Abstract
Objective
The efficacy of intraoperative radiotherapy (IORT) as boost dose following neoadjuvant chemotherapy (NACT) is not clearly established yet. This trial evaluated the outcome and complications of IORT boost dose after NACT in patients with breast cancer.
Methods and Materials
This study was a single-arm phase II clinical trial conducted at tertiary referral centers including Omid and Imam Reza Educational Hospitals and Pasteur Private Hospital, Mashhad, Iran. Twenty-four women with breast cancer underwent breast-preservation surgery following the neoadjuvant chemotherapy were enrolled. During BCS, a 20 Gy dose was prescribed to the tumor bed using intrabeam system Zeiss followed by 50 Gy/2 Gy whole breast radiotherapy. Patients were followed for complications of treatment and disease recurrence at 2 years.
Results
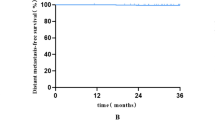
Toxicity criteria of the RTOG revealed there was only one case of acute toxicity grade 4 as necrosis. During the follow up, two cases of grade III fibrosis were observed. With a median follow up of 29.5 months, one patient died due to cancer with the median survival time of 23.93 months and the 2-year survival rate of 95.5 ± 4.4%. Moreover, one localized relapses in the breast, one recurrent in axillary region and distant metastases, and two distant metastases were diagnosed. The mean free survival was 23.23 months, and the 2-year free survival rate was 87.1%.
Conclusion
The results of this study showed that in the short-term follow up, IORT results in terms of local control, side effects and cosmetic outcome after neoadjuvant chemotherapy in breast cancer patients are acceptable.

Similar content being viewed by others
References
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;26(suppl_5):v8-v30.
Kuerer HM. FACS reviewing Early Breast Cancer Trialists' Collaborative Group (EBCTCG). NEJM Journal Watch, Waltham, Massachusetts. 2011 https://www.jwatch.org/oh201111150000004/2011/11/15/benefits-radiotherapy-after-breast-conserving. Accessed 27 May 2019.
Faverly DR, Hendriks JH, Holland R. Breast carcinomas of limited extent: frequency, radiologic-pathologic characteristics, and surgical margin requirements. Cancer. 2001;91(4):647–59.
Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1–2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer. 1985;56(5):979–90.
Vaidya JS, Tobias JS, Baum M, Keshtgar M, Joseph D, Wenz F, et al. Intraoperative radiotherapy for breast cancer. Lancet Oncol. 2010;5(3):165–73.
Bartelink H, Horiot JC, Poortmans P, Struikmans H, Van den Bogaert W, Barillot I, et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Eng J Med. 2001;345(19):1378–87. https://doi.org/10.1056/NEJMoa010874.
Kindts I, Laenen A, Depuydt T, Weltens C. Tumour bed boost radiotherapy for women after breast-conserving surgery. Cochrane Database Syst Rev. 2017;11:Cd011987. https://doi.org/10.1002/14651858.CD011987.pub2.
Goyal S, Buchholz TA, Haffty BG. Breast cancer, Early Stage. In: Halperin EC, Wazer DE, Perez CA, Brady LW, editors. Perez and Brady's principles and practice of radiation oncology. 7th ed. Philadelphia, PA: Wolters Kluwer; 2018. p. 1268–1378.
Moran MS, Truong P. Breast cancer, Locally advanced, part 2. In: Halperin EC, Wazer DE, Perez CA, Brady LW, editors. Perez and Brady's principles and practice of radiation oncology. 7th ed. Philadelphia, PA: Wolters Kluwer; 2018. p. 1395–1418.
Shiloh RY, Mahal BA, Wong S, Khan A, Bellon JR. Breast cancer, Locally advanced, part 1. In: Halperin EC, Wazer DE, Perez CA, Brady LW, editors. Perez and Brady's principles and practice of radiation oncology. 7th ed. Philadelphia, PA: Wolters Kluwer; 2018. p. 1379–1394.
Jalali R, Singh S, Budrukkar A. Techniques of tumour bed boost irradiation in breast conserving therapy: current evidence and suggested guidelines. Acta Oncol. 2007;46(7):879–92. https://doi.org/10.1080/02841860701441798.
Van Limbergen E. Indications and technical aspects of brachytherapy in breast conserving treatment of breast cancer. Cancer/Radiothérapie. 2003;7(2):107–20. https://doi.org/10.1016/S1278-3218(03)00015-5.
Sedlmayer F, Reitsamer R, Wenz F, Sperk E, Fussl C, Kaiser J, et al. Intraoperative radiotherapy (IORT) as boost in breast cancer. Radiat Oncol. 2017;12(1):23. https://doi.org/10.1186/s13014-016-0749-9.
Nairz O, Deutschmann H, Kopp M, Wurstbauer K, Kametriser G, Fastner G, et al. A dosimetric comparison of IORT techniques in limited-stage breast cancer. Strahlenther Onkol. 2006;182(6):342–8. https://doi.org/10.1007/s00066-006-1580-2.
Fastner G, Hauser-Kronberger C, Moder A, Reitsamer R, Zehentmayr F, Kopp P, et al. Survival and local control rates of triple-negative breast cancer patients treated with boost-IOERT during breast-conserving surgery. Strahlenther Onkol. 2016;192(1):1–7. https://doi.org/10.1007/s00066-015-0895-2.
