Abstract
Bioavailability evaluations can be an important tool to determine the quality of dredging sediments. Sequential extractions are commonly used to assess the different chemical forms of trace metals in coastal sediments; however, sometimes they are expensive and time-consuming. Additionally, these analyses do not represent possible changes due to resuspension during and after dredging activity. Newer, easier options to evaluate bioavailability are necessary to understand and preview bioavailability changes resulting from trace metals in dredging and disposal areas. This study aims to evaluate changes in Cd and Zn bioavailability due to sediment resuspension in a highly contaminated area in Sepetiba Bay, Brazil. Twelve surface sediment samples from this location were submitted to a resuspension experiment during two different time intervals (1 and 24 h). The extraction of potentially bioavailable (1 mol L−1 HCl-extractable) fractions and strongly bound (concentrated HNO3-extractable) fractions were sequentially carried out. More than 50 % of the concentrations were in potentially bioavailable fractions. Bioavailability change indexes were proposed; these were estimated as relative changes in bioavailability percentage after resuspension. However, two of these stations presented increases in the Cd and Zn HCl-extractable fractions after sediment resuspension above effects range low and effects range medium National Oceanic and Atmospheric Administration which are the same limits adopted for Brazilian environmental jurisdiction. Positive correlation of Zn and Cd with TOC in strongly bound fractions evidenced the importance of organic matter binding to preserve metals in this fraction. Fe and Mn partitioning was a major geochemical control on bioavailability of Cd and Zn upon short-time resuspension periods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sediment resuspension events in coastal environments can occur due to natural processes or be induced by human activities, which are known as factors affecting trace metal bioavailability and their associated environmental risks (Eggleton and Thomas 2004; Roberts 2012). This concern is particularly important for areas that combine metal contamination history with frequent sediment resuspension events, such as harbors, where periodical sediment dredging occurs to support navigation. Assessments of environmental risks associated with metal contamination in these areas can involve the use of sediment resuspension experiments combined with extraction techniques that allow metal bioavailability estimation (Caetano et al. 2003; Machado et al. 2011). Studies comparing strong chemical extraction data with sediment quality guidelines (SQGs) have been frequently done in sediments from dredging areas (Huerta-Diaz et al. 2008; Buruaem et al. 2012). However, the use of resuspension experiments can improve results and be a useful tool for the management of dredging sediments.
Assessment of sediment quality is recognized as a critical step for estimating the risk associated with man-made pollution in aquatic systems (Castillo et al. 2013). Environmental regulatory agencies from many countries recognize the importance of sediment quality evaluation before dredging activities begin at an area or any other remediation activity at aquatic ecosystems (e.g., Chapman and Mann 1999). In Brazil, trace metals SQGs used for the licensing process of dredging activities were established in 2004 (CONAMA 2004), adopting the safety levels proposed by Long et al. (1995), where Level 1 corresponds to 1.2 and 150 mg/kg−1 for Cd and Zn, respectively (ERL). In 2012, the Brazilian legislation was reviewed and incorporated rules for the final disposal of dredged material (CONAMA 2012). The new restrictions drove environmental authorities to demand safer dredging procedures (Wasserman et al. 2013) and include new tests, such as bioaccumulation tests, when the sediment has total concentrations of trace metals above the safety levels. However, chemistry bioavailability tests or geochemistry mobility tests were not included in the legislation.
The effects of dredging on geochemical mobility of contaminants and their availability to biota are poorly known, especially in tropical ecosystems. To improve this knowledge, changes in metal geochemical mobility can be measured in resuspension assays, which simulate the dredging effects using different time intervals (Morse 1994; Van den Berg et al. 2001; Caetano et al. 2003; Cappuyns et al. 2006; Cantwell et al. 2008; Machado et al. 2011; Acquavita et al. 2012).
