Abstract
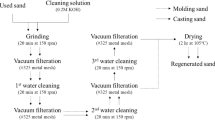
Due to its thermodynamic stability, silica sand is traditionally used during the metal casting process in foundries. The sand-casting process involves a wide range of organic and inorganic binders. Due to ease in mold making process and less adverse environmental impact, sodium silicate has emerged as the most popular binder. Sand castings exposed to elevated temperatures lose the binding property and can no longer be used in the foundries. Present work reports the formation of cristobalite, a polymorph of quartz in sodium silicate bonded sand exposed to temperature of 1000 °C. It was believed that sodium silicate is responsible for the conversion of silica sand into cristobalite, leading to the loss of binding property. To avoid cristobalite formation, further, the binder was replaced with sodium aluminate. The mineralogical characterization of such sand also showed the presence of cristobalite under the same experimental conditions. It was suspected that apart from sodium, other cations could also be responsible for cristobalite formation. To support this hypothesis, the sand was mixed with a solution of different cations, namely NaCl, KCl, KOH, CaCl2, Al2(SO4)3 and FeCl3 were calcined at two different temperatures. It was found that the conversion of silica sand to cristobalite takes place at a temperature of 1000 °C in the presence of monovalent cation sodium. In the presence of all other cations, the transformations occur at a temperature of 1250 °C. These conversions were supported using XRD, SEM-EDS and FTIR analyses. Reclamation of such sand is not possible due to its inertness.
Graphical Abstract







Similar content being viewed by others
References
K.J. Hodder, R.J. Chalaturnyk, Addit. Manuf. (2019). https://doi.org/10.1016/j.addma.2019.06.008
N. Kivi, A. Moore, K. Dyar, S. Haaf, Devitrification rates of fused silica in the presence of trace impurities, chancellor’s honors program projects. University of Tennessee, Knoxville (2016). https://trace.tennessee.edu/utk_chanhonoproj/1972
A. Arulrajah, E. Yaghoubi, I. Monzur, S. Horpibulsuk, Sustain. Cities Soc. (2017). https://doi.org/10.1016/j.scs.2016.10.009
“Foundry informatics centre, profile of indian foundry industry” 2022, http://foundryinfo-india.org/profile_of_indian.aspx. Accessed 02nd August 2022
V. Merta, J. Beňo, T. Obzina, F. Radkovský, I. Kroupová, P. Lichý, M. Folta, K. Janovská, I. Nguyenová, M. Dostál, Metals. (2021). https://doi.org/10.3390/met11050733
N. Ünlü, A. Odabaş, Inter. Metalcast. (2018). https://doi.org/10.1007/s40962-018-0210-y
Y.A. Owusu, Adv. Colloid. Interf. Sci. (1982). https://doi.org/10.1016/0001-8686(82)85031-8
M. Holtzer, A. Kmita, Mold and core sands in metalcasting: chemistry and ecology. Sustain. Dev. (2020). https://doi.org/10.1007/978-3-030-53210-9_9
J.S. Kowalski, Arch. Foundry Eng. 10–3, 111–114 (2010)
I. Vasková, L. Varga, I. Prass, V. Dargai, M. Conev, M. Hrubovčáková, M. Bartošová, B. Buľko, P. Demeter, Metals. (2020). https://doi.org/10.3390/met10020235
M. Dapiaggi, L. Pagliari, A. Pavese, L. Sciascia, M. Merli, F. Francescon, J. Eur. Ceram. Soc. (2015). https://doi.org/10.1016/j.jeurceramsoc.2015.08.015
A.M. Venezia, V. La Parola, A. Longo, A. Martorana, J. Solid State Chem. (2001). https://doi.org/10.1006/jssc.2001.9345
N.P. Hoolikantimath, K.G. Guptha, R.K. Rao, P.A. Ghorpade, JOM. (2022). https://doi.org/10.1007/s11837-021-05056-4
