Abstract
Thermal conductivity is an important property for cast components produced from different types of cast iron. Development of a general widely-accepted thermal conductivity model for compacted and lamellar graphite irons poses a research challenge. The present study extends the modeling approach introduced earlier for pearlitic lamellar graphite iron toward compacted graphite iron and ferritic lamellar graphite iron. The proposed thermal conductivity model of the bulk material is based on the alloy microstructure and Si segregation between eutectic cells and non-cell regions, at the main assumption that the heat paths in the eutectic cells are formed by connected graphite phases surrounded by ferrite phases. The overall thermal resistance of these heat paths is determined by the hydraulic diameter of the interdendritic region. The uncertainties both for the modeled and for experimentally derived thermal conductivities have been estimated. The importance of considering the Si segregation in the model has been discussed. For the investigated samples, the agreement between modeled and measured thermal conductivities has been achieved within 4% on the average, at the same value of the single fitting parameter found for pearlitic, pearlitic–ferritic lamellar, and compacted graphite iron alloys. The results contribute to the understanding of the material microstructure effects on the cast iron thermal conductivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lamellar graphite iron (LGI) has for some decades been a common material for engine and cylinder blocks. Recent introduction of compacted graphite iron (CGI) as the material for diesel engine blocks in trucks and heavy vehicles created the new challenges in how to enable the desired thermal conductivity of the cast iron components, where the heat should be effectively conducted toward the engine coolant channels. Nodularity and graphite morphology are acknowledged to influence the thermal properties of a CGI alloy.1,2,3 Ferrite and pearlite fractions as well as Si segregation are the other factors that determine thermal conductivity of both CGI and LGI components.1,4,5
Measurements and modeling efforts on thermal conductivity of cast irons have been done in the previous research. A linear relationship between the thermal diffusivity and aspect ratio of graphite flakes has been established.6 The dependence of thermal conductivity on microstructure has been discussed for LGI.7 The modeling efforts on thermal conductivity of cast irons have been reviewed,8 where the importance of morphology and individual properties of the compounds has been admitted. The difference in heat transfer between spheroidal graphite iron (SGI) and LGI has been analyzed,9 accounting for the heat transfer parallel to the basal planes in graphite. The effects of the alloying elements, microstructural constituents, and temperature on the thermal conductivity have also been discussed in the previous research.1,10,11,12
Cast iron can be considered a multi-phase composite comprising graphite, ferrite, and/or pearlite, etc. The classical models of thermal conductivity for multi-phase composites and polymers such as Maxwell model,13 Bruggeman model,14 and percolation theory-based models15 have been found to supply reasonable values for the SGI.1
Furthermore, the cast iron can be treated as a combination of an anisotropic phase (graphite), isotropic phase (ferrite), and weakly anisotropic phase (pearlite). In the thermal conductivity model,16 a shape factor is introduced for graphite to account for the deviation from the spherical shape.
The modeling of CGI and LGI including interconnected graphite phases represents a challenge due to the complex graphite shape and interconnection. A simplified interconnected graphite shape has been considered to develop a thermal conductivity model for the LGI.17
Giving the credit to the research efforts to develop thermal conductivity models for cast irons, a general approach to the thermal conductivity modeling for all the types of cast iron has, to the best of our knowledge, not been presented. An experimentally supported method to model thermal conductivity of the pearlitic LGI has been introduced.18 The new general method is based on the thermal resistance network to represent the heat transfer within a eutectic cell and in the matrix between the cells.
The present work aims to prove and extend the applicability of this approach to CGI and ferritic–pearlitic LGI and improve understanding of how as-cast alloy microstructure affects the thermal conductivity. The model is developed under the assumption that the input microstructural parameters can either be predicted by solidification simulation or be measured.
A common modeling framework for LGI and CGI is described. After that, the equivalent thermal resistance network is introduced, and a theoretical thermal conductivity model is presented. The experimental part includes measurement of the microstructural parameters and phase fractions for the investigated cast iron samples, serving as model inputs. The same alloy composition is studied at different holding times to obtain different microstructure. The calculated thermal conductivity values are compared to the experimentally derived thermal conductivities of the studied samples, followed by the discussion of the results and modeling parameters.