It is known that trace metals which typically undergo high incorporation into pyrite are susceptible to changes in their geochemical partitioning upon sediment resuspension in oxidizing water. The pyrite can become a source of these elements to the water column if oxidized (Morse 1994; Saulnier and Mucci 2000). However, potential changes in the bioavailability of trace metals that generally do not present elevated pyritization degree (such as Cd and Zn; Huerta-Diaz and Morse 1992), can be also an issue of concern in areas strongly contaminated by these elements. This is the case with sites affected by metallurgical activities, as observed in Sepetiba Bay (SE Brazil), a tropical coastal system influenced by combined effects of severe Cd and Zn contamination (principally due to past activities of a Zn smelting plant), urbanization and harboring activities (Barcellos et al. 1991; Machado et al. 2008; Ribeiro et al. 2013). Cd and Zn emissions from this major industrial source were estimated as 24 and 3660 t year−1, respectively, until 1997 when the metallurgical plant was closed. Contaminants were mainly transferred to the bay by a tidal creek, Saco do Engenho Creek, which received the drainage from a large refuse pile of the past metallurgical plant (Molisani et al. 2004).
This study aimed to evaluate possible changes on trace metal bioavailability in coastal sediments due to resuspension events by simulating a scenario of final disposal of dredging material in which the highly contaminated sediment would be exposed to an oxidant water column. This exposure of anoxic sediment to an oxidant water column can increase the mobility and, consequently, the potential bioavailability of trace metals which could be a threat to local biota. The hypothesis was that the trace metals become more bioavailable after resuspension in an oxidant water column in a laboratory assay using Cd and Zn contaminated sediments samples from Sepetiba Bay as consequence of geochemical partitioning changes.
Materials and methods
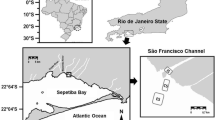
The study area is highly impacted by Cd and Zn contamination, located between Saco do Engenho Creek (SEC) and Itaguaí Harbor, northern region of Sepetiba Bay, Rio de Janeiro Brazil. In March 2011, surface sediment samples (nearly 0–10 cm depth) were collected using a Van Veen grab at 12 sampling stations (Fig. 1), while surface estuarine water was sampled in station 12 at approximately 15 cm depth, using acid-cleaned glass bottles. Sampled water salinity was 32, pH was 8.1 and dissolved oxygen concentration was 5.77 mg L−1, as measured using an YSI sonde. Resuspension experiments were carried out at room temperature (~25 °C). The experiment compared short and long time periods of resuspension (1 vs. 24 h). Wet sediment subsamples (7 g) were transferred to 125-mL Erlenmeyer flasks and were shaken in 100 mL of unfiltered estuarine water in contact with atmosphere. The experiment was made in duplicate. As blanks, two Erlenmeyers containing only unfiltered estuarine water were used for each time period. Reactive geochemical phase of Cd and Zn were extracted by 16-h agitation in 1 mol L−1 HCl solution, a usual approach for metal bioavailability evaluation (Huerta-Diaz and Morse 1990). HCl-extracted subsamples were washed with deionized water and centrifuged three times to remove HCl residues prior to a second-step extraction of strongly bound metal fractions, as previously carried out by Machado et al. (2011). The strongly extraction bound metals method chosen was a microwave-assisted digestion in concentrated HNO3 (USEPA method 3051A) (USEPA 1994). Cd, Zn, Mn and Fe concentrations were determined by ICP OES. The accuracy was checked using certified samples of NIST 2782. The detection limits were: Cd: 0.01 mg/kg, Fe: 3.00 mg/kg, Mn: 0.02 mg/kg and Zn: 0.01 mg/kg. Sediment grain size was characterized using a particle size analyzer CILAS 1064. After carbonate removal by acidification, total organic carbon (TOC) contents were determined using a Shimadzu TOC analyzer. Possible associations between studied variables were evaluated using correlation analysis. A significance level of 0.05 was accepted.
For the evaluation of possible differences in bioavailability after resuspension, the relative change in the percentage of trace metals in HCl-extractable fractions was calculated, hereafter called as bioavailability change index (BCI), as expressed by the formula:
where %HClAR is the percentage in the HCl-extractable fraction after resuspension, while %HClBR is the percentage in HCl-extractable fraction before resuspension.
Contamination factors (CF) were calculated in relation to mean background values (Håkanson 1980). These values were obtained from 210Pb-dated sediment cores sampled in Sepetiba, as reported by Gomes et al. (2009) and Marques et al. (2006). The averages of background levels derived from these two studies were 0.27 and 72.5 mg/kg−1 for Cd and Zn, respectively.