Kolberg H-C, Lövey G, Akpolat-Basci L, Stephanou M, Fasching P, Untch M, et al. Targeted intraoperative radiotherapy tumour bed boost during breast-conserving surgery after neoadjuvant chemotherapy—a subgroup analysis of hormone receptor-positive HER2-negative breast cancer. Breast Care (Basel). 2017;12(5):318–23. https://doi.org/10.1159/000479424.
Kolberg HC, Loevey G, Akpolat-Basci L, Stephanou M, Fasching PA, Untch M, et al. Targeted intraoperative radiotherapy tumour bed boost during breast conserving surgery after neoadjuvant chemotherapy in HER2 positive and triple negative breast cancer. Rev Recent Clin Trials. 2017;12(2):93–100. https://doi.org/10.2174/1574887112666170201142458.
Zur M, Shai A, Leviov M, Bitterman A, Shiloni E, Ben Yosef R, et al. Short-term complications of intra-operative radiotherapy for early breast cancer. J Surg Oncol. 2016;113(4):370–3. https://doi.org/10.1002/jso.24157.
Cracco S, Semprini G, Cattin F, Gregoraci G, Zeppieri M, Isola M, et al. Impact of intraoperative radiotherapy on cosmetic outcome and complications after oncoplastic breast surgery. Breast J. 2015;21(3):285–90. https://doi.org/10.1111/tbj.12402.
Cedolini C, Bertozzi S, Seriau L, Londero AP, Concina S, Moretti E, et al. Feasibility of concervative breast surgery and intraoperative radiation therapy for early breast cancer: a single-center, open, non-randomized, prospective pilot study. Oncol Rep. 2014;31(4):1539–46. https://doi.org/10.3892/or.2014.3018.
Chua BH, Henderson MA, Milner AD. Intraoperative radiotherapy in women with early breast cancer treated by breast-conserving therapy. ANZ J Surg. 2011;81(1–2):65–9. https://doi.org/10.1111/j.1445-2197.2010.05431.x.
Fazilat-Panah D, Vakili Ahrari Roudi S, Keramati A, Fanipakdel A, Sadeghian M, Homaei-Shandiz F, et al. Changes in cytokeratin during neoadjuvant chemotherapy of breast cancer: a prospective study. Iran J Pathol. 2020;1:12–24. https://doi.org/10.30699/ijp.2020.116238.2261.
National Comprehensive Cancer Network. Breast Cancer (Version 3.2019). 2019. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed June 10, 2019.
Fanipakdel A, Seilanian Toussi M, Rezazadeh F, Mohamadian Roshan N, Javadinia SA. Overexpression of cancer-testis antigen melanoma-associated antigen A1 in lung cancer: a novel biomarker for prognosis, and a possible target for immunotherapy. J Cell Physiol. 2019;234(7):12080–6. https://doi.org/10.1002/jcp.27884.
Javadinia SA, Shahidsales S, Fanipakdel A, Joudi-Mashhad M, Mehramiz M, Talebian S, et al. Therapeutic potential of targeting the Wnt/β-catenin pathway in the treatment of pancreatic cancer. J Cell Biochem. 2019;120(5):6833–40. https://doi.org/10.1002/jcb.27835.
Javadinia SA, Shahidsales S, Fanipakdel A, Mostafapour A, Joudi-Mashhad M, Ferns GA, et al. The esophageal cancer and the PI3K/AKT/mTOR signaling regulatory micrornas: a novel marker for prognosis, and a possible target for immunotherapy. Curr Pharm Des. 2018;24(39):4646–51. https://doi.org/10.2174/1381612825666190110143258.
Javadinia SA, Gholami A, Joudi Mashhad M, Ferns GA, Shahidsales S, Avan A, et al. Anti-tumoral effects of low molecular weight heparins: a focus on the treatment of esophageal cancer. J Cell Physiol. 2018;233(10):6523–9. https://doi.org/10.1002/jcp.26613.
Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet (London, England). 2014;383(9917):603–13. https://doi.org/10.1016/s0140-6736(13)61950-9.
Veronesi U, Orecchia R, Luini A, Galimberti V, Zurrida S, Intra M, et al. Intraoperative radiotherapy during breast conserving surgery: a study on 1,822 cases treated with electrons. Breast Cancer Res Treat. 2010;124(1):141–51.
Fastner G, Reitsamer R, Ziegler I, Zehentmayr F, Fussl C, Kopp P, et al. IOERT as anticipated tumor bed boost during breast-conserving surgery after neoadjuvant chemotherapy in locally advanced breast cancer–results of a case series after 5-year follow-up. Int J Cancer. 2015;136(5):1193–201. https://doi.org/10.1002/ijc.29064.
Spaich S, Tuschy B, Sperk E, Wenz F, Sütterlin M. Initial experience of intraoperative radiotherapy as tumour bed boost after neoadjuvant chemotherapy in breast cancer patients. Transl Cancer Res. 2017;6(2):416–23.
Acknowledgements
The authors would like to thank staffs of Reza Radiotherapy & Oncology Center of Mashhad.
Funding
This work was supported by the Mashhad University of Medical Sciences [Grant numbers 950995, 2017].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Homaei Shandiz, F., Fanipakdel, A., Forghani, M.N. et al. Clinical Efficacy and Side Effects of IORT as Tumor Bed Boost During Breast-Conserving Surgery in Breast Cancer Patients Following Neoadjuvant Chemotherapy. Indian J Gynecol Oncolog 18, 46 (2020). https://doi.org/10.1007/s40944-020-00389-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-020-00389-5