Modeling Framework
This section introduces the common modeling framework for CGI and LGI based on the typical LGI and CGI microstructure observations, followed by the equivalent thermal circuit to represent the heat transfer paths across the eutectic cells and intercellular space. The approach to modeling of thermal conductivity for pearlitic LGI has been described.18
It is recognized in the research community that the heat conduction in LGI and CGI occurs primarily through graphite phases arranged in the metallic matrix including flaky and compacted graphite particles, respectively. The standard ISO 16112 defined CGI may also contain restricted fraction of spherical graphite particles (max 20%). The spatial distribution of the graphite phases can be described considering the solidification structure of the metallic matrix. The primary austenite and eutectic austenite are generically interconnected in austenite grains.19 They may be distinguished due to the elemental segregation of alloying elements, such as Si.20,21 The spatial distribution of the flaky and the compacted graphite is delimited to the space between the primary austenite network, forming eutectic cells (referred to also as eutectic colonies) in combination with the eutectic austenite.22,23 The eutectic cells may also be distinguished due to the elemental segregation of alloying elements such as Si.24 The spatial distribution of the spherical graphite is mainly delimited to the domains between the eutectic cells engulfed in the metallic matrix. The primary austenite grains including the primary and eutectic austenite are transformed to ferrite, perlite, or a mixture of them during the solid-state transformation.
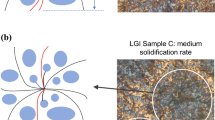
Generalization of the modeling approach suggests that the cast iron sample, Figure 1, comprises eutectic cell (c) and non-cell (nc) regions. The cells contain either flaky or compacted graphite (black-colored), and ferrite phases (αc) surrounding the graphite inside the cells. Furthermore, austenite dendrite arms (dc) and eutectic (pearlite) regions (pc) are found in the cells.
The non-cell region is represented by the pearlitic matrix (pnc), where primary austenite dendrite arms (dnc) and spheroidal graphite nodules (black-colored circles in Figure 1) are located. Ferrite phases (αnc) surround the graphite nodules in the non-cell region.
A typical CGI microstructure can be viewed as a superposition of spherical graphite iron (SGI) and LGI microstructures. Spherical graphite is found in the non-cell region, and the interconnected graphite is in the cell region. In eutectic cells, the heat path along the basal plane of the graphite phases has the lowest thermal resistance. Even though the flaky/compacted graphite can be oriented differently, it is reasonable to assume that in each heat transfer direction, there exist graphite phases with the basal planes oriented along the heat flow direction, delivering the maximum thermal conductivity typical for a pyrolytic crystal structure.10,25,26
Matrix bridges (non-cell region) between the eutectic cell boundaries pose additional resistance to fracture of LGI and CGI as has been shown.27 Since the non-cell region has far lower thermal conductivity than graphite, the non-cell region also exhibits additional resistance to the heat flow. In the present work, parameter s represents a measure of the matrix bridges, Figure 1.
Consider a one-dimensional heat transfer under the assumption of the isotropic distribution of eutectic cells in the bulk cast iron. The heat transfer path comprises one eutectic cell with diameter d and the gap of width s between two neighboring eutectic cells, Figure 1.
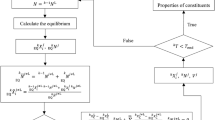
An equivalent thermal resistance network is presented in Figure 2. It corresponds to the above description of the alloy microstructure. The heat flow q = (T1 – T2) / Rtot is conducted across the total thermal resistance, Rtot. Thermal resistance Rtot is a series combination of Rpncs, the thermal resistance of the pearlitic matrix along the path of length s between the interconnected graphite regions inside the eutectic cells, and the block of parallel thermal resistances representing the parallel heat paths of length d within the eutectic cell. It comprises thermal resistances Rpcd (eutectics and primary dendrite arms along the heat transfer path of length d), Rgd, the thermal resistance of the interconnected graphite region along the heat transfer path of length d, and Rαcd, the thermal resistance of the ferrite phase formed around the graphite phases inside the eutectic cells.
