compared to placebo, those treated with mepolizumab demonstrated an improvement in lung function, had evidence of improved asthma control with a lower ACQ-5 score (although less than the minimal clinically significant change of 0.5 [39]), and had significant improvement in quality of life (QOL) as measured by a numerical decrease on the St. Georges Respiratory Questionnaire (SGRQ). Mepolizumab reduced overall exacerbation rates from 1.74 to 0.93 exacerbations per patient year in the IV group (47% reduction, 95% CI 28–60%; p < 0.001) and 0.83 in the SQ group (53% reduction, 95% CI 36–65%; p < 0.001). Efficacy was again noted to be linked with markers of eosinophilic inflammation, with those subjects with peripheral blood eosinophil counts of over 500 cells/μL having the greatest reduction in exacerbations. The most commonly reported adverse events were injection-site reactions. This study further supports the notion that, with careful patient selection, mepolizumab can be effective in a specific population of patients with severe eosinophilic asthma.
This early suggestion that mepolizumab could be useful as a glucocorticoid-sparing agent [35] was later confirmed by the larger Steroid Reduction with Mepolizumab Study (SIRIUS) [41], in which 135 patients with glucocorticoid-dependent eosinophilic asthma were randomized to mepolizumab 100 mg SQ or placebo every four weeks for 20 weeks as an additive therapy. Similar to MENSA [37], the SIRIUS investigators defined eosinophilic asthma as the presence of a peripheral blood eosinophil level of least 300 cells/μL on initial screening, or at least 150 cells/μL during the run-in phase of the trial. Administration of mepolizumab resulted in a significant reduction in daily glucocorticoid dose (50% median reduction from baseline compared to no reduction in the placebo group, p = 0.007), a reduced annualized rate of exacerbation (1.44 exacerbations per patient year versus 2.12, p = 0.04), as well as improvements in the ACQ-5 and SGRQ. Previous studies [34, 36, 37] did not demonstrate this improvement in asthma control with mepolizumab, suggesting that there may be a greater potential for symptomatic control in those patients who are steroid dependent. There was no significant difference in ability to wean completely off glucocorticoid therapy, but the trial’s short length may have limited the ability to completely assess this or its long-term effect on exacerbations. The safety profile for mepolizumab was similar to placebo, but six (4%) participants in the two study groups developed non-neutralizing antibodies to mepolizumab.
Overall, a 2013 meta-analysis of seven randomized controlled trials comparing mepolizumab to placebo concluded that mepolizumab “appears to be useful for control of exacerbations and improve asthma-related quality of life in individuals with persistent airway eosinophilia” [42]. The authors did not find a significant difference in pooled change in FEV1 [42], although the analysis was published prior to the MENSA study, so additional statistical analysis may be warranted in the future. The reductions in both peripheral [33–36, 42] and sputum [34, 35, 42] eosinophil counts in those receiving mepolizumab were significant and, given the specificity of monoclonal-antibody-targeted therapy, is supportive of the causal relationship between reduction in eosinophilic inflammation and rate of exacerbation.
Based on the growing body of literature [33–37, 41, 42], mepolizumab was approved for clinical use in the European Union by the European Medicines Agency (EMA) on September 24, 2015 [43], and in the United States by the Food and Drug Administration (FDA) on November 4, 2015 [44]. It is approved as an add-on maintenance treatment of patients with severe asthma with an eosinophilic phenotype (peripheral blood eosinophil count ≥300 cells/μL or sputum eosinophil count ≥3%) aged 12 years and older. The recommended dose is 100 mg administered by subcutaneous injection of the thigh, upper arm, or abdomen every four weeks. There were few serious side effects reported in clinical trials of mepolizumab [44]. The most common adverse effect was headache (19% vs. 18% placebo), followed by injection site reaction (8% vs. 3% placebo). Severe hypersensitivity reactions have been reported in pooled data (7% of those who received placebo versus 10% who received mepolizumab), as has herpes zoster infection (two cases during clinical trials) [44].
