Abstract
A polyetheretherketone (PEEK) cranial implant is one of the most well-known polymeric implants used in cranioplasty. However, most off-the-shelf PEEK cranial implants are developed by molding and then sized into the patient's defect anatomy by machining, which is time-consuming and capital-intensive. On the contrary, 3D printing, specifically material extrusion, can develop patient-specific cranial implants that precisely fit the defect anatomy, ensuring stable fixation and restoring esthetic cranial symmetry. However, 3D printing high-quality, mechanically robust PEEK implants are challenging due to the high thermal processing conditions required for PEEK printing, its high melt viscosity, and its susceptibility to incomplete crystallization. If appropriately attuned, an optimized set of 3D printing conditions can yield high-quality patient-specific PEEK cranial implants with clinically relevant mechanical properties. Hence, in this study, we comprehensively analyzed the effect of essential 3D printing conditions on cranial implants' material and mechanical properties. Specifically, we varied critical 3D printing material extrusion parameters, such as build orientation, nozzle, bedplate, chamber temperature, and print speed, and analyzed their effect on the implants' impact strength. We also used microscopy and Finite Element Analysis to understand the implants' fracture patterns with the impact indentor's impact. Based on our research, we determined an optimized set of 3D printing conditions to yield cranial implants with appropriate impact strength. Our results revealed that specimens printed at 0° build orientation, i.e., parallel to the bedplate, with optimum printing parameters, such as nozzle, bedplate, chamber temperature, and print speed, sustained a peak force of 2034 N. We envision that this study will help implant manufacturers utilize high-temperature material extrusion 3D printing to develop patient-specific PEEK cranial implants with clinically viable mechanical properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cranioplasty is the surgical repair of a skull bone defect resulting from a traumatic injury, existing deformity, or disease. Currently, cranioplasty or skull reconstruction is widely used in ‘decompressive craniectomies’, a critical procedure to relieve intracranial pressure resulting from brain swelling due to trauma, cerebrovascular accidents, or diseases. The surgical procedure involves lifting the scalp and restoring the skull contour with either an original skull bone, referred to as autologous grafts, or a custom-contoured synthetic implant. Autologous bone grafts have been the standard for reconstructive cranioplasty and have been used for decades [1]. However, autologous bone grafts exhibit high protrusion and infection rates, resorption, and high chances of donor site morbidity [2]. In addition, it is challenging to get bone grafts that match the skull's defective contour. On the contrary, synthetic cranial implants can be designed according to the shape and size of the skull defect and are less prone to immune rejections than autografts [3]. The most common synthetic materials used to develop cranial implants are metals, such as titanium alloys (Ti6Al4V), polymers, such as polyetheretherketone (PEEK), and ceramics, such as hydroxyapatite (HA) [4].
Although cranial metal implants have been used extensively for many years, they have several disadvantages. First, highly stiff Ti6Al4V implants do not match the stiffness or elasticity of skull bone, resulting in stress shielding where the rigid metallic implant bears most of the load and does not allow the healthy bone to regenerate [5, 6]. While the latter is not a significant concern in the case of cranial implants, metal ions leaching from the implants due to corrosion have a high chance of inducing cytotoxic reactions and are proven to cause soft tissue atrophy and negatively affect the human body's immunological system [7]. Finally, metallic implants interfere with medical diagnostics using computer tomography, magnetic resonance imaging (MRI), and cone beam X-ray imaging. Metals also absorb or scatter radiation, making postoperative radiation therapy difficult [7, 8]. Bioceramics, such as hydroxyapatite (HA) and tri-calcium phosphate (TCP), have been used for cranial implants; however, the brittleness of ceramic materials is a significant concern [9]. Moreover, injectable/moldable bioceramic (calcium phosphate)-based bone cement can result in exothermic reactions while setting, harming the tissues around the autograft [10].
Some of these issues with metals and ceramics have led to the use of polymers, such as polymethylmethacrylate (PMMA) and polyetheretherketone (PEEK). However, PMMA has poor impact resistance [11]. In addition, it also has poor wear and chemical resistance, and it has been evidenced to result in high exothermic reactions at the implantation site, affecting the neighboring tissue [12, 13]. On the contrary, PEEK is a high-performance polymer with outstanding strength, high wear, and chemical resistance. It is widely used in the medical device industry due to several advantages. First, PEEK exhibits mechanical properties that are similar to those of bone. For instance, PEEK exhibits stiffness in the range of 3–5 GPa and can be modified to match the stiffness of bone [14]. Second, PEEK has high impact and compressive strength (> 100 MPa). Third, PEEK has less density and hence exhibits a high strength-to-weight ratio. Finally, PEEK is radiolucent, making radiographic assessment easy [15, 16]. All these properties have promoted PEEK as the material of choice for cranial implants [17]. Consequently, there are several commercially available PEEK implants in the market, and there has been an increasing trend in the usage of PEEK implants. However, conventional processing, such as molding, is used to develop most PEEK cranial implants. The implant design is patient-specific, using customized, prefabricated molds per the patient's defect contour or by cutting and reshaping a stock cranial implant using conventional machining methods. However, there are some significant disadvantages to this process. Notably, traditional manufacturing methods require a considerable turnaround time to make the implants, prolonging the implants' logistics. Usually, a patient undergoing craniectomy requires the implant promptly, but with such manufacturing methods, that becomes challenging [18, 19].