Thermal circuit in Figure 2 contains multiple parallel paths Rgd and Rαcd to account for the effective number n(\({D}_{{\text{IR}}}^{{\text{Hyd}}}\)) of the interconnected graphite flakes surrounded by ferrite that can pass in the space between the primary dendrite arms. This number depends on the hydraulic diameter of the interdendritic region \({D}_{{\text{IR}}}^{{\text{Hyd}}}\). The hydraulic diameter of the interdendritic region is a microstructural parameter, characterizing the space between dendrite arms, which can be estimated either from solidification simulation or from measurements taken on a two-dimensional micrograph of the etched cast iron samples.28 It is expressed as Eqn. (1):
where \({A}_{{\text{IR}}}\) is the area of the interdendritic region and \({P}_{\upgamma }\) is the perimeter of the dendrite–eutectic interface. The value of the effective number n(\({D}_{{\text{IR}}}^{{\text{Hyd}}}\)) ranges between 0 and 1; n = 1 gives the maximum thermal conduction of the parallel combination of Rgd and Rαcd. A more detailed explanation of the effective number n(\({D}_{{\text{IR}}}^{{\text{Hyd}}}\)) is provided in.18
The following assumptions and simplifications were adopted in the thermal resistance network, describing the heat transfer in the cast iron. Thermal conductivities of the dendrite arms and pearlite in the cells were assumed to be the same. However, due to different Si segregation in the cells and in the non-cell region, pearlite thermal conductivity values will differ between the cells and the non-cell region. The contribution of the nodular graphite and the surrounding ferrite phases in the non-cell region to the thermal conduction is neglected, due to the discontinuous nature of these inclusions in the investigated samples. The thermal conduction in the matrix is assumed to be dominated by Rpncs.
Considering the thermal resistance network in Figure 2, the total thermal resistance coefficient \({R}_{{\text{tot}}}\) = \(\frac{s+d}{{k}_{{\text{eff}}} }\) can be expressed in terms of the area-independent thermal resistance coefficients (m2 K W−1), as Eqn. (2):
where \({R}_{{\text{pnc}}}^{{\text{s}}}=\frac{s}{{k}_{{\text{pnc}}}}\); \({R}_{{\text{pc}}}^{{\text{d}}}=\frac{d}{{k}_{{\text{pc}}}{f}_{{\text{pc}}}(1-{f}_{{\text{g}}}(1-{f}_{{\text{nod}}}))}\); \({R}_{\alpha\mathrm{ c}}^{{\text{d}}}=\frac{d}{{k}_{\alpha\mathrm{ c}}{f}_{\alpha\mathrm{ c}}(1-{f}_{{\text{g}}}(1-{f}_{{\text{nod}}}))}\); \({R}_{{\text{g}}}^{{\text{d}}}=\frac{d}{{k}_{{\text{g}}}{f}_{{\text{g}}}(1-{f}_{{\text{nod}}})};\) and \(\frac{1}{{R}_{||}}=\frac{1}{{R}_{{\text{pc}}}^{{\text{d}}}}+\frac{n{(D}_{{\text{IR}}}^{{\text{Hyd}}})}{{R}_{{\text{g}}}^{{\text{d}}}}+\frac{n{(D}_{{\text{IR}}}^{{\text{Hyd}}})}{{R}_{\alpha\mathrm{ c}}^{{\text{d}}}}.\)
In the above equations, kpc is the thermal conductivity of the pearlite in the eutectic cells, kpnc is the thermal conductivity of the pearlite in the non-cell region, kαc is the thermal conductivity of the ferrite in the eutectic cells, and kg is the thermal basal plane conductivity of the graphite.
Furthermore, fg is the graphite volume fraction and fnod is the fraction of nodular graphite in the total graphite volume fraction fg, assuming that the graphite nodules are mainly located in the non-cell region (matrix). Notice that fnod is approximately equal to nodularity (%) measured according to ISO 16112: Compacted (vermicular) graphite cast irons—Classification, divided by 100. Therefore, (1 − fnod) is the fraction of flaky/compacted graphite in the total graphite volume fraction fg, assuming that the flaky/compacted graphite is mainly located in the eutectic cells. According to the adopted notation, fg(1− fnod) is the volume fraction of flaky/compacted graphite in the alloy, physically located in the eutectic cells, and (1− fg(1 − fnod)) is the volume fraction of pearlite and ferrite in the cells. Defining fpc and fαc as shares of pearlite and ferrite in the cells, respectively (fpc+ fαc = 1), one can easily derive the thermal resistance coefficients \({R}_{{\text{pc}}}^{{\text{d}}}\) and \({R}_{\alpha\mathrm{ c}}^{{\text{d}}}\) describing the parallel heat transfer paths across pearlite and ferrite phases, respectively, in the eutectic cells.