Results
Sediment grain size and TOC content results are presented in Table 1, while metal concentrations observed before and after resuspension experiments are shown in Table 2. Studied sediments are essentially composed of fine particles (upper 70 % of the silt and clay), while TOC contents ranged from 1.66 to 4.23 %. There was a clear trend of higher Cd and Zn concentration at the stations closer to the shore and to SEC (reaching values one order of magnitude above concentrations found in other stations), partly coinciding with a generally higher HCl-extractable concentration of Mn in stations 1 and 2. These trends were not observed for Fe; that was the only metal showing lower concentration at station 6, which had a more relevant sand content, suggesting sand dilution effect. There was no significant correlation of Cd and Zn concentrations with grain size data. Cadmium and Zn HNO3-extractable phases were significantly correlated with sediment TOC content (r = 0.62 to 0.78), while Cd HCl-extractable phases were significantly correlated with HCl-extractable Mn (r = 0.63 to 0.82) for all datasets. Zinc HCl-extractable phases were significantly correlated with HCl-extractable Mn before resuspension and after 1-h resuspension (r = 0.64 and 0.80, respectively). Cadmium HCl-extractable phases were correlated with Fe HCl-extractable before resuspension (r = 0.59) and after 1-h resuspension (r = 0.64).
The relative contributions of HCl-extraction phase to the total concentration are presented in Table 3. All metals presented a general tendency of slight increases in the relative contributions of HCl-extractable phase after sediment resuspension for both time intervals. There was a decreasing tendency in contributions from the closest stations to SEC. Cd showed percentages in HCl-extraction phase above 55 % after resuspension, whereas these estimates were above 80 %.
Relative changes in metal bioavailability are represented in Table 4 estimated as BCI values. These values were up to 16 % for Cd and 20 % for Zn after 1-hr resuspension, and up to 13 % for Cd and 18 % for Zn after 24-h resuspension, which indicate a low relative susceptibility of Cd and Zn to become more bioavailable after resuspension. The spatial distribution, according to the study, were characterized by a clear trend of increasing values with the distance from SEC for Zn. Cadmium followed this trend to a less accentuated extent. Zinc bioavailability changes were correlated with those of Fe after both resuspension times (r = 0.62 and 0.67 for 1- and 24-h resuspension times, respectively). Cadmium bioavailability changes were also correlated with Fe bioavailability changes after 24-h resuspension (r = 0.63), but not for the 1-h resuspension experiment.
The anthropogenic contribution of Cd and Zn were calculated as the ratio between metal concentrations in superficial sediments from the sampling stations and the respective background levels. The results obtained for these contamination factors (CF) can be classified, according to Håkanson (1980), as follows:
CF <1 = there is no contamination or it is low.
CF 1 >3 = moderate contamination.
CF 3 >6 = considerable high contamination.
CF >6 = very high contamination.
Table 5 presents CFs results. For Cd, the maximum CF was found to be 71 (very high contamination) at station 2, and the minimum was 5.4 (considerable high contamination) at station 11. There was a trend of decreased CF values with the distance from SEC mouth. Zinc showed a similar pattern, with the maximum CF value found at station 2 (55.8; very high contamination) and the minimum found at station 11 (2.4; moderate contamination). Stations 10 and 11 were classified as moderate contamination and stations 9 and 12 were (classified as considerable high contamination; all the other stations showed very high contamination. CFs were significantly correlated with relative contributions of potentially bioavailable phases for Cd (r = 0.78) and Zn (r = 0.67).
Discussion
In cases of dredging activities, the first evaluation is the comparison of the results with the safety levels established on actual legislation. According to Brazilian Federal Legislation, the studied samples presented Cd and Zn levels (Table 2) exceeding CONAMA Level 1, indicating they may promote adverse effects. Sums of HCl and HNO3 extractions were up to 16-times higher the SQG for Cd and 27-times higher the SQG for Zn, with HCl-extractable phase predominant for both metals. For the SQG of Level 2, 7.2 and 410 mg/kg−1 for Cd and Zn, respectively, values correspond to the threshold above which there is major probability of adverse effects occurring on biota. Sampling stations 1, 2 and 3 exceeded the limit for Cd, and stations 1 to 8 exceeded the limit for Zn, being 1.4–2.7 times higher the SQG for Cd, and 1.1–9.9 times higher than the SQG for Zn. According to these regulations, such results request additional investigation if dredging is intended, such as toxicity tests and/or bioaccumulation assays (CONAMA 2012).