On the contrary, 3D printing, or additive manufacturing, can develop implants that match the patient's cranial defect anatomy. Hence, 3D-printed patient-specific implants perfectly fit the patient's anatomy, fill a defect, ensure stable fixation, and restore esthetic cranial symmetry. Patient-specific cranial implants also decrease the risk of complications, prevent side effects, and minimize surgery and recovery time [20, 21]. However, manufacturing mechanically robust implants by 3D printing is a challenge. This is because the implants are developed in a layer-by-layer format in 3D printing, and poor interlayer adhesion can significantly affect the overall strength of the implant. 3D printing techniques like selective laser sintering (SLS) have been used to develop PEEK cranial implants; however, SLS of PEEK can be challenging due to the variation in PEEK's particle size, which can lead to inhomogeneous particle fusion and poor mechanical properties of the final part. In addition, manufacturing implants with SLS is complex, labor- and capital-intensive.
Fused Filament Fabrication (FFF) is a simple yet powerful material extrusion 3D printing technique that can efficiently develop high-quality patient-specific implants. However, utilizing FFF to develop mechanically robust PEEK implants is challenging because FFF of PEEK requires high thermal processing conditions [22]. For instance, PEEK can only be printed with nozzle temperatures > 370 °C, bedplate temperatures > 150 °C, and chamber temperatures > 90 °C [22]. In addition, PEEK is prone to rapid crystallization. The high processing temperatures and the PEEK's high crystallization speed cause excessive thermal stress, which can get unevenly distributed between printed layers, resulting in thermal cracks, poor interlayer adhesion, and part warpage in the 3-D printed PEEK parts. Finally, PEEK has high melt viscosity, which can lead to non-uniform extrusion during 3D printing, resulting in voids and defects in the printed part [23]. Hence, it is evident that the FFF of PEEK is challenging and requires careful processing and expert manufacturing skills to develop mechanically robust implants [24]. Some efforts have been made to develop PEEK cranial implants using the FFF process; however, no studies to date have performed a comprehensive analysis of all the effects of vital FFF conditions on the mechanical properties of the cranial implants. In the past two years, significant efforts have been made to develop clinically viable (in terms of mechanical durability and strength) PEEK implants [22, 23, 25,26,27,28,29,30]. In this study, we selected the critical FFF parameters based on our prior studies and analyzed the effect of those parameters on the mechanical properties of the PEEK cranial implants. We also analyzed the various fracture patterns of the FFF-printed parts developed with varying printing conditions. Finally, we perform finite element analysis to understand the fracture patterns of the PEEK cranial implants.
2 Materials and methods
2.1 Raw materials for 3D printing
A commercially available PEEK filament with a diameter of 1.75 mm (Thermax™ PEEK Natural, 3DXTECH) was used for 3D printing the cranial implants. The filaments were dried at 120 °C for 5 h before using them for 3D printing.
2.2 FFF-3D printing parameters
A FUNMAT HT Enhanced (Intamsys Technology Ltd., Shanghai, China) 3D printer was used for manufacturing the cranial implants. The orientations of the printed specimens were varied, with printing occurring at 0º, 45º, and 90º. All other FFF parameters were kept constant. The details for the processing parameters are shown in Table 1. For all the samples, the infill rate was 100%. All the implants were printed adjacent to the bedplate, with the Z-axis serving as the thickness or height of the specimens.
2.3 Design of experiments
The Taguchi method optimizes design criteria, simplifies the experimental plan and interactions between process parameters [31]. A minimal set of combinations of parameters and levels for each experiment was found using orthogonal arrays. This approach is helpful for PEEK 3D printing, where the cost of producing prototypes is high due to expensive materials [32]. The present study investigated four processing parameters at three levels, as depicted in Table 1.
The L9 Taguchi orthogonal array (shown in Table 2) method was employed in this study based on the number of processing parameters, levels, and calculated degrees of freedom. Table 3 presents the experimental plan, designed using an L9 orthogonal array.
2.4 Mechanical property analyses
The impact tests were carried out on the Drop Tower Impact System (Instron CEAST9350), which fulfills the requirements of ISO 6603-2. Data acquisition was done with the attached computer system and the related Instron software. The testing speed was set to 4.43 m/s, and an impact mass of 15.392 kg was used, resulting in a drop height of 1 m and 151 J impact energy. A 22 kN piezo striker model M2098 with a Ø16 mm hemispherical head was used. The implant was securely placed on the base plate (as shown in Fig. 1), and the drop tower impact testing was performed at room temperature. During the impact test, the force applied to the implant increases until it reaches a maximum value known as the peak force (PF). This peak force corresponds to the highest load the implant could sustain during the impact test. The peak force's magnitude reflects the impact's severity and the implant's ability to absorb and dissipate energy, and a higher peak force indicates higher impact strength. Representative force–displacement curves were compared for each printing condition. After impact testing, the fractured specimens were analyzed for the various failure mechanisms with the help of digital images. A stereomicroscope (AM Scope, California, USA) was also used to analyze the implants' fracture patterns.
2.5 Thermal property analyses
The thermal properties of the 3D-printed cranial implants were investigated using Differential Scanning Calorimetry (DSC) (DSC 250, TA instruments, New Castle DE, USA). Samples were collected from the cross section of cranial implants. The degree of crystallinity (Xcw%) was calculated using the following equation: \(Xcw(\%)=\frac{{H}_{m}}{{w}_{f}\times {H}_{c}}\times 100\%\), where Hm is the melt enthalpy as obtained from the DSC scan, wf is the weight fraction of PEEK (1), and Hc is the melt enthalpy of 100% crystalline PEEK (130 J.g−1).