It is worth noting that ferrite in the cell exists in the form of “envelope” surrounding the flaky/compacted graphite and mimics its structure that protrudes between dendrite arms. That is why the effective number n(\({D}_{{\text{IR}}}^{{\text{Hyd}}}\)) is applied to both heat transfer paths in the cells, i.e., across graphite and across ferrite.
The effective thermal conductivity \({k}_{{\text{eff}}}\) of the heat transfer path, represented by the thermal resistance network in Figure 2, is derived from Eqn. (2) resulting in Eqn. (3):
where \({F}_{{\text{S}}}=\frac{s}{d+s}\), and \(({1-F}_{{\text{S}}})\) is the connectivity factor that characterizes the connectivity between the flaky graphite phases located in the neighboring eutectic cells. It approaches 1 when the eutectic cell diameter is large compared to the distance between the cells.
\({F}_{{\text{C}}}=n\left({D}_{{\text{IR}}}^{{\text{Hyd}}}\right)={F}_{{\text{D}}}\cdot 0.5\cdot {\text{erfc}}(6.0\cdot {F}_{{\text{D}}}-C)\) is termed as the ”hydraulic conductivity” function (C is the fitting parameter). It contains \({F}_{{\text{D}}}=\frac{{D}_{{\text{IR}}}^{{\text{Hyd}}}}{{D}_{{\text{IR}},\mathrm{ max}}^{{\text{Hyd}}}}\), where the maximum hydraulic diameter value \({D}_{{\text{IR}},\mathrm{ max}}^{{\text{Hyd}}}=90\, \upmu \mathrm{m}\) corresponds to the longest practical solidification time for iron castings.29
The sigmoid-shaped curve defined by the complementary error function, erfc(⋅), is designed to dampen the thermal conductivity when the hydraulic diameter increases beyond the limiting value, which is explained in detail in.18
Modeling and Validation Parameters
The methodology to obtain the required model inputs and the validation metric (thermal conductivity of the samples) are described in this section.
Basic Microstructural Parameters
A re-melting experiment was performed for production of cast iron samples with nodularity between 0 and 30%. The casting experiment is described in more detail in.3 Ductile iron base alloy was re-melted and held under isothermal conditions. The holding time, representing the fading time of Mg, was modified to provide the targeted nodularity. The cylindrical iron samples had dimensions of Ø38 × 42 mm, and the experiment was performed under argon atmosphere. Table 1 shows the chemical composition of the base alloy.
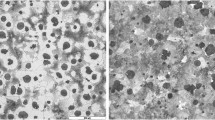
The samples for microstructural analysis were color-etched in a picric acid-based solution to reveal the eutectic grains and the dendrites of primary austenite. The etching reagent contained picric acid, NaOH, KOH, and distilled water at a ratio of 1:1:4:5, respectively. The etching was performed at 110 °C. Figure 3 shows typical microstructures for the studied samples obtained at different holding times. The primary dendrites were colored black in the image manipulation software GIMP,30 and the microstructural parameters AIR and Pγ were estimated from the binary image via the open-source software ImageJ.31
Factor \({F}_{{\text{S}}}\) was calculated from the simple ruler measurements of distances s and d performed on the micrograph. Technically, on the two-dimensional micrograph, one should choose neighboring eutectic cells with the largest size. This would approximate the three-dimensional situation with the actual distance between the eutectic cells. The method is also described in.18
Total area fractions of graphite (fg), pearlite (fp), and ferrite (fα) were measured on as-polished and nital-etched samples, respectively, using the open-source software ImageJ. It was found for all samples that ferrite phase dominated in the eutectic cells, such that the share of the ferrite in the eutectic cells fαc ≈ 0.95.
Measurement results regarding microstructural parameters for the studied alloys are presented in Table 2.
Si Segregation
The average wt% Si in the cells and in the non-cell region, presented in Table 3, was estimated using the Scheil Eqn. 4, which had been shown to apply well for Si in cast irons.32 The input for the calculation was the mass fraction of dendrites \({f}_{d}\) (estimated from the measured area fraction), wt% Si from Table 1, w0, and the partition coefficient for Si, k = 1.12.33 The non-cell area fraction fnc was assumed to equate the nodularity fraction fnod.
where \({f}_{M}={f}_{d}+{f}_{c}(1-{f}_{d})+{f}_{nc}(1-{f}_{d})\) and \({f}_{c}\) is the cell fraction of the matrix.