Reslizumab
Similar to mepolizumab, reslizumab is a humanized monoclonal antibody targeted against IL-5, preventing binding to eosinophil targets [45]. Several randomized controlled trials comparing reslizumab to placebo have been completed. The first enrolled 106 adults with poorly controlled eosinophilic asthma defined as ≥3% eosinophils on induced sputum and an ACQ-7 score of 1.5 or higher despite high-dose inhaled corticosteroid therapy (at least 880 μg fluticasone or equivalent daily) plus an additional controller [46]. Over a short 15-week observation period, there was a trend towards improvement in mean ACQ-7 score with reslizumab administration, but this did not reach statistical significance except in the subgroup of participants with nasal polyps (p = 0.0119). Participants who received reslizumab showed significantly greater improvements in lung function, including FEV1 and forced vital capacity (FVC), and reductions in both peripheral blood (40% reduction) and induced sputum (95% reduction) eosinophil counts than those who received placebo. The improvement in FEV1 was most pronounced in those subjects with peripheral blood eosinophils of at least 500 cells/μL or more at baseline, further highlighting the need to clearly establish the appropriate phenotype in which these agents are likely to be effective.
A more recent publication described data collected from two duplicate multicenter, randomized, placebo-controlled trials that included adolescents and adults with uncontrolled eosinophilic asthma defined by an ACQ-7 score ≥1.5 and at least 440 μg fluticasone or equivalent daily with or without an additional controller agent and with a peripheral blood eosinophil count ≥400 cells/μL. A total of 953 participants were randomized to receive either reslizumab 3 mg/kg or placebo IV every four weeks for one year. In pooled data from both trials, those who received reslizumab had a significant improvement in overall exacerbation rates to 0.84 exacerbations per patient per year versus 1.81 in the placebo group (reduction of 54%). Reslizumab treatment also improved lung function, AQLQ scores (improvement of 1.08 versus 0.81 in those who received placebo) and ACQ-7 scores (reduction of 1.02 versus 0.77 in those who received placebo) over the 52-week study period. While reslizumab had a similar effect on rates of asthma exacerbation as those reported for mepolizumab in DREAM [36] and MENSA [37], the improvements in asthma control and lung function were more notable. The authors speculate that the higher eosinophil threshold of ≥400 cells/μL for inclusion in these studies may more accurately represent airway eosinophilia [47], and may ultimately better predict which patients will respond to inhibition of IL-5. With the exception of two patients who suffered from anaphylaxis that was believed to be related to the study medication, adverse events were similar in the two groups [48].
A confirmatory trial comparing two doses of reslizumab [49] in patients with poorly controlled eosinophilic asthma (blood eosinophils ≥400 cells/µL) randomized patients 1:1:1 to 0.3 mg/kg reslizumab, 3 mg/kg reslizumab, and placebo administered IV once every four weeks for 16 weeks. Reslizumab improved lung function, asthma symptoms, and quality of life when compared to placebo, but there was no significant difference in asthma control. The effect was generally greater at the 3.0 mg/kg dose, without an increase in adverse events. The improvement in FEV1 did not correlate with baseline eosinophil levels of ≥400 cells/µL [49]. Finally, Corren et al. evaluated the effect of reslizumab in a population of adults with inadequately controlled asthma unselected for eosinophilia [50]. When compared to placebo, there was no difference in FEV1, ACQ scores, or rescue inhaler use between groups. The study was not powered for detailed subgroup analyses, but in those participants with a peripheral blood eosinophil count of ≥400 cells/μL, there was a significant improvement in FEV1 (270 mL) compared to placebo, supporting a blood eosinophil threshold of ≥400 cells/μL for clinical use.
Based on the previous body of work [46, 48–50], reslizumab was approved for use in the US on March 23, 2016 by the FDA [51] and the EU on June 23, 2016 by the EMA [52] for add-on maintenance therapy for severe asthma in adults aged 18 years or older with an eosinophilic phenotype. The recommended dose is 3 mg/kg every four weeks administered by intravenous infusion over 20–50 min [53]. Anaphylaxis was reported in 0.3% of patients included in all clinical trials (0% in placebo). Other adverse effects include oropharyngeal pain (2.6% vs. 2.2% placebo) and creatine phosphokinase (CPK) elevation (14% vs. 9% placebo) [53].
