Abstract
Purpose
To identify prognostic factors and to achieve the internal validation of a predictive model for overall survival in patients with cervical cancer at any stage.
Methods
A prospective cohort study was conducted between January 2010 and January 2019 on 229 women with cervical cancer. We performed a survival analysis using the Kaplan–Meier method and log-rank test, and, finally, developed a Cox model.
Results
Overall survival was 41 (IQR = 57.5) months (R = 1–264), with a cancer-specific mortality rate of 26.2%. We found significant differences in the median overall survival between the early and the locally advanced stages (43 versus 11 months, P = 0.001) and between the early and the advanced stages (40 versus 11 months, P = 0.003). There were no significant differences in the 5-year overall survival between the monotherapy based on types B and C2 radical hysterectomy (P = 0.1) and between the radical and the extrafascial hysterectomy (P = 0.2). Regarding the surgical approach for type B radical and total extrafascial hysterectomy, we could not study differences between laparotomic and laparoscopic routes due to a lack of enough amount of power. We developed a model with a Harrell´s concordance index of 0.87. The predictors of the model were primary surgery, maximum tumor diameter, and type of therapeutic response.
Conclusion
The cancer-specific mortality rate was 26.2%. We developed a model that, once statistical power was increased and externally validated, might provide useful prognostic information for both patients and oncologists at first consultation.



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to the fact that their containing information could compromise the privacy of research participants.
Code Availability
Not applicable.
Abbreviations
- ACC:
-
Advanced cervical cancer
- BMI:
-
Body mass index
- CC:
-
Cervical cancer
- CI:
-
Confidence interval
- C-index:
-
Harrell´s concordance index
- C-section:
-
Cesarean section
- DF:
-
Degrees of freedom
- DSI:
-
Deep stromal invasion
- ECC:
-
Early cervical cancer
- ECOG:
-
Eastern Cooperative Oncology Group
- EE:
-
Error estimate
- Exp(βn):
-
Relative risk
- FIGO:
-
International Federation of Gynecology and Obstetrics
- GR:
-
Grade of recommendation
- HPV:
-
Human papillomavirus
- HR:
-
High-risk
- IQR:
-
Interquartile range
- LACC:
-
Laparoscopic approach to cervical cancer
- LCC:
-
Locally advanced cervical cancer
- LE:
-
Level of evidence
- LHR:
-
Likelihood ratio
- LVI:
-
Lymphovascular invasion
- Me :
-
Median
- MLN:
-
Metastatic lymph node
- MPALN:
-
Metastatic para-aortic lymph node
- MPLN:
-
Metastatic pelvic lymph node
- MTD:
-
Maximum tumor diameter
- N:
-
Sample size
- NA:
-
Not available
- NCI:
-
National Cancer Institute
- OS:
-
Overall survival
- PAL:
-
Para-aortic lymphadenectomy
- PI:
-
Parametrial invasion
- PL:
-
Pelvic lymphadenectomy
- PS:
-
Performance status
- R:
-
Range
- RFS:
-
Recurrence-free survival
- RH:
-
Radical hysterectomy
- SD:
-
Standard deviation
- SEER:
-
Surveillance, Epidemiology, and End Results
- SIGN:
-
Scottish Intercollegiate Guidelines Network
- SLNB:
-
Sentinel lymph node biopsy
-
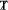 :
: -
Arithmetic mean
- TEH:
-
Total extrafascial hysterectomy
- VIF:
-
Variance inflation factor
- Z-value:
-
Test de Wald
- βn :
-
Predictor regression coefficient
References
Instituto de Salud Carlos III [Internet]. Madrid: ISCIII; c2019. Ariadna; [cited on October 28th, 2022]. Available in: http://www.ariadna.cne.isciii.es
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
Wang W, Liu X, Meng Q, Zhang F, Hu K. Nomograms predicting survival and patterns of failure in patients with cervical cancer treated with concurrent chemoradiotherapy: a special focus on lymph nodes metastases. PLoS One. 2019;14(4):e0214498. DOI: https://doi.org/10.1371/journal.pone.0214498
Yan DD, Tang Q, Chen JH, Tu YQ, Lv XJ. Prognostic value of the 2018 FIGO staging system for cervical cancer patients with surgical risk factors. Cancer Manag Res. 2019;11:5473–80. https://doi.org/10.2147/CMAR.S203059.