The average wt% Si was first determined in dendrites, \({w}_{d}\), and the non-graphite (ng) non-dendrite (nd) part of cells, \({w}_{ng,nd,c}\), and the non-graphite non-dendrite part of the non-cell region, \({w}_{ng,nd,nc}\), by averaging over respective region of the curve as illustrated in Figure 4.
Given that cell and non-cell regions contain a mixture of dendrites and eutectic, concentrations in these regions were then calculated by averaging across their dendrite and non-dendrite portions, as described by Eqns. (5) and (6).
Thermal Conductivity
The average thermal conductivities of pearlite and ferrite, Table 4, were calculated considering actual Mn, C contents, and Si segregation in the alloy, Table 3, using the method proposed in.16 Typical measurement uncertainties for wt% Si and wt% Mn in the alloy, 4% and 3%, respectively, were assumed to estimate the uncertainty in the thermal conductivity values for the alloyed ferrite and pearlite, as shown in Table 4. The uncertainties will propagate to the uncertainty of the effective thermal conductivity, keff, via Eqn. (3).
The basal plane thermal conductivity of the pyrolytic graphite, kg = 1950 W m−1 K−1, was adopted from.10,34
The thermal conductivity (km) at room temperature is calculated from Eqn. (7), based on the measured (subscript “m”) data, as follows:
The thermal diffusivity (α) was measured by a Netzsch LFA 427 laser flash apparatus which is based on the principle presented in.35
Specific heat, \({c}_{{\text{p}}}\), and density, \(\rho \), were obtained by using a thermodynamic calculation software, Thermo-Calc (ver. 2023a, TCFE12 database), since the measurement of the specific heat with a differential scanning calorimeter and density measurement by the Archimedes method resulted in a large variation due to the small size of extracted samples, which would have resulted in the large uncertainty in the thermal conductivity values.
In the thermodynamic calculations, the alloy composition and measured graphite, pearlite, and ferrite fractions in the samples were used, and the specific heat and density were obtained by applying the rule of mixtures. It allowed us to come up with reasonable values of the specific heat and density for the studied samples.
The measured thermal diffusivity, the calculated specific heat and density, and the derived thermal conductivity are summarized in Table 5.
The uncertainty of the thermal conductivity was obtained by estimating the typical uncertainties in the thermal diffusivity, density, and specific heat as 3%, 0.1%, and 2.5%, respectively.
Modeling Results and Discussion
The calculated thermal conductivity values were compared with the thermal conductivity of the samples derived from the measurements, followed by the discussion of the model.
Model Calibration Principles
It is worth noting that the value of the fitting parameter C = 3.2 determined earlier in18 for several pearlitic LGI castings was found valid for the CGI and ferritic LGI castings studied in the present work. In this sense, the model given by Eqn. (3) is the natural extension of the thermal conductivity model to a more complex material, based on microstructural parameters, using the same general approach. The shape of the curve given by the upper Eqn. (8) reflects the saturation trend for the thermal conductivity at a limiting value of the hydraulic diameter, which was studied in18 for relatively small \({D}_{{\text{IR}}}^{{\text{Hyd}}}\) values. However, the behavior of this function for the larger \({D}_{{\text{IR}}}^{{\text{Hyd}}}\) values valid for the samples studied in the present work should be adjusted by introducing the constant level corresponding to the maximum value, 0.35, of the hydraulic conductivity function, FC, that is reached at \({D}_{{\text{IR}}}^{{\text{Hyd}}}=\) 38 \(\upmu \mathrm{m}\). Without doing such an adjustment, the FC and, thus, thermal conductivity saturation trend would, for the relatively large \({D}_{{\text{IR}}}^{{\text{Hyd}}}\) values, change to the decay of the FC function, Figure 5, which would lead to the unreasonably low thermal conductivity values given by Eqn. (3). Therefore, the final expression for the hydraulic conductivity function, valid both for the alloys studied in18 and in the present work, is given by Eqn. (8).
Furthermore, the model calibration process led to the need of tuning the experimentally derived thermal conductivity values, described in the previous section, which resulted in a relatively small uncertainty propagated to the final thermal conductivity values, as reported in the last column of Table 5.
Comparison with Measurements
The modeling results for keff with fitting parameter C = 3.2 were compared with the measured thermal conductivity, km, values for the six investigated cases. The comparison plots are provided in Figure 6.