2.6 Finite element analysis (FEA)
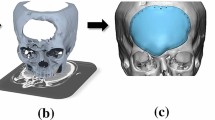
A finite element model of the cranial implant was developed in ABAQUS Software (Simulia, Dassault Systèmes, Paris, France) to evaluate the stresses, deformation, and damage response of the cranial implant under impact loads. Steps followed in the simulation process are briefly described below. It is worth noting that the complexity of the microstructure and the strong anisotropic properties inherent to FDM parts as a function of the printing parameters and fabrication processes pose a significant challenge in effectively predicting the behavior of the cranial implant using FEA [33]. The primary purpose of incorporating FEA into this study is to complement the experimental results and provide a comprehensive understanding of the behavior of the cranial implant under impact loads.
First, the solid geometry of the implant was developed from an STL file used to print the 3D samples of the PEEK cranial implants, and the PEEK material and corresponding damage behavior were modeled using the Johnson–Cook (J–C) model already available in the ABAQUS material constitutive model database. This model has been widely used in finite element analysis (FEA) to simulate the behavior of materials under high-strain rate loading conditions, such as those encountered in impact events [34, 35]. A detailed description of the J–C model and the process of obtaining its parameters can be found in the work of Banerjee et al. (2015), and Gkolfinopoulos and Chijiwa (2022). The damage evolution in the J–C model is often expressed as a damage initiation criterion, followed by a damage growth equation; the initiation criterion defines the conditions under which damage begins to occur, while the growth equation governs the rate at which damage progresses after initiation. In Abaqus FEA, the damage dissipation energy can be used to quantify the extent of degradation in the material, with values ranging from 0 (undamaged) to 1 and above to indicate complete damage. Table 4 shows the applied numerical values of the J–C model [36, 37].
For the boundary and loading conditions, the edges of the cranial implant were all fixed against translation and rotation in the x-, y-, and z-axes, as shown in Fig. 2a; the impact hammer was represented as a rigid material, and precisely positioned to deliver a direct impact close to the center of the implant. In an attempt to mimic the shape of the impact hammer, the total length of the hammer was segmented as shown in later Fig. 13. This translates to the possibility of the FEA impact force increasing even after the initial penetration, as the larger segments beyond the indentor also contact and penetrate the cranial implant. After conducting a mesh convergence study, a total of 28,770 continuum 3D, 8-noded elements (C3D8) was used for the cranial implant; dynamic explicit analysis was performed throughout the simulation process with applied velocities similar to the experiment. Figure 2b shows the meshed configuration of the cranial implant geometry.
2.7 Statistical analysis
We used the Taguchi Design of Experiments (DOE) methodology to optimize the total number of runs. Specifically, we utilized the L9 array to efficiently explore the effects of multiple factors while minimizing the number of experimental runs required. We could maximize the information gained from each experimental run by strategically selecting the experimental design. This allowed us to optimize the use of resources while maintaining statistical rigor. We used a General Full Factorial Design approach to calculate the sample size, as shown in Fig. 3. Based on pilot data, we assumed an alpha level (α) of 0.05 and a standard deviation 340. We were able to determine the appropriate sample size to ensure adequate statistical power by creating a model with four factors, each having three levels, and considering terms in the model up through the fourth order. Initially, the design included two replicates, but we increased it to three for enhanced reliability and precision in our analysis. We used a one-way analysis of variance with the Tukey test to determine the statistical difference between different specimen groups, and ρ < 0.05 was considered significant.
3 Results and discussion
3.1 Effect of Build Orientation on the implants' fracture patterns and impact strength
First, the cranial implants were printed at 0°, 45°, and 90° with respect to the build plate, while all the other printing parameters were kept identical. The specimens printed at 0° required the highest amount of support structures shown in Fig. 4-ia; the entire inner surface area of the implant parallel to the bedplate comprised support structures. On the contrary, the specimens printed at 90° required the least support structures, as shown in Fig. 4-ic, with only one edge of the cranial implant supported on the bedplate. Implants developed at an inclination of 45° had higher support structures than the 90° but significantly less than the 0° specimen, as shown in Fig. 4-ib.
(i) Effect of different build orientations on the cranial implants. The as-printed cranial implants with support structures (above) and the top view of the as-printed cranial implants (below) show the crystalline and amorphous region between build orientation printed at a 0° b 45o and c 90° build orientation. ii Shows the comparison of crystallinity of different region in the same cranial implants
We also observed dark brown, indicative of amorphous regions, and natural beige regions, indicative of semi-crystalline regions in one single implant (Fig. 4-ii); this is attributed to the uneven heat distribution to the implant while it was being printed, which leads to variations in cooling rates and material solidification. Amorphous regions in a material indicate that it has cooled rapidly, forming a non-crystalline structure. Another explanation is that the dark brown regions do not receive sufficient heat in the print chamber, resulting in incomplete crystallization. On the other hand, crystalline regions suggest that the cooling process was slower, which allowed for the orderly arrangement of molecular chains that are characteristic of crystalline materials. Moreover, these regions received the necessary heat in the print chamber to completely crystallize. Polymer crystallinity has essential implications for the material's properties, including its mechanical strength, thermal conductivity, and surface finish. Understanding the relationship between heat distribution and material structure is crucial for optimizing printing parameters and achieving the desired material performance. We observed the least amount of amorphous regions (dark brown regions) in the implants printed at 90°, compared to the ones printed at 0° and 45°. This observation indicates that the build orientation significantly influences the extent of heat the specimen receives while it is being printed, which in turn dictates the crystallinity of the parts.
In addition to the parts' crystallinity, qualitatively, the implants printed at 0° orientation exhibited the highest outer surface roughness, as shown in Fig. 5a, because regular profiles are obtained at low-build orientation angles with circular filaments with sharp edges corresponding to layer height, increasing the surface roughness.