Kilic C, Cakir C, Yuksel D, et al. Which factors predict parametrial involvement in early stage cervical cancer? A Turkish multicenter study. Eur J Obstet Gynecol Reprod Biol. 2019;243:63–6. https://doi.org/10.1016/j.ejogrb.2019.10.033.
Hsu HC, Tai YJ, Chen YL, Chiang YC, Chen CA, Cheng WF. Factors predicting parametrial invasion in patients with early-stage cervical carcinomas. PLoS One. 2018;13(10):e0204950. https://doi.org/10.1371/journal.pone.0204950
Matsuo K, Shimada M, Nakamura K, et al. Predictors for pathological parametrial invasion in clinical stage IIB cervical cancer. Eur J Surg Oncol. 2019;45(8):1417–24. https://doi.org/10.1016/j.ejso.2019.02.019.
Queiroz ACM, Fabri V, Mantoan H, et al. Risk factors for pelvic and distant recurrence in locally advanced cervical cancer. J Obstet Gynecol Reprod Biol. 2019;235:6–12. https://doi.org/10.1016/j.ejogrb.2019.01.028.
Hellebrekers BW, Zwinderman AH, Kenter GG, et al. Surgically treated early cervical cancer: prognostic factors and the significance of depth of tumor invasion. Int J Gynecol Cancer. 1999;9(3):212–9. https://doi.org/10.1046/j.1525-1438.1999.99023.x.
Sun H, Cao D, Shen K, et al. Piver type II vs. type III hysterectomy in the treatment of early-stage cervical cancer: midterm follow-up results of a randomized controlled trial. Front Oncol. 2018;8:568–79. https://doi.org/10.3389/fonc.2018.00568
Wang YZ, Deng L, Xu HC, Zhang Y, Liang ZQ. Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer. 2015;15:928. https://doi.org/10.1186/s12885-015-1818-4.
Cao T, Feng Y, Huang Q, Wan T, Liu J. Prognostic and safety roles in laparoscopic versus abdominal radical hysterectomy in cervical cancer: a meta-analysis. J Laparoendosc Adv Surg Tech A. 2015;25(12):990–8. https://doi.org/10.1089/lap.2015.0390.
Laterza RM, Uccella S, Casarin J, et al. Recurrence of early stage cervical cancer after laparoscopic versus open radical surgery. Int J Gynecol Cancer. 2016;3:547–52. https://doi.org/10.1097/IGC.0000000000000627.
Mendivil AA, Rettenmaier MA, Abaid LN, et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: a five year experience. Surg Oncol. 2016;25(1):66–71. https://doi.org/10.1016/j.suronc.2015.09.004.
Ramírez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379(20):1895–904. https://doi.org/10.1056/NEJMoa1806395.
Melamed A, Rauh-Hain JA, Ramírez PT. Minimally invasive radical hysterectomy for cervical cancer: when adoption of a novel treatment precedes prospective, randomized evidence. J Clin Oncol. 2019;37(33):3069–74. https://doi.org/10.1200/JCO.19.01164.
Kim JH, Kim K, Park SJ, et al. Comparative effectiveness of abdominal versus laparoscopic radical hysterectomy for cervical cancer in the postdissemination era. Cancer Res Treat. 2019;51(2):788–96. https://doi.org/10.4143/crt.2018.120.
Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. 2019;221(6):619.e1-619.e24. https://doi.org/10.1016/j.ajog.2019.07.009.
Uppal S, Spencer R. Modify or abandon: minimally invasive radical hysterectomy for early-stage cervical cancer. Int J Gynecol Cancer. 2019;29(5):843–4. https://doi.org/10.1136/ijgc-2019-000574.
Uppal S, Gehrig PA, Peng K, et al. Recurrence rates in patients with cervical cancer treated with abdominal versus minimally invasive radical hysterectomy: a multi-institutional retrospective review study. J Clin Oncol. 2020;38(10):1030–40. https://doi.org/10.1200/JCO.19.03012.