The error bars for the calculated thermal conductivity values were estimated by considering the propagation error of the factors in Eqn. (3). In Eqn. (3), propagation errors were calculated for the thermal conductivity of the ferrite and pearlite, for which uncertainty estimation was possible. For the other factors, it was assumed that there was no uncertainty since it was hard to estimate it, and thus, note that the uncertainty in the effective thermal conductivity is underestimated.
As can be seen in Figure 6, the error bars for the corresponding predicted and experimental thermal conductivity values overlap for all the investigated samples, such that all the calculated values are in reasonable agreement with the experimental values.
The samples with higher connectivity factor, \(({1-F}_{{\text{S}}})\), in general exhibit a higher thermal conductivity, due to the relatively large size of the cell region compared to the size of the non-cell region. Since the high-conductivity interconnected graphite phases are in the cell region, it leads to the overall higher thermal conductivity of the cast iron.
The importance to consider the Si segregation in the model is evaluated in Figure 7, where the wt% Si in the eutectic cells and in the non-cell region was assumed to be 2.64% (square markers), i.e., corresponding to the alloy chemical composition. The comparison expectedly revealed that considering Si segregation is especially important when the actual Si content in the non-cell region (provided along the horizontal axis) is much smaller than the average Si content in the alloy, as is the case for samples 5 and 6. When Si content is low, the resulting thermal conductivity of pearlite in the matrix becomes high. Notice that the graph is not applicable for comparison of thermal conductivity values between the studied samples, since the other factors have much stronger effect on the cast iron thermal conductivity.
In the investigated samples, the ferrite phase was found mainly in the eutectic cells, which makes the term \({k}_{{\text{pc}}}{f}_{{\text{pc}}}\) in Eqn. (3) negligible. The term was still included in the model, since the ferrite phases in the industrial cast iron alloys are found mainly in the non-cell regions, and thus, pearlite contribution to the thermal conduction in the eutectic cells would be more significant.
The investigated samples have moderate nodularity, and the graphite nodules located in the non-cell region are sparse and disconnected. This fact allowed us to neglect the role the graphite nodules and surrounding ferrite phase play in the heat transfer across the matrix. At a high nodularity, the nodules would be located closer to each other, such that ferrite regions surrounding the nodules may overlap. The overall combination of the ferrite and graphite in the non-cell region has a higher thermal conductivity than the pearlitic matrix and primary austenite dendrite arms. Therefore, continuous heat transfer paths would be formed in parallel to the pearlitic matrix. This case is addressed in Eqn. (2). The term \({R}_{{\text{pnc}}}^{{\text{s}}}\) must be replaced by the parallel combination of the \({R}_{{\text{pnc}}}^{{\text{s}}}\) and a series combination of \({R}_{{\text{gnc}}}^{{\text{s}}}\) and \({R}_{\alpha\mathrm{ nc}}^{{\text{s}}}\), the thermal resistance coefficients for non-cell nodular graphite and the non-cell ferrite, respectively. Such a modification, however, requires additional research efforts, regarding the thermal conductivity of the nodular graphite and the degree of continuity of the thermal path formed by graphite nodules enveloped in ferrite phases within the distance s between the eutectic cells.
The presented thermal conductivity model is expected to be applicable at elevated temperatures, which, however, requires additional investigation, via substituting the input thermal conductivities at the specific elevated temperature. The dependence of the microstructural parameters s and d on the temperature is expected to be negligible, due to the negligible variation (within several percent units) in thermal expansion of cast irons at elevated temperatures.
Conclusions
The microstructure-based approach to modeling of thermal conductivity of pearlitic LGI was extended to the CGI and ferritic–pearlitic LGI.18 The model was built as the thermal resistance network describing the heat transfer through a eutectic cell and the region between the cells, followed by derivation of the equation for the effective thermal conductivity of the bulk material. The microstructural parameters employed in the thermal conductivity model were measured.
The key assumption in the model is that the dominant heat paths are formed by the interconnected graphite phases in the eutectic cells, enclosed by ferrite phase, and being determined by the hydraulic diameter of interdendritic region. The assumption was confirmed by comparison between the predicted and experimentally derived values of thermal conductivity for six iron cast samples. The studied samples had the same chemical composition. The different nodularity levels were achieved at different holding times. The agreement on the average within 4% was achieved between predicted and experimentally derived thermal conductivities. The influence of Si segregation on the thermal conductivity of the pearlite and ferrite phases was considered in the model and judged as important to consider at low Si contents in the matrix, for the studied samples.