Meanwhile, the implants printed at 90° showed a smooth surface finish with a glossy appearance, as shown in Fig. 5c. This is because the distance between consecutive filaments increases at high print orientation angles, resulting in lower surface roughness [38]. The implants printed at 45° exhibited a slightly lower surface finish (higher surface roughness) than the 90° implants, but the surface roughness was less than that of the 0° specimens, as shown in Fig. 5b.
Impact testing of the implants printed at different build orientations exhibited essential results. In the case of 0° orientation, the layers were printed parallel to the bedplate and the implant's surface, as illustrated in Fig. 6a, allowing them a large surface contact area and strong adhesion with the prior layer. Each layer showed significant resistance to tensile failure during testing, as all implants were kept parallel to the horizontal surface. The impact load was applied perpendicular to the implant's surface, as shown in Fig. 6d at the center portion of the implant. As a result, the specimens printed at 0° displayed the maximum impact strength, and the force–displacement curve showed that the peak force was a high 2034 N.
On the other hand, when a 45o or 90° build orientation was used, the printed layers were aligned parallel to the printer's bedplate but at 45° and 90° [39] inclinations relative to the implant's surface [38], as shown in Fig. 6b, c, respectively. Of note, compared to the 0° orientation, 45° and 90° orientations provided a much smaller surface contact area of adhesion for the print layers. Thus, when the impact load was applied, it was applied parallel to the print layers of the 90° specimens (Fig. 6f), and this caused the implant to undergo shear failure. Due to the implant's minimal surface contact area, it exhibited the weakest adhesion between adjacent layers, leading to an impact strength of 1076 N, significantly less than the 0° specimens. In the case of the 45° angle specimens, the impact load was applied at a 45° angle to the direction of the printed layers, causing the impact load to be divided into two components: shear force and tensile force, as shown in Fig. 6e. Hence, the printed layers exhibited shear and tensile failures due to this dual-loading [40].
Figures 7a–c shows the distinct fracture patterns observed in the fractured implants printed at 0°, 45°, and 90° orientations. In the case of the 0° implant, as shown in Fig. 7a, complete fracture occurs perpendicular to the print layers. Moreover, layer delamination is not the predominant failure mechanism because the print layers strongly adhere to one another with the highest surface area. Conversely, a complete fracture pattern parallel to (or along) the print layers is evident in the implants printed at 45° and 90°. The predominant fracture pattern is complete delamination of the print layers, as the print layers have a minimal surface area of contact with each other.
We used stereomicroscopy to identify particular differences in the peak force values and associated fracture patterns among the evaluated implants. Implants printed with 0° build orientations displayed consistent interlayer connectivity, homogenous PEEK fusion, and strong interlayer bonding, as shown in Fig. 8a. Importantly, the print layer adhesion is evident in the images, highlighting the vital role of interlayer bonding strength in influencing the impact strength of the cranial implants. As opposed, only the clean fractured surface of the print layer was seen in the implants printed at 45° and 90° build orientations (Fig. 8b, c). Moreover, the raster angle and print trajectory could be seen in the clean fractured surface of these specimens.
Petersmann, et al. [41] investigated the effects of printing parameters (build orientation and airflow temperature) in FFF of patient-specific cranial implants (PSCI) using PEEK, and they confirmed that horizontally printed implants exhibit higher mechanical integrity but lower surface quality compared to other orientations (45° and 90°). The authors also observed lower airflow temperatures lead to implant discoloration and increased energy absorption during impact. Berretta, et al. [42] analyzed the effect of different build orientations on patient-specific laser-sintered PEEK cranial implants. They also confirmed that horizontal and inverted horizontal orientations exhibited the slightest deviation and highest compressive strength of 793.7 N. Another study reported the relationship between processing parameters and mechanical behavior for the clinical implementation of cranial implants; the study highlighted that a 0° configuration yields superior performance with a maximum tensile strength of 95.4 MPa compared to 45° and 90° build configurations [43]. The results from prior studies are similar to ours, which indicates that the 0° build orientations results in cranial implants with the highest mechanical properties. Furthermore, the surface roughness of the implants printed at 0° exhibits the highest roughness compared to the other build orientations (45° and 90°), which is beneficial to prevent skin or muscle slippage and implant exposure, a critical problem in cranioplasty. Such complications may occur for several reasons, including infection, insufficient soft tissue coverage, poor wound healing, or problems with the implant [44]. In addition to being unsightly, implant exposure raises the possibility of infection and implant failure. Patients who have had their implants exposed may feel pain, discomfort, and worry, which can lower their quality of life overall [45]. Surgery is frequently necessary for managing and treating implant exposure, including implant removal, wound debridement, and replacement, which adds financial burden to the patient and raises healthcare expenditures [46]. Based on our analysis, we envision that cranial implants 3D-printed at 0° will be appropriate to develop clinically viable implants.