Two of twelve sampling stations presented an increase on HCl-extractable Cd (3.86 and 2.38 mg/kg−1 in stations 2 and 6, respectively) and Zn (819 and 178 mg/kg−1 in stations 2 and 6, respectively) above the referred SQGs after 1-h resuspension. This pattern was also observed for Zn (275 and 158 mg/kg−1 in stations 2 and 6, respectively) after 24-h experiments. Even then with a slight increase after resuspension, this increase was enough to exceed the safety levels and transform into potentially bioavailable forms a significant amount of Cd and Zn that were strongly bound to sediments before. This may exemplify situations in which small relative differences correspond to environmentally relevant absolute differences.
Considering the severity of this contamination, bioavailability-based data can be additional tools to identify management priorities related to activities that promote sediment resuspension, since relatively small changes in high concentrations can imply in ecological risks and increasing biological metals uptake. For example, during resuspension events, metals may become partly available in the dissolved phase (which may often be of short duration; e.g., Maddock et al. 2007) and metals recently associated to the solid phase can present a weaker association than occurred before resuspension (e.g., by adsorption to particles surfaces; e.g., Caetano et al. 2003). The mechanisms involved in such transition between geochemical phases are frequently related to the production of metal oxides and hydroxides upon sediment resuspension, e.g., as a consequence of sulfides oxidation (Simpson et al. 2000; Cantwell et al. 2008). The observed correlations between Cd and Zn data with Mn and Fe data support that these mechanisms were important geochemical controls on the behavior of studied metal contaminants. Morse (1994) and Machado et al. (2011) showed also in their results after resuspension that the Cu in sediments was in a bioavailable phase.
The study of trace metals bioavailability is of great interest for management decisions concerning dredging activities and the final disposal of their residuals. However, high trace metals concentrations in sediment will not always be a consequence of dredging activity. Many other physical–chemical factors might capture metals from the water column. Carvalho and Lacerda (1992) found very low trace metals concentrations on biota of Guanabara Bay, despite the extremely high trace metal contamination due to anthropogenic activities in this ecosystem. The authors also compared the results with trace metals found in organisms from other two non-contaminated areas, Arraial do Cabo and Angra dos Reis, both located in Rio de Janeiro State. Here, higher concentrations were described to both uncontaminated areas. The reasons pointed by the authors for this difference are the high organic matter which regulates the bioavailability of trace metals in Guanabara Bay. The same result was found by Kehrig et al. (2002) when they analyzed MeHg and THg in fish species in Guanabara Bay. Rodrigues et al. (2011) have found similar results to Carvalho and Lacerda (1992), where Guanabara Bay had lower mercury concentrations in fish species than Ribeira bay, an uncontaminated site.
Considering that environmental legislations are revised periodically, the coupled use of different lines of evidence has been recognized as important tools to be incorporated, e.g., toxicity tests and bioavailability (Torres et al. 2009). The results discussed above also evidence that additional studies concerning geochemical partitioning may improve risk assessment, such as utilizing conventional uses of total (or near-total) metal concentration comparison together with SQGs. While fluctuations in environmental physical–chemical characteristics which influence metal bioavailability can be used to adapt SQGs (Hübner et al. 2009), the use of bioavailability-based sediment quality analysis in addition to current uses of SQGs would be an alternative and advantageous approach.
Conclusions
Low relative changes in the bioavailability of studied trace metals were found in the study site. Two of twelve sampling stations presented relevant increases in the Cd and Zn HCl-extractable fractions after sediment resuspension, being above SQGs adopted in the ERL and ERM. The positive correlation of Zn and Cd with TOC in strongly bound forms evidenced the importance of organic matter binding to preserve metals in this fraction. Fe and Mn partitioning was a major geochemical control on bioavailability of Cd and Zn upon short-time resuspension periods, but this role seems to be dependent on resuspension time, as observed for Cd. The formation of oxy-hydroxides after resuspension regulated the bioavailability of trace metals in bioavailable fraction.