The value of the single fitting parameter included in the hydraulic conductivity function determined earlier for the pearlitic LGI was found valid for the CGI with comparable hydraulic diameter of interdendritic region. The adjustment was made for the alloy microstructures characterized by a relatively large values of the hydraulic diameter. Applicability of the model to predict thermal conductivity of both LGI and CGI alloys contributes to the physical assumptions and modeling principles being general.
The results are believed to improve understanding of how as-cast alloy microstructure affects the thermal conductivity of the bulk material of CGI and LGI. Furthermore, the presented model allows the material and process engineers to strive for a material microstructure that enables the required thermal conductivity of iron cast components.
References
T. Matsushita, A.G. Saro, L. Elmquist, A.E.W. Jarfors, On the thermal conductivity of CGI and SGI cast irons. Int. J. Cast Met. Res. 31, 135–143 (2018). https://doi.org/10.1080/13640461.2017.1379263
D. Holmgren, A. Diószegi, I.L. Svensson, Effects of transition from lamellar to compacted graphite on thermal conductivity of cast iron. Inter Metalcast 19(6), 303–313 (2006). https://doi.org/10.1179/136404607X176203
V. Fourlakidis, J.C. Hernando, D. Holmgren, A. Diószegi, Relationship between thermal conductivity and tensile strength in cast irons. Inter Metalcast 17(4), 2862–2867 (2023). https://doi.org/10.1007/s40962-023-00970-6
D. Holmgren, I.L. Svensson, Thermal conductivity–structure relationships in grey cast iron. Inter Metalcast 18(6), 321–330 (2005). https://doi.org/10.1179/136404605225023162
G.-H. Wang, Y.-X. Li, Thermal conductivity of cast iron-A review. China Foundry 17, 85–95 (2020)
R.L. Hecht, R.B. Dinwiddie, H. Wang, The effect of graphite flake morphology on the thermal diffusivity of gray cast irons used for automotive brake discs. J. Mater. Sci. 34, 4775–4781 (1999). https://doi.org/10.4271/96212
D. Holmgren, I.L. Svensson, Thermal conductivity—structure relationships in grey cast iron. Int J Cast Metal Res. 18, 321–330 (2005)
D. Holmgren, I.L. Svensson, Review of the thermal conductivity of cast iron. Int. J. Cast. Metal Res. 18, 331–345 (2005)
H. Kempers, Steelplant ingot molds of nodular iron. Giesserei 53(1), 15–18 (1966)
G. Wang, Y. Li, Thermal conductivity of cast iron—a review. China Foundry 17, 85–95 (2020). https://doi.org/10.1007/s41230-020-9112-8
D. Holmgren, A. Diószegi, I.L. Svensson, Effects of transition from lamellar to compacted graphite on thermal conductivity of cast iron. Inter Metalcast 19(6), 303–313 (2006)
K. Jalava, K. Soivio, J. Laine, J. Orkas, Effect of silicon and microstructure on spheroidal graphite cast iron thermal conductivity at elevated temperatures. Int Metalcast 12, 480–486 (2018)
J.C. Maxwell, A Treatise on Electricity and Magnetism (Clarendon Press, Oxford, 1873)
D.A.G. Bruggeman, Dielectric constant and conductivity of mixtures of isotropic materials. Ann. Phys. (Leipzig) 24, 636–679 (1935)
N. Mehra, L. Mu, T. Ji, X. Yang, J. Kong, J. Gu, J. Zhu, Thermal transport in polymeric materials and across composite interfaces. Appl. Mater. Today 12, 92–130 (2018). https://doi.org/10.1016/j.apmt.2018.04.004
J. Helsing, G. Grimvall, Thermal conductivity of cast iron: Models and analysis of experiments. Appl. Phys. 70, 1198–1206 (1991). https://doi.org/10.1063/1.349573
G. Wang, Y. Li, Calculating the effective thermal conductivity of gray cast iron by using an interconnected graphite model. China Foundry 17, 183–189 (2020). https://doi.org/10.1007/s41230-020-0029-z
I. Belov, V. Fourlakidis, B. Domeij et al., A thermal conductivity model for grey iron. Int Metalcast (2023). https://doi.org/10.1007/s40962-023-01157-9