3.2 Effect of thermal processing parameters
3.3 Effect of nozzle temperatures
In the current study, we used nozzle temperatures ranging from 410 to 450 ºC, and we observed that the higher nozzle or extrusion temperatures act as a localized thermal source, delivering more energy to the PEEK crystallization process and enhancing the material's mechanical characteristics, resulting in a peak force of 2034 N at 450 °C. Specimens printed at 450 °C exhibited a crystallinity of 23%, while the ones printed at 410 °C exhibited a crystallinity of 19.5%. The average peak forces at various nozzle temperatures that the implant could withstand before breaking are shown in Fig. 9a. The combination of thermal 3D printing parameters greatly influences implants' resolution and mechanical properties. Our study revealed that we can achieve distinct mechanical strengths and properties in PEEK implants by varying the chamber and bed temperature combinations during printing at 450 °C nozzle temperature. Implants printed with a chamber temperature of 50 °C and bed temperature of 130 °C displayed superior mechanical strength due to enhanced adhesion and structural integrity. However, implants printed with higher chamber temperatures (> 50 ºC) and lower bed temperatures (< 130 ºC) showed weaker adhesion and reduced mechanical strength. Additionally, higher nozzle temperatures reduce the melt viscosity of PEEK and raise its flowability, which improves the material's rheological characteristics in the molten state and promotes robust interlayer adhesion [47]. Also, we noted that increasing the nozzle temperature improved the load-bearing capability and strength of the printed items, which aligns with our previous studies [22]. Our results are similar to prior published reports. Vaezi and Yang [48] found that nozzle temperatures between 400 and 430 °C were ideal for 3D printing PEEK. However, temperatures beyond 430 °C lead to either material degradation over an extended extrusion or considerable filament deformation. Also, El Magri et al. [49] observed a higher degree of crystallinity in the PEEK specimens when the printing temperature was increased from 380 to 420 °C.
3.4 Effect of bedplate temperatures
Figure 9b shows the effect of variation in bedplate temperature on the average values of peak force carried by the implant before fracture. The bedplate temperatures we employed ranged from 100 to 160 °C. Notably, we found that the implant printed at 130 °C showed the maximum impact force, whereas the implant produced at 100 °C showed the lowest impact force. The bedplate temperature plays a crucial role in the fabrication of FFF parts because it binds the initial layer of the print to the bedplate and holds the entire part until the end of the print. Moreover, a higher bedplate temperature will provide more heat energy for better adhesion between the print layer and encourage a greater crystallization rate, improving mechanical qualities. In our prior study, we observed excellent interlayer adhesion and minimal weld lines near the bedplate when a tensile specimen printed at 130 °C bedplate temperature but reduced adhesion in upper layers and confirmed that the bedplate temperature could effectively affect the printed layers up to a specific distance along the build direction (z-axis) [22]. We also noticed that a higher surface area contact facilitates increased heat transfer to the upper layers than smaller surface area contact. In the current study, the cranial implants printed at 00 exhibited a significantly high surface area in contact with the bedplate. Consequently, higher bedplate temperatures provided greater energy to the print layers during printing, improving interlayer adherence. However, we observed a slight decrease in the peak force for the implants printed at 1600C compared to the ones printed at 1300C; this can be attributed to the overriding influence of other thermal conditions, such as nozzle and chamber temperatures.
3.5 Effect of chamber temperatures
PEEK is a semi-crystalline polymer and is prone to rapid crystallization, so a higher chamber temperature is vital in yielding parts with crystalline PEEK parts. A higher chamber temperature facilitates thermal energy transfer to the amorphous polymer chains, leading to isothermal crystallization during the printing of parts. A reduced or absent chamber temperature fails to provide sufficient heat, leading to incomplete crystallization and the formation of mechanically weak amorphous regions. Figure 9c shows the effect of various chamber temperatures on the impact strength of the implant. We observed the maximum impact force value at 50 °C, followed by 90 °C and 70 °C. Correspondingly, we achieved a crystallinity of 23% in the case of 50 °C, 21% in the case of 90 °C, and 19% in the case of 70 °C. In contrast, Yang et al. [50] observed a rise in crystallinity from 17 to 31% with an increase in chamber temperature from 25 to 200 °C. The current results also contradict our previous results, which indicate that higher chamber temperatures result in parts with higher mechanical properties [51]. This is because, even though the chamber temperature was high, it was not homogeneously and effectively transmitted to the cranial implant during the printing process, resulting in specimens with low crystallinity.
In our previous studies, we printed tensile, flexural, and compressive specimens [51], dental [23], or spinal fusion cages [30], which are significantly smaller than the cranial implants in the present study. Due to the large size of the cranial implant, the heat distribution to the implants was not homogenous, resulting in varied amorphous regions (brown regions) and crystalline regions (beige-colored regions) in the cranial implant (Figs. 4 and 5). Thus, not only higher chamber temperatures, but homogenous heat distribution inside the printing chamber and effective heat transmission to the implant are vital to achieving isothermal crystallization in PEEK parts.
Figure 10a shows the combined effect of the nozzle, bedplate, and chamber temperature on the maximum peak force that could be withstood by the cranial implants. The graph indicates the printing combination with the parameters nozzle temperature: 450 °C, bed plate temperature: 130 °C, and chamber temperature: 50 °C yields implants with the highest peak force, indicative of the highest impact strength. The force–displacement curve of this implant undergoing impact testing is shown in Fig. 10b and provides significant data on the implant's dynamic response to impact loading conditions. As the test initiates, the implant experiences an initial displacement with minimal force, signifying the early stages of impact absorption. As the impact progresses, the force exerted on the implant increases, reaching a peak value. This peak force corresponds to the maximum impact force sustained by the implant during the test. Following the peak force, there is typically a rapid decrease in force accompanied by a continued displacement. This phase represents the post-impact deformation and the energy absorption mechanisms within the material. The force gradually diminishes as the material absorbs and dissipates the impact energy. Figure 11a shows the cranial implant's peak force and displacement plot printed with a 0º build orientation. Variations in peak force are observed across all the specimens. Specimen 9 displays higher peak forces at lower displacements than the other specimens. All specimens exhibit ascending slopes, which indicate a rise in stiffness. The peak points represent the material's ultimate strength before failing or deforming significantly. In Fig. 11b, the peak force and the displacement plot show cranial implants printed at fixed temperature settings: 450 °C, 130 °C, and 50 °C as the nozzle, bedplate, and chamber temperatures, respectively, and different build orientations (0º, 45º, and 90º). The variation in peak force across different build orientations reveals distinct mechanical behaviors of the material. Notably, the 0º orientation shows consistent peak force increase with displacement, indicating strong resistance to deformation. On the other hand, the 45º and 90º orientations exhibit peak forces at different displacement points, which suggests varying degrees of material ductility. These findings highlight the anisotropic nature of the material, where properties differ based on the direction of the load. Overall, the 0º orientation demonstrates the highest peak force, followed by the 45º orientation, with the 90º orientation showing the lowest peak force. These findings indicate that the material has different ductility levels and deformation resistance across different build orientations.
(a) The peak force and the displacement diagram for the cranial implant printed with 0 º build orientation with the different printing temperature combinations. (b) The peak force and displacement diagram for the cranial implant were printed at 450 °C, 130 °C, and 50 °C as nozzle, bedplate, and chamber temperature, respectively, with different build orientations
Our comprehensive analysis indicates that in FFF of PEEK cranial implants, a single thermal processing condition might not significantly impact the impact strength of the implants. In our previous studies pertaining to FFF of PEEK parts and implants, we noticed that a single type of thermal processing condition would have a linear effect on the parts' mechanical properties, i.e., a, higher thermal processing parameters, such as nozzle, bedplate, and chamber temperatures, will help in increasing the strength of the PEEK parts [22, 27]. However, in the present study, we did not observe that. Instead, the combined effect of FFF thermal processing conditions plays a significant role in the impact strength of the cranial implants; we deduce that the larger size of the cranial implants and the longer printing duration, as opposed to other implants or standard-specific parts, is responsible for this.
3.6 Effect of printing speed
Printing speed significantly impacts the printed implants' strength, print quality, and part resolution. We explored three distinct printing speeds, 20 mm/s, 30 mm/s, and 40 mm/s, to investigate their impact on the printed cranial implants. Notably, the implants printed at 40 mm/s exhibited a peak force of 2034 N, as shown in Fig. 12, suggesting that higher printing speeds positively correlate with improved impact strength. Also, higher print rates and extrusion speeds increase melt pressure, directly influencing the surface morphology and extrusion diameter of the PEEK filament in the nozzle. Increased melt pressure, a benefit of higher print rates, also reduces surface imperfections in the extruded PEEK filament [22, 52].
Furthermore, the implants printed at 40 mm/s demonstrated superior resolution, indicating a positive relationship between printing speed and the precision of filament deposition [53]. The rapid movement of the extruding nozzle at higher speeds reduced the chances of void formation and layer defects, promoting filament density and enhancing the printed implants' overall resolution. Achieving this accurate filament deposition is essential to provide desirable structural integrity under load-bearing conditions [54].
In contrast, lower printing speeds, 20 mm/s, resulted in a lower peak force value of 955 N. The slow movement or extrusion of the filament at lower speeds can enhance thermal expansion, increase the likelihood of irregularities in print layers, and result in unevenness and defects in the deposited layers [49]. These factors can negatively impact the bonding between layers, ultimately degrading the mechanical properties of the printed cranial implants [53]. It is crucial to consider factors, such as print time, associated costs, and desired part quality, when determining the most suitable printing speed for fabricating cranial implants using FFF technology.
Creating customized cranial implants that fit a patient's unique anatomy is crucial for successful outcomes in cranial implant surgery. This process requires careful planning and execution to ensure precise fitting and optimal functional integration. To capture detailed anatomical data, various imaging techniques such as CT scans or MRI are used. This data is then used to design and manufacture an implant that accurately matches the patient's skull's defect anatomy. FFF is a powerful yet seamless manufacturing technique that can utilize the design and develop implants matching the defect's anatomy, i.e., patient-specific implants. Our study aims to contribute to this process of patient-specific implant manufacturing by identifying critical parameters in the FFF of PEEK cranial implants. We have systematically analyzed the effects of critical FFF parameters on the mechanical properties of PEEK implants to offer valuable insights into optimizing the manufacturing process. Our findings have highlighted the importance of build orientation, nozzle temperature, bedplate temperature, and chamber temperature to mitigate thermal stresses and ensure uniform interlayer adhesion. Additionally, our analysis of fracture patterns provides crucial guidance for enhancing the structural integrity of FFF-printed PEEK implants. By incorporating these insights into the design and fabrication of personalized implants, implant manufacturers can select an optimized set of FFF conditions and develop mechanically robust PEEK cranial implants. Utilizing FFF to develop PEEK implants will significantly reduce manufacturing time and implant development costs while seamlessly developing implants that match the defect anatomy. Clinicians can rely on such FFF-developed patient-specific PEEK cranial implants to achieve better implant fit, stability, and long-term performance outcomes. Our research lays the groundwork for further refinement of utilizing FFF of patient-specific cranial implants, ultimately benefiting patients by improving surgical precision and postoperative recovery.
3.7 FEA stress distribution
The FE simulations focused on monitoring the gradual changes in the effective Von Mises stress, the vertical displacement/deformation of the implant, the peak force sustained by the implant, and the damage energy (from time = 0 up to the end of the analysis). Here, the J–C model employs a damage parameter that quantifies the extent of degradation in the material, with values ranging from 0 (undamaged) to 1 (completely damaged). This is also evidenced in the separation of the (and possible deletion) of the elements during penetration of the impactor. The critical aspects of the simulation occurred between the first time of contact between the drop hammer and the implant up to the end (where damage was excessive). Figure 13 shows the changes in the stress at selected instances in the simulation. Prior to any observable damage, i.e., loss in continuity of the implant constituent elements, the maximum vertical displacement induced in the implant was 1.437 mm, with a corresponding stress of 99.74 MPa. Subsequent penetration of the impact hammer shows a reduction in the effective/maximum stress. Contrary to the experimental results, the absolute peak force evaluated from the simulation was ~ 650 N; this could be due to the differences in the assumed material properties for the FEA and the actual experiments. Figure 14 shows the (a) plan view and (b) side view of the cranial implant section. Also, it is worth mentioning that the FEA utilized the Elastic modulus based on the material point stress–strain behavior (from the dog-bone specimen). Figure 15 demonstrates the distribution of the impact force that the implant can withstand over time. In the simulation, two peak forces were observed. The first peak occurred upon the initial penetration of the indentor, the second peak occurred as the larger portion of the hammer beyond the indentor also contacted and penetrated the cranial implant. In a separate case, when the hammer was not segmented, a single peak of approximately 795N was observed in the FEA simulations.