Sánchez-Periut E, Muro-Toledo G, Losada-Guerra J, Reyes-Almeida L. La nefrostomía percutánea en el carcinoma cérvico-uterino avanzado con uropatía obstructiva. [Percutaneous nephrostomy in advanced cervical cancer with obstructive uropathy] (in Spanish). Revista Mexicana de Urología 2016;76(4):207–12. https://doi.org/10.1016/j.uromx.2016.04.002
Nogueira Diasn Genta ML, Sadalla JC, De Carvalho JPM, et al. Multiple HPV genotype infection impact on invasive cervical cancer presentation and survival. PLoS One. 2017 Aug;12(8):e0182854. https://doi.org/10.1371/journal.pone.0182854
Jeon W, Koh HK, Kim HJ, Wu HG, Kim JH, Chung HH. Salvage radiotherapy for lymph node recurrence after radical surgery in cervical cancer. J Gynecol Oncol. 2012;23(3):168–74. https://doi.org/10.3802/jgo.2012.23.3.168.
Uno T, Kanazawa A, Nemoto MW, et al. Radiation therapy for extrapelvic lymph node recurrence after curative treatment for cervical cancer. Anticancer Res. 2019;39(2):891–6. https://doi.org/10.21873/anticanres.13190.
Ho KC, Wang CC, Qiu JT, et al. Identification of prognostic factors in patients with cervical cancer and supraclavicular lymph node recurrence. Gynecol Oncol. 2011;123(2):253–6. https://doi.org/10.1016/j.ygyno.2011.07.020.
Polterauer S, Grimm C, Hofstetter G, et al. Nomogram prediction for overall survival of patients diagnosed with cervical cancer. Br J Cancer. 2012;107(6):918–24. https://doi.org/10.1038/bjc.2012.340.
R: a language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2017 [cited on 11th Feb 2022]. Available in: http://www.R-project.org
Dienstmann R, da Silva PC, Pereira MT, Small IA, Ferreira CG. Palliative percutaneous nephrostomy in recurrent cervical cancer: a retrospective analysis of 50 consecutive cases. J Pain Symptom Manage. 2008;36(2):185–90. https://doi.org/10.1016/j.jpainsymman.2007.09.010.
Kim JY, Nam BH, Lee JA. Is human papillomavirus genotype an influencing factor on radiotherapy outcome? Ambiguity caused by an association of HPV 18 genotype and adenocarcinoma histology. J Gynecol Oncol. 2011;22(1):32–8. https://doi.org/10.3802/jgo.2011.22.1.32.
Onuki M, Matsumoto K, Tenjimbayashi Y, et al. Human papillomavirus genotype and prognosis of cervical cancer: Favorable survival of patients with HPV16-positive tumors. Papillomavirus Res. 2018;6:41–5. https://doi.org/10.1016/j.pvr.2018.10.005.
Lai CH, Chang CJ, Huang HJ, et al. Role of human papillomavirus genotype in prognosis of early-stage cervical cancer undergoing primary surgery. J Clin Oncol. 2007;25(24):3628–34. https://doi.org/10.1200/JCO.2007.11.2995.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors met the authorship criteria. JCG: Investigation, Conceptualization, Methodology, Writing-Original draft preparation, Visualization, Writing-Reviewing and Editing. LRP: Methodology, Software, Data Curation, Formal analysis, Validation, Writing-Reviewing and Editing, and Supervision. MCRR: Supervision. FMM: Resources and Supervision. IRJ: Resources and Supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest or financial support.
Informed Consent
The research assistant obtained a written informed consent from each subject before each interview to participate and publish her data; participants were assured that they could stop the study process at any time and were assured that nonparticipation would have no consequences for their follow-up care or therapy. There was no refund for the participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix I
Statistical analysis of the confounding factors of the association between the overall survival and the type of surgery/surgical approach. P-values are presented in the Table.
BRH vs. C2 RH | Laparoscopy | Laparotomy | 2/TEH 19 | Laparoscopy | Laparotomy | Surgical approach |
|---|---|---|---|---|---|---|
34 | 13 | 34 | 13 | N | ||
BRH 4+5 | BRH + 2CRH 15 | BRH + C2RH 8/TEH 5 | Type of surgery | |||
1 | 0.358 | 0.184 | Age | |||
1 | 0.676 | 0.187 | Comorbidities | |||
1 | 0.473 | – | Baseline PS | |||
1 | 0.357 | 0.699 | FIGO stage | |||
0.22 | 0.199 | 0.596 | Histological type | |||
0.877 | 0.421 | 0.285 | Histological grade | |||
1 | 0.42 | 0.418 | LVI | |||
0.928 | 0.942 | 0.1 | DSI | |||
0.207 | 0.326 | 0.156 | MTD | |||
– | 0.695 | 0.695 | MTD ≥ 20 mm | |||
– | – | 0.521 | Surgical technique | |||
1 | 0.446 | 0.027 | PL | |||
0.17 | 0.451 | 0.381 | PLNs removed | |||
– | – | 0.119 | PALNs removed | |||
1 | 1 | 1 | MLNs | |||
1 | 0.25 | 1 | Recurrence | |||
- | 0.156 | 0.002 | Follow-up duration | |||
DSI deep stromal invasion, TEH total extrafascial hysterectomy, FIGO International Federation of Gynecology and Obstetrics, LVI lymphovascular invasion, MLN metastatic lymph node, MTD maximum tumor diameter, N sample size, PL pelvic lymphadenectomy, PLN pelvic lymph node, PALN para-aortic lymph node, PS performance status, RH radical hysterectomy.