G.L. Rivera, R.E. Boeri, J.A. Sikora, Solidification of gray cast iron. Scr. Mater. 50(3), 331–335 (2004). https://doi.org/10.1016/j.scriptamat.2003.10.019
S. Vazehrad, J. Elfsberg, A. Diószegi, Study of microstructure and silicon segregation in cast iron using color etching and electron microprobe analysis. Mater Charact 104, 132–138 (2015). https://doi.org/10.1016/j.matchar.2014.09.008
J.M. Motz, Microsegregations—an easily unnoticed influencing variable in the structural description of cast materials. Pract. Metallogr. 25(6), 285–293 (1988)
A. Hultgren, Y. Lindblom, E. Rudberg, Eutectic solidification in grey, white, and mottled hypo-eutectic cast irons. J. Iron Steel Inst. 176, 365–374 (1954)
B. Domeij, J. Elfsberg, A. Diószegi, Evolution of dendritic austenite in parallel with eutectic in compacted graphite iron under three cooling conditions. Metall. Mater. Trans. B 54, 2395–2410 (2023). https://doi.org/10.1007/s11663-023-02842-0
B. Domeij, A. Diószegi, Depletion of Si in the early solidification structure of Gray Cast Iron. in Proceedings of the12th International Symposium on the Science and Processing of Cast Iron (SPCI-XII), 9-12 Nov. 2021, Muroran, Japan.
G. Paul, The role of interfacial energies in the crystallisation of graphite in hard iron. J. S. Afr. Inst. Min. Metall. 72(6), 165–170 (1972)
J.E. Gruzleski, On the growth of spherulitic graphite in nodular cast iron. Carbon 13(3), 167–173 (1975)
R.C. Voigt, S.D. Holmgren, Crack initiation and propagation in gray and compacted graphite iron (CG) cast irons. Trans. Am. Foundary’s Soc. 91, 213–225 (1990)
V. Fourlakidis, A. Diószegi, A generic model to predict the ultimate tensile strength in pearlitic lamellar graphite iron. Mater. Sci. Eng. A 618, 161–167 (2014)
J.C. Hernando, J. Elfsberg, A.K. Dahle, A. Diószegi, Evolution of primary austenite during coarsening and impact on eutectic microstructure in Fe–C–Si alloys. Materialia 7, 100391 (2019). https://doi.org/10.1016/j.mtla.2019.100391
The GIMP Development Team. GIMP (2019). Retrieved from https://www.gimp.org.
C.T. Rueden, J. Schindelin, M.C. Hiner, B.E. DeZonia, A.E. Walter, E.T. Arena, K.W. Eliceiri, Image J2: imageJ for the next generation of scientific image data. BMC Bioinform. 18, 1–26 (2017)
C. Selig, A. Lacaze, Study of microsegregation buildup during solidification of spheroidal graphite cast iron. Metall. Mater. Trans. B 31, 827–836 (2000). https://doi.org/10.1007/s11663-000-0119-7
B. Domeij, A. Diószegi. Inferring the development of microsegregation and microstructure in Spheroidal and Compacted Graphite Iron using EPMA-WDS. In Proceedings of the 6th Decennial Conf. on Solidification Processing (SP17), 25-28 Jul. 2017, Old Windsor, London, UK. BCAST, Brunel University London 2017, pp. 455-458
C.Y. Ho, R.W. Powell, P.E. Liley, Thermal conductivity of the elements. J. Phys. Chem. Ref. Data 1, 279–421 (1972). https://doi.org/10.1063/1.3253100
W.J. Parker et al., Flash method of determining thermal diffusivity, heat capacity, and thermal conductivity. Appl. Phys. 32, 1679–1684 (1961). https://doi.org/10.1063/1.1728417
Acknowledgements
The work was performed within projects LeanCast (Grant number 20180033, Swedish Knowledge Foundation) and IFT: Jönköping (Grant number 20210082, Swedish Knowledge Foundation)
Funding
Open access funding provided by Jönköping University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Belov, I., Fourlakidis, V., Domeij, B. et al. A Thermal Conductivity Model for Lamellar and Compacted Graphite Irons. Inter Metalcast (2024). https://doi.org/10.1007/s40962-024-01373-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40962-024-01373-x