4 Conclusion
In this study, we explored the effect of critical FFF-3D printing factors on PEEK cranial implants' material and mechanical properties. We observed that the printing orientation is an essential factor, and implants printed at a 0° orientation demonstrated the highest mechanical strength and exhibited a peak force of 2034 N. The 0° orientation promotes robust interlayer adhesion and superior impact resistance due to the raster angle being perpendicular to the tensile direction. Conversely, 45° and 90° showed weaker interlayer adhesion, decreasing the implant's impact strength and resistance to applied forces. Furthermore, the influence of thermal processing parameters was critical in influencing the implant's strength, with elevated nozzle temperatures (410 °C to 450 °C), a bedplate temperature of 130 °C, and a chamber temperature of 50 °C proving optimal for enhancing the implant's strength. Thus, this study provides a comprehensive analysis and forms the basis of developing robust PEEK cranial implants using FFF. This study's findings apply to the manufacturing industry, especially implant manufacturers who are moving away from conventional machining and prefer to utilize high-throughput FFF to develop high-quality PEEK implants seamlessly and efficiently.
Data Availability
More additional data will be made available on request.
References
Mustafa MA, et al (2023) Health-related quality of life following cranioplasty–a systematic review. Br J Neurosurg pp 1–11
Zhang J, et al (2023) Clinical guidelines for indications, techniques, and complications of autogenous bone grafting. Chin Med J pp 10.1097
Linder LKB et al (2019) Patient-specific titanium-reinforced calcium phosphate implant for the repair and healing of complex cranial defects. World Neurosurg 122:e399–e407
Jindal P et al (2023) Optimizing cranial implant and fixture design using different materials in cranioplasty. Proc Inst Mech Eng Part L J Mater Des Appl 237(1):107–121
Gautam S et al (2022) Recent advancements in nanomaterials for biomedical implants. Biomed Eng Adv 3:100029
Aufa A, Hassan MZ, Ismail Z (2022) Recent advances in Ti-6Al-4V additively manufactured by selective laser melting for biomedical implants: Prospect development. J Alloy Compd 896:163072
Tibau AV et al (2019) Titanium exposure and human health. Oral Science International 16(1):15–24
Vallittu PK (2017) Bioactive glass-containing cranial implants: an overview. J Mater Sci 52(15):8772–8784
Pietak AM et al (2007) Silicon substitution in the calcium phosphate bioceramics. Biomaterials 28(28):4023–4032
Kucko NW et al (2019) Calcium phosphate bioceramics and cements. Principles of Regenerative Medicine. Elsevier, pp 591–611
Mallya PK, Juneja M (2021) Rapid prototyping of orthopedic implant materials for cranio-facial reconstruction: a survey. Mater Today Proc 45:5207–5213
Ramakrishna S et al (2001) Biomedical applications of polymer-composite materials: a review. Compos Sci Technol 61(9):1189–1224
Unterhofer C et al (2017) Reconstruction of large cranial defects with poly-methyl-methacrylate (PMMA) using a rapid prototyping model and a new technique for intraoperative implant modeling. Neurol Neurochir Pol 51(3):214–220
Skirbutis G et al (2018) PEEK polymer’s properties and its use in prosthodontics. A review Stomatologija 20(2):54–58
Skirbutis G et al (2017) A review of PEEK polymer’s properties and its use in prosthodontics. Stomatologija 19(1):19–23
Ma H et al (2021) PEEK (Polyether-ether-ketone) and its composite materials in orthopedic implantation. Arab J Chem 14(3):102977
Altiok E et al (2019) Applications of polyetheretherketone in craniomaxillofacial surgical reconstruction. PEEK Biomaterials Handbook. Elsevier, pp 319–331
Tevlin R et al (2014) Biomaterials for craniofacial bone engineering. J Dent Res 93(12):1187–1195
Attaran M (2017) The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus Horiz 60(5):677–688
Mian SH et al (2022) Adaptive mechanism for designing a personalized cranial implant and its 3D printing using PEEK. Polymers 14(6):1266
Sharma N et al (2021) Quantitative assessment of point-of-care 3D-printed patient-specific polyetheretherketone (PEEK) cranial implants. Int J Mol Sci 22(16):8521
Sikder P, Challa BT, Gummadi SK (2022) A comprehensive analysis on the processing-structure-property relationships of FDM-based 3-D printed polyetheretherketone (PEEK) structures. Materialia 22:101427
Sonaye SY et al (2022) Patient-specific 3D printed Poly-ether-ether-ketone (PEEK) dental implant system. J Mech Behav Biomed Mater 136:105510
Wang P et al (2019) Effects of printing parameters of fused deposition modeling on mechanical properties, surface quality, and microstructure of PEEK. J Mater Process Technol 271:62–74
Bokam VK et al (2023) Effect of milling on the compounding of poly-ether-ether ketone (PEEK) and amorphous magnesium phosphate (AMP) composites. Powder Technol 427:118747
Bokam VK, et al (2023) Extrusion of uniform-diameter polyetheretherketone-magnesium phosphate bio-composite filaments for 3D printing of design-specific multi-functional implants. Mater Adv
Challa BT, et al (2022) In-house processing of 3-D printable polyetheretherketone (PEEK) filaments and the effect of fused deposition modelling parameters on 3D Printed PEEK structures
Gummadi SK, et al (2022) Mechanical properties of 3D-printed porous poly-ether-ether-ketone (PEEK) orthopedic scaffolds. In: JOM, 2022, pp 1–13
Naganaboyina HPS, et al (2023) In-house processing of Carbon Fiber Reinforced Polyetheretherketone (CFR-PEEK) 3D printable filaments and fused filament fabrication-3d printing of CFR-PEEK parts
Saini A et al (2022) Fused filament fabrication-3D printing of poly-ether-ether-ketone (PEEK) spinal fusion cages. Mater Lett 328:133206
Davis R, John P (2018) Application of Taguchi-based design of experiments for industrial chemical processes. Statistical approaches with emphasis on design of experiments applied to chemical processes, p 137. InTech. Available at: https://doi.org/10.5772/intechopen.69501
Deng X et al (2018) Mechanical properties optimization of poly-ether-ether-ketone via fused deposition modeling. Materials 11(2):216
Baikerikar, P.J. and C.J. Turner. Comparison of as-built FEA simulations and experimental results for additively manufactured dogbone geometries. in International Design Engineering Technical Conferences and Computers and Information in Engineering Conference. 2017. American Society of Mechanical Engineers.