Benralizumab
Rather than directly binding and inhibiting IL-5, benralizumab is an investigational humanized monoclonal antibody that is directed at a subunit of the IL-5 receptor (IL-5R) located on eosinophils and basophils that induces apoptosis [54]. In a small phase-one trial, intravenous and subcutaneous benralizumab was shown to effectively decrease eosinophil counts in both peripheral blood and sputum, with a trend toward a reduction in peripheral blood basophil counts compared to placebo [55]. In a phase two, randomized, double-blind, placebo-controlled parallel group study, patients with physician-diagnosed asthma and at least one exacerbation requiring urgent care in the preceding 12 months who presented to the emergency department with an acute asthma exacerbation were randomized to either a single dose of benralizumab (0.3 or 1.0 mg/kg) or placebo in addition to usual care administered at the time of presentation, and were followed over a 12-week period [56]. Patients were enrolled regardless of peripheral blood eosinophil levels. There was no difference between groups in number of exacerbations at four, 12, or 24 weeks, FEV1, ACQ, or AQLQ scores. However, pooled analysis found that benralizumab significantly decreased the rate of exacerbations when compared to placebo (p = 0.01). Interestingly, the effect of benralizumab was not related to blood eosinophil count in this study.
A second larger randomized, controlled, dose-ranging trial enrolled adults with uncontrolled moderate-to-severe asthma and a history of recurrent exacerbations to receive benralizumab over one year at varying doses [57]. Patients were classified as either eosinophilic or non-eosinophilic based on an elevated FeNo (>50 ppb) in combination with a mathematical algorithm to predict sputum eosinophils. A significant reduction in annual exacerbation rate was found in those subjects with an eosinophilic subtype who received 100 mg benralizumab versus those who received placebo, although, due to the nature and goals of the study, the authors accepted p < 0.169 as significant rather than the traditional 0.05. Additionally, there were significant improvements in FEV1 and ACQ-6 scores in all subjects, regardless of phenotype, who received benralizumab compared to those who did not. The activity in the non-eosinophilic subtype suggests that benralizumab may be active towards other IL-5R-expressing cells that contribute to inflammation [57].
Dupilumab
Dupilumab is an investigational humanized monoclonal antibody against the IL-4 receptor α-subunit present in two distinct receptors on lymphocytes (type I receptor) and bone marrow precursors (type II receptors) [27, 58–60]. The type I receptor present on lymphocytic precursors is predominantly responsible for Th2 cell differentiation and maturation [27, 60], so inhibition of this differentiation should reduce the downstream effects of eosinophilic maturation and activation. A phase-two randomized controlled trial [61] enrolled 104 adults with uncontrolled moderate-to-severe eosinophilic asthma (peripheral blood eosinophil count ≥300 cells/μL or induced sputum eosinophil count ≥3%). Participants were randomized to receive subcutaneous dupilumab 300 mg weekly or placebo over 12 weeks in addition to inhaled fluticasone and salmeterol. After 4 weeks, participants were advised to discontinue salmeterol and tapered off fluticasone. Compared to placebo, dupilumab significantly reduced the number of exacerbations over the study period (odds ratio 0.08; 95% CI, 0.02–0.28; p < 0.001), despite discontinuation of inhaled corticosteroid and long-acting beta-agonist therapy. Dupilumab also resulted in improved lung function and asthma control, but the short length of the study did not allow the investigators to duplicate clinical practices [61]. Although dupilumab effectively reduced FeNo, serum IgE, and other serum biomarkers supporting biologic activity, there was no change in peripheral blood eosinophil counts, suggesting that reducing eosinophils is not essential for reducing disease activity.
Lebrikizumab
Interleukin-13 (IL-13) is a pro-inflammatory Th2 cytokine secreted by activated T-cells, eosinophils, natural killer cells, mast cells, and basophils that acts as a potent stimulator of mucus production, airway fibrotic changes, and airway eosinophilia [62]. Serum levels are elevated in asthma, and this is thought to be a mechanism of steroid resistance [63]. One of the mechanisms by which IL-13 induces airway fibrosis and remodeling is by upregulating the secretion of periostin [64], which has been linked as a systemic biomarker specific for airway eosinophilia [65]. Lebrikizumab is a humanized monoclonal antibody that binds to IL-13, which is under investigation for the treatment of uncontrolled asthma [66, 67]. Early studies indicate that lebrikizumab may improve lung function (percent improvement in baseline FEV1 compared to placebo ranged from 8.2% to 10.7% in three phase-two trials [66, 67], although this improvement seems to be limited to those patients with high baseline levels of systemic periostin (≥50 ng/mL). In secondary analysis, lebrikizumab was also shown to decrease FeNo, another marker of eosinophilic airway inflammation [68].
Conclusion
Asthma is a complex chronic inflammatory condition where patients with severe eosinophilic airway disease pose a particular challenge for clinicians. As new biologic therapies targeted at specific subtypes of asthma become available, it will be increasingly important to be able to readily identify those subgroups of patients who will be the most likely to respond. Our concept of asthma phenotyping has evolved from emphasizing broad clinical classifications to much more specific biologic characteristics that can more clearly link the underlying pathology to the phenotype.
The place of these new biologic agents in our armamentarium of options for patients with uncontrolled disease is still being defined, but the decision to begin such therapies should carefully weigh the cost of the agent, its safety profile, and the target population. Standard asthma therapies should be optimized according to national guidelines [69, 70], with attention paid to adherence and control of comorbid conditions before the addition of biologics. For currently approved therapies [44, 53], we recommend consideration prior to the initiation of chronic systemic steroids to reduce the risk of exacerbations, or to use them in add-on therapies as potential steroid-sparing agents. While strategies for long-term monitoring of these agents have not been defined, we recommend a year-long trial for those patients who tolerate the medications, with the monitoring of frequency of exacerbations and healthcare utilization, the assessment of asthma control with standardized questionnaires, and the ability to wean systemic corticosteroids as primary goals.
References
Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat 3. 2012;35:1–58.
Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–73.
Lane S, Molina J, Plusa T. An international observational prospective study to determine the cost of asthma exacerbations (COAX). Respir Med. 2006;100(3):434–50.
O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW, Group SI. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179(1):19–24.
The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J. 2003;22(3):470–7.
Heaney LG, Brightling CE, Menzies-Gow A, Stevenson M, Niven RM, Network BTSDA. Refractory asthma in the UK: cross-sectional findings from a UK multicentre registry. Thorax. 2010;65(9):787–94.
Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–13.
Custovic A, Johnston SL, Pavord I, Gaga M, Fabbri L, Bel EH, et al. EAACI position statement on asthma exacerbations and severe asthma. Allergy. 2013;68(12):1520–31.
Agache I, Akdis C, Jutel M, Virchow JC. Untangling asthma phenotypes and endotypes. Allergy. 2012;67(7):835–46.
Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–25.
Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42(5):650–8.
Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–24.
Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160(3):1001–8.
Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57(10):875–9.
Efraim AHNB, Levi-Schaffer F. Tissue remodeling and angiogenesis in asthma: the role of the eosinophil. Ther Adv Respir Dis. 2008;2(3):163–71.
Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Gossage D, et al. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract. 2014;2(6):741–50.
Tran TN, Khatry DB, Ke X, Ward CK, Gossage D. High blood eosinophil count is associated with more frequent asthma attacks in asthma patients. Ann Allergy Asthma Immunol. 2014;113(1):19–24.
Malinovschi A, Fonseca JA, Jacinto T, Alving K, Janson C. Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J Allergy Clin Immunol. 2013;132(4):821–7 (e1–5).
Ulrik CS. Peripheral eosinophil counts as a marker of disease activity in intrinsic and extrinsic asthma. Clin Exp Allergy. 1995;25(9):820–7.
Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–21.
Chlumský J, Striz I, Terl M, Vondracek J. Strategy aimed at reduction of sputum eosinophils decreases exacerbation rate in patients with asthma. J Int Med Res. 2006;34(2):129–39.
Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemière C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27(3):483–94.
Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–92.
Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95.
Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, et al. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988;167(1):43–56.
Boyman O, Kaegi C, Akdis M, Bavbek S, Bossios A, Chatzipetrou A, et al. EAACI IG Biologicals Task Force paper on the use of biologic agents in allergic disorders. Allergy. 2015;70(7):727–54.
Gnanakumaran G, Babu KS. Technology evaluation: mepolizumab, GlaxoSmithKline. Curr Opin Mol Ther. 2003;5(3):321–5.
Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112(7):1029–36.