Appendix II
Statistical analysis of the background variables and confounding factors of the association between the overall survival and the surgical approach in patients submitted to type B radical hysterectomy and in patients who underwent radical surgery alone vs. total extrafascial hysterectomy
Variables | Type B RH laparotomy (N = 4) vs. laparoscopy (N = 5) (p) | Radical surgery alone (N = 16) vs. TEH (N = 30) (p) |
|---|---|---|
Age at diagnosis of CC | 0.358 | 0.753 |
Menopausal state at diagnosis | 0.919 | 0.794 |
Smoking prior to CC | 0.072 | 0.794 |
BMI | 0.07 | 0.228 |
Origin | 0.268 | 0.289 |
Nationality | 0.089 | 0.29 |
Marital status at survey | 0.789 | 0.48 |
Religion | 0.333 | 0.697 |
Social stratum | 0.829 | 0.539 |
Educational level | 0.176 | 0.67 |
Occupation | 0.402 | 0.671 |
Comorbidities | 0.676 | 0.877 |
Depression prior to CC | 0.61 | 0.457 |
Prior C-section | 0.409 | 0.39 |
Histological type | 0.199 | 0.342 |
Histological grade | 0.421 | 0.005 Low 43.75% < 76.67% High 43.75% > 6.67% |
LVI | 0.42 | 0.457 |
MTD | 0.326 | 0.002 21.6 vs. 5.88 mm |
Stromal invasion | 0.942 | 0.001 < 1/3 31.25% < 73.33% |
CC stage | 0.357 | < 0.0001 IA1 18.75% < 83.33% IB1 10% < 93.75% |
Baseline PS score | 0.473 | 1 |
Ovarian preservation | 0.632 | 0.457 |
SLNB | 0.012 Laparoscopy > laparotomy | < 0.0001 43.75% > 0% |
PL | 0.446 | < 0.0001 100% > 13.33% |
PAL | 0.007 Laparotomy > laparoscopy | 0.026 18.75 > 0% |
Number of PLNs removed | 0.451 | 0.85 |
Number of MPLNs removed | 1 | 1 |
Intraoperative complications | 0.729 | 0.731 |
Postoperative complications | 0.025 27.27 vs. 64.52% | 0.413 Overall complications 0.342 |
Time interval to diagnosis of surgical complication | 0.036 21 vs. 5.5 days | 0.502 |
Length of hospital stay | 0.007 6.11 vs. 3.22 days | 0.018 5.07 vs. 2.68 |
Number of recurrences | 0.25 | 1 |
Follow-up time > 2 years | < 0.0001 100% vs. 71.88% | 0.363 |
BMI body mass index, CC cervical cancer, C-section cesarean section, LVI lymphovascular invasion, MPLN metastatic pelvic lymph node, MTD maximum tumor diameter, PL pelvic lymphadenectomy, PAL para-aortic lymphadenectomy, PLN pelvic lymph node, PS performance status, RH radical hysterectomy, SLNB sentinel lymph node biopsy, TEH total extrafascial hysterectomy.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cea García, J., Márquez Maraver, F., Rodríguez Jiménez, I. et al. Internal Validation of a Predictive Model for Overall Survival in Patients with FIGO stages I–IV Cervical Cancer. Indian J Gynecol Oncolog 21, 67 (2023). https://doi.org/10.1007/s40944-023-00744-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-023-00744-2


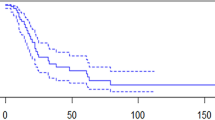
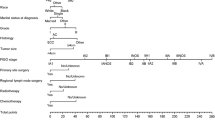

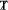 :
: