Abstract
Background
The infiltrating margins of carcinomas are associated with the presence of inflammatory cell infiltrate which are an integral part of the tumor microenvironment. Among the inflammatory cells, tumor-associated macrophages (TAMs) play a key role in the tumorigenesis. This study elucidates the density of TAMs in invasive mammary carcinomas and attempts to establish an association with the following pathological variables: tumor size, histological grade, nodal status, hormonal expression status and Her2Neu overexpression.
Materials and Methods
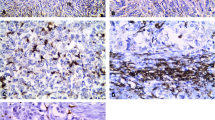
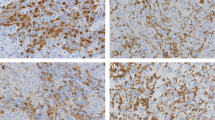
Ninety diagnosed archival cases of invasive mammary carcinomas at a tertiary care center were included. Density of TAMs was assessed by using CD68 which is a pan-macrophage marker by immunohistochemistry on the archival tissue blocks. The density of TAMs (CD68 positive cells) was dichotomized into high (> 50 CD68 positive cells/HPF) and low (< 50 CD68 positive cells/HPF) and compared with the above-mentioned pathological variables using appropriate statistical tests.
Results
The density of TAMs was significantly higher around the infiltrating edge of the carcinoma in comparison with the adjoining normal terminal duct lobular units. The density of TAMs was more in the infiltrating edge of the tumor than within the tumor nodule/nests. A higher TAM density showed a significant association in tumors having large tumor size, higher histological grade, nodal metastasis, the absence of ER and PR expression and Her2Neu overexpression (p value < 0.05).
Conclusion
TAMs play an important role in tumor progression in invasive mammary carcinomas. This is as a result of the multiple roles enacted by TAMs in the various stages of tumor development starting from tumor growth, invasion, angiogenesis and metastases. Targeted therapy against TAMs has great potential in being important components of future treatment strategies against breast carcinomas.








Similar content being viewed by others
References
Mbeunkui F, Johann DJ Jr. Cancer and the tumor microenvironment a review of an essential relationship. Cancer Chemother Pharamacol. 2009;53:571–82.
Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12.
Bolli E, Movahedi K, Laoui D, Van Ginderachter JA. Novel insights in the regulation and function of macrophages in the tumor microenvironment. Curr Opin Oncol. 2017;29:55–61.
Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6.
Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306.
Zhang Y, Cheng S, Zhang M, Zhen L, Pang D, Zhang Q, et al. High-infiltration of tumor-associated macrophages predicts unfavorable clinical outcome for node-negative breast cancer. PLoS ONE. 2013;8:e76147.
Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2:15025.
Lahmar Q, Keirsse J, Laoui D, Movahedi K, Van Overmeire E, Van Ginderachter JA. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochim Biophys Acta (BBA)—Rev Cancer. 2016;1865:23–34.
Malvia S, Bagadi SA, Dubey US, Saxena S. Epidemiology of breast cancer in Indian women. Asia Pacific J Clin Oncol. 2017;13:289–295.
Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev. 2016;99:180–5.
Georgoudaki A-M, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Östling J, et al. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016;15:2000–11.
Guo Q, Jin Z, Yuan Y, Liu R, Xu T, Wei H, et al. New mechanisms of tumor-associated macrophages on promoting tumor progression: recent research advances and potential targets for tumor immunotherapy. J Immunol Res. 2016;2016, Article ID 9720912, 12 pages.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10.
Micklem K, Rigney E, Cordell J, Simmons D, Stross P, Turley H, et al. A human macrophage-associated antigen (CD68) detected by six different monoclonal antibodies. Br J Haematol. 1989;73:6–11.
Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci. 2011;108:12425–30.
Yang J, Liao D, Chen C, Liu Y, Chuang T, Xiang R, et al. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2013;31:248–58.
Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. In: Seminars in cancer biology, 2006;38–52.
Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–6.
Raggi C, Mousa HS, Correnti M, Sica A, Invernizzi P. Cancer stem cells and tumor-associated macrophages: a roadmap for multitargeting strategies. Oncogene. 2016;35:671–82.
Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–56.
Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–55.
Häuselmann I, Roblek M, Protsyuk D, Huck V, Knopfova L, Grässle S, et al. Monocyte induction of E-selectin–mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade. Cancer Res. 2016;76:5302–12.
Pignatelli J, Bravo-Cordero JJ, Roh-Johnson M, Gandhi SJ, Wang Y, Chen X, et al. Macrophage-dependent tumor cell transendothelial migration is mediated by Notch1/MenaINV-initiated invadopodium formation. Sci Rep. 2016;6:37874.
Niu M, Valdes S, Naguib YW, Hursting SD, Cui Z. Tumor-associated macrophage-mediated targeted therapy of triple-negative breast cancer. Mol Pharm. 2016;13:1833–42.
Achkova D, Maher J. Role of the colony-stimulating factor (CSF)/CSF-1 receptor axis in cancer. Biochem Soc Trans. 2016;44:333–41.
Rennert G, Pinchev M, Gronich N, Saliba W, Flugelman A, Lavi I, et al. Oral bisphosphonates and improved survival of breast cancer. Clin Cancer Res. 2016;23:0547.
Salazar LG, Lu H, Reichow JL, Childs JS, Coveler AL, Higgins DM, et al. Topical imiquimod plus nab-paclitaxel for breast cancer cutaneous metastases: a phase 2 clinical trial. JAMA Oncol. 2017;3:969–973.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author A (Dr Imitiaz Ahmed) declares that he has no conflict of interest. Author B (Dr Manoj Gopal Madakshira) declares that he has no conflict of interest. Author C (Dr Puja Dudeja) declares that she has no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
“Informed consent was obtained from all individual participants included in the study.”
Rights and permissions
About this article
Cite this article
Ahmed, I., Madakshira, M.G. & Dudeja, P. Tumor-Associated Macrophages: Oblivious Confederates in Invasive Mammary Carcinoma. Indian J Gynecol Oncolog 15, 75 (2017). https://doi.org/10.1007/s40944-017-0169-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-017-0169-2