Menzies-Gow A, Flood-Page P, Sehmi R, Burman J, Hamid Q, Robinson DS, et al. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003;111(4):714–9.
Leckie MJ, ten Brinke A, Khan J, Diamant Z, O’Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356(9248):2144–8.
Kips JC, O’Connor BJ, Langley SJ, Woodcock A, Kerstjens HA, Postma DS, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med. 2003;167(12):1655–9.
Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176(11):1062–71.
Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–84.
Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360(10):985–93.
Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–9.
Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–207.
American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162(6):2341–51.
Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–8.
Haldar P, Brightling CE, Singapuri A, Hargadon B, Gupta S, Monteiro W, et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. J Allergy Clin Immunol. 2014;133(3):921–3.
Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–97.
Liu Y, Zhang S, Li DW, Jiang SJ. Efficacy of anti-interleukin-5 therapy with mepolizumab in patients with asthma: a meta-analysis of randomized placebo-controlled trials. PLoS One. 2013;8(3):e59872.
European Medicines Agency. Summary of opinion on mepolizumab (press release); 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/003860/WC500194118.pdf. Accessed 15 July 2016.
GlaxoSmithKline. Nucala (package insert); March 2016. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125526Orig1s000Lbl.pdf. Accessed 15 July 2016.
Egan RW, Athwal D, Bodmer MW, Carter JM, Chapman RW, Chou CC, et al. Effect of Sch 55700, a humanized monoclonal antibody to human interleukin-5, on eosinophilic responses and bronchial hyperreactivity. Arzneimittelforschung. 1999;49(9):779–90.
Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125–32.
Fowler SJ, Tavernier G, Niven R. High blood eosinophil counts predict sputum eosinophilia in patients with severe asthma. J Allergy Clin Immunol. 2015;135(3):822–4 (e2).
Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–66.
Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;S0012–3692(16):47551–3. doi:10.1016/j.chest.2016.03.032.
Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;S0012–3692(16):45715–6. doi:10.1016/j.chest.2016.03.018.
US Food and Drug Administration. FDA approves Cinqair to treat severe asthma (press release); 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491980.htm. Accessed 15 July 2016.
European Medicines Agency. Summary of opinion on reslizumab (press release); 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/003912/WC500209307.pdf. Accessed 15 July 2016.
Teva Pharmaceutical Industries Ltd. Cinqair (package insert); June 2016. http://www.cinqair.com/pdf/PrescribingInformation.pdf. Accessed 15 July 2016.
Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–53 (e2).
Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132(5):1086–96 (e5).
Nowak RM, Parker JM, Silverman RA, Rowe BH, Smithline H, Khan F, et al. A randomized trial of benralizumab, an antiinterleukin 5 receptor α monoclonal antibody, after acute asthma. Am J Emerg Med. 2015;33(1):14–20.
Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014;2(11):879–90.
Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282(5397):2258–61.
Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282(5397):2261–3.
Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal. 2008;1(51):pe55.
Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–66.
Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111(4):677–90 (quiz 91).
Saha SK, Berry MA, Parker D, Siddiqui S, Morgan A, May R, et al. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121(3):685–91.
Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118(1):98–104.
Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130(3):647–54 (e10).
Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365(12):1088–98.
Hanania NA, Noonan M, Corren J, Korenblat P, Zheng Y, Fischer SK, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015;70(8):748–56.
Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15.
Global Initiative for Asthma. Global strategy for asthma management and prevention; 2016. http://www.ginasthma.org/2016-gina-report-global-strategy-for-asthma-management-and-prevention/. Accessed 18 July 2016.
US Department of Health and Human Services, National Institutes of Health. Expert Panel Report 3: guidelines for the diagnosis and management of asthma (EPR-3); July 2007. http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines. Accessed 17 July 2016.
Lötvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355–60.
Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–23.
Acknowledgments
No funding or sponsorship was received for this study or the publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Bryan R. Hay, Carleen M. Risaliti, and Jennifer W. McCallister have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/F7E4F060562F83DF.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hay, B.R., Risaliti, C.M. & McCallister, J.W. Emerging Biological Therapies in Severe Eosinophilic Asthma. Pulm Ther 2, 153–169 (2016). https://doi.org/10.1007/s41030-016-0019-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-016-0019-x