Garcia-Gonzalez D et al (2015) Mechanical impact behavior of polyether–ether–ketone (PEEK). Compos Struct 124:88–99
El Halabi F et al (2011) Mechanical characterization and numerical simulation of polyether–ether–ketone (PEEK) cranial implants. J Mech Behav Biomed Mater 4(8):1819–1832
Banerjee A et al (2015) Determination of Johnson cook material and failure model constants and numerical modelling of Charpy impact test of armour steel. Mater Sci Eng, A 640:200–209
Gkolfinopoulos I, Chijiwa N (2022) Determination of Johnson-Cook material and failure model constants for high-tensile-strength tendon steel in post-tensioned concrete members. Appl Sci 12(15):7774
Buj-Corral I, Domínguez-Fernández A, Durán-Llucià R (2019) Influence of print orientation on surface roughness in fused deposition modeling (FDM) processes. Materials 12(23):3834
Solomon IJ, Sevvel P, Gunasekaran J (2021) A review on the various processing parameters in FDM. Mater Today Proc 37:509–514
Ravindrababu S et al (2018) Evaluation of the influence of build and print orientations of unmanned aerial vehicle parts fabricated using fused deposition modeling process. J Manuf Process 34:659–666
Petersmann S et al (2023) Material extrusion-based additive manufacturing of polyetheretherketone cranial implants: Mechanical performance and print quality. J Market Res 22:642–657
Berretta S, Evans K, Ghita O (2018) Additive manufacture of PEEK cranial implants: Manufacturing considerations versus accuracy and mechanical performance. Mater Des 139:141–152
Zhao Y et al (2020) Mechanical characterization of biocompatible PEEK by FDM. J Manuf Process 56:28–42
Alkhaibary A et al (2020) Cranioplasty: a comprehensive review of the history, materials, surgical aspects, and complications. World Neurosurg 139:445–452
Xu H et al (2015) Early cranioplasty vs. late cranioplasty for the treatment of cranial defect: a systematic review. Clin Neurol Neurosurg 136:33–40
Shibahashi K et al (2017) Cranioplasty outcomes and analysis of the factors influencing surgical site infection: a retrospective review of more than 10 years of institutional experience. World Neurosurg 101:20–25
Elhattab K et al (2020) Fabrication and evaluation of 3-D printed PEEK scaffolds containing Macropores by design. Mater Lett 263:127227
Vaezi M, Yang S (2015) Extrusion-based additive manufacturing of PEEK for biomedical applications. Virtual Phys Prototyping 10(3):123–135
El Magri A et al (2020) Optimization of printing parameters for improvement of mechanical and thermal performances of 3D printed poly (ether ether ketone) parts. J Appl Polym Sci 137(37):49087
Yang C et al (2017) Influence of thermal processing conditions in 3D printing on the crystallinity and mechanical properties of PEEK material. J Mater Process Technol 248:1–7
Sikder P, Challa BT, Gummadi SK (2022) A comprehensive analysis on the processing-structure-property relationships of FDM-based 3-D printed polyetheretherketone (PEEK) structures. Materialia 22:p 101427
Geng P et al (2019) Effects of extrusion speed and printing speed on the 3D printing stability of extruded PEEK filament. J Manuf Process 37:266–273
Basgul C et al (2018) Structure–property relationships for 3D-printed PEEK intervertebral lumbar cages produced using fused filament fabrication. J Mater Res 33(14):2040–2051
Rybachuk M et al (2017) Anisotropic mechanical properties of fused deposition modeled parts fabricated by using acrylonitrile butadiene styrene polymer. J Polym Eng 37(7):699–706
Funding
This work was funded by Cleveland State University to Prabaha Sikder with Grant number STARTUP06.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sonaye, S.Y., Mack, J.P., Tan, KT. et al. A comprehensive analysis of high-temperature material extrusion 3D printing parameters on fracture patterns and strength of polyetheretherketone cranial implants. Prog Addit Manuf (2024). https://doi.org/10.1007/s40964-024-00688-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40964-024-00688-9