Z.T. Fan, N.Y. Huang, X.P. Dong, Int. J. Cast. Metal. Res. (2004). https://doi.org/10.1179/136404604225020551
Q.Z. Sun, H. Du, P.Q. Zhang, Z.K. Zhao, J.G. Yan, Adv. Mat. Res. (2014). https://doi.org/10.4028/www.scientific.net/AMR.1004-1005.1008
S. Balbay, China Foundry. (2019). https://doi.org/10.1007/s41230-019-8144-4
M. Fertani-Gmati, K. Brahim, I. Khattech, M. Jemal, Thermochim. Acta. (2014). https://doi.org/10.1016/j.tca.2014.09.003
J. Theil, American foundry society proceedings, Schaumburg, IL USA. 11 (2011)
A. Kazemia, M.A. Faghihi-Sani, H.R. Alizadeh, J. Eur. Ceram. Soc. (2013). https://doi.org/10.1016/j.jeurceramsoc.2013.06.025
L. Pagliari, M. Dapiaggi, A. Pavese, F. Francescon, J. Eur. Ceram. Soc. (2013). https://doi.org/10.1016/j.jeurceramsoc.2013.06.014
C.J. Horwell, B.J. Williamson, K. Donaldson, J.S. Le Blond, D.E. Damby, L. Bowen, Part. Fibre. Toxicol. (2012). https://doi.org/10.1186/1743-8977-9-44
S. Hillier, D. Lumsdon, Clay Miner. (2008). https://doi.org/10.1180/claymin.2008.043.3.11
G. Anbalagan, A.R. Prabakaran, S. Gunasekaran, J. Appl. Spectrosc. (2010). https://doi.org/10.1007/s10812-010-9297-5
R. Zheng, Z. Ren, H. Gao, A. Zhang, Z. Bian, J. Alloys Compd. (2018). https://doi.org/10.1016/j.jallcom.2018.05.010
Z. Qiao, Q. Liu, S. Zhang, Y. Wu, Solid State Sci. (2019). https://doi.org/10.1016/j.solidstatesciences.2019.105948
E.R. Lippincott, A.V. Valkenburg, C.E. Weir, E.N. Bunting, J. Res. Natl. Inst. Stan. (1958). https://doi.org/10.6028/JRES.061.009
B.K. Awuah, E.V. Kiti, I. Nkrumah, R.E. Ikyreve, I. Radecka, C. Williams, Desalin. Water. Treat. (2016). https://doi.org/10.1080/19443994.2015.1128361
A.S. Khan, H. Khalid, Z. Sarfraz, M. Khan, J. Iqbal, N. Muhammad, M.A. Fareed, I.U. Rehman, Appl. Spectrosc. Rev. (2017). https://doi.org/10.1080/05704928.2016.1244069
M.Y.A. Mollah, S. Promreuk, R. Schennach, D.L. Cocke, R. Güler, Fuel (1999). https://doi.org/10.1016/S0016-2361(99)00057-5
U. Nurbaiti, S. Pratapa, J. Phys. Conf. Ser. (2018). https://doi.org/10.1088/1742-6596/983/1/012014
Acknowledgment
The authors like to acknowledge Aqua Alloys Pvt. Ltd. Chandgad, Kolhapur, India, for providing Foundry facilities to carry out the research. The authors would also like to acknowledge USIC and SAIF from Karnatak University Dharwad, Karnataka, India, for providing support with instrumentation. The authors sincerely thank the department of chemistry/CMS, KLE Technological University, for extending the FTIR facility, which is purchased from Grant No.: GRD-540, Vision Group on Science and Technology, Karnataka, India.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Conceptualization, formal analysis, investigation and writing original draft was carried out by Nayana P. Hoolikantimath; Formal analysis and investigation was performed by Sanjeevkumar Dodamani; Methodology, supervision, project administration, and visualization was done by Dr. K. G. Guptha; Conceptualization, validation, supervision, resources, project administration was supported by Dr. Raghuraj K. Rao; Conceptualization, methodology, validation, writing original draft, visualization, supervision and project administration was carried out by Dr. Praveen A. Ghorpade.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoolikantimath, N.P., Dodamani, S., Guptha, K.G. et al. Influence of Metal Casting Temperature and Cations on Phase Transformation of Silica Sand to Cristobalite. Inter Metalcast 17, 2038–2049 (2023). https://doi.org/10.1007/s40962-022-00921-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-022-00921-7