The use of BCI showed a important and efficient tool for showing changes in bioavailability of trace metals before and after resuspension and can be used in evaluation of ecological risk of sites. The prediction of behavior of trace metals in sediment on site is a form for relatively quickly assessing the risks associated with dredging before the activity occurs. Good planning can decrease the environmental problems associated with bioavailability of trace metals in sediment or in water column after dredging activities.
The use of different tools for bioavailability prediction in contaminated areas susceptible to resuspension is a way to improve the SQGs, to support the management decisions for contaminated areas and to bring new possibilities for reviewing regulatory legislation.
References
Acquavita A, Emili A, Covelli S, Faganeli J, Predonzani S, Koron N, Carrasco L (2012) The effects of resuspension on the fate of Hg in contaminated sediments (Marano and Grado Lagoon, Italy): Short-term simulation experiments. Estuar Costal Shelf 113:32–40
Barcellos C, Rezende CE, Pfeiffer WC (1991) Zn and Cd production and pollution in a Brazilian coastal region. Mar Pollut Bull 22:558–561
Buruaem LM, Hortellani MA, Sarkis JE, Costa-Lotufo, LV Abessa DMS (2012) Contamination of port zone sediments by metals from Large Marine Ecosystems of Brazil. Marine Pollut Bull 64:479–488
Caetano M, Madureira MJ, Vale C (2003) Metal remobilisation during resuspension of anoxic contaminated sediment: short-term laboratory study. Water Air Soil Pollut 143:23–40
Cantwell MG, Burgess RM, King JW (2008) Resuspension of contaminated field and formulated reference sediments Part I: evaluation of metal release under controlled laboratory conditions. Chemosphere 73:1824–1831
Cappuyns V, Swennen R, Devivier A (2006) Dredged river sediments: potential chemical time bombs? A case study. Water Soil Pollut 171:49–66
Carvalho EV, Lacerda LD (1992) Heavy metals in the Guanabara Bay biota: why such low concentrations? J Braz Assoc Adv Sci 44:184–186
Castillo MLA, Trujillo SI, Alonso EV, Torres AG, Pávon JMC (2013) Bioavailability of heavy metals in water and sediments from a typical Mediterranean Bay (Málaga Bay, Region of Andalucía, Southern Spain) Marine Pollut 76:427–434
Chapman PM, Mann GS (1999) Sediment quality values (SQVs) and ecological risk assessment (ERA). Marine Pollut 38:339–344
CONAMA (2004) Procedures for the evaluation of dredging materials in Brazilian jurisdictional waters. Resolution #344/2004 from Conselho Nacional do Meio Ambiente. Diário Oficial da República Federativa do Brasil, Brasília (in Portuguese)
CONAMA (2012) Procedures for the evaluation of dredging materials in Brazilian jurisdictional waters. Resolution #454/2012 from ConselhoNacional do MeioAmbiente. Diário Oficial da República Federativa do Brasil, Brasília (in Portuguese)
Eggleton J, Thomas KV (2004) A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ Int 30:973–980
Gomes FC, Godoy JM, Godoy MLDP, Carvalho ZL, Lopes TR, Sanchez-Cabeza JA, Lacerda LD, Wasserman JC (2009) Metal concentrations, fluxes, inventories and chronologies in sediments from Sepetiba and Ribeira Bays: a comparative study. Marine Pollut Bull 59:123–133
Håkanson L (1980) The quantitative impact of pH, bioproduction and Hg contamination on the Hg content of fish (pike). Environ Pollut 1:285–304
Hübner R, Astin KB, Herbert RJH (2009) Comparison of sediment quality guidelines (SQGs) for the assessment of metal contamination in marine and estuarine environments. J Environ Monit 11:713–722
Huerta-Diaz MA, Morse JW (1990) A quantitative method for determination of trace metal concentration in sedimentary pyrite. Mar Chem 29:119–144
Huerta-Diaz MA, Morse JW (1992) Pyritization of trace metals in anoxic marine sediments. Geochimica Cosmochimica Acta. 56:2681–2702
Huerta-Diaz MA, Delgadillo-Hinojosa F, Hernández-Ayón M, Segovia-Zavala JA, García-Esquivel Z, López-Zárate H, Siqueiros-Valencia A, Galindo-Bect S (2008) Diagnosis of trace metal contamination in sediments: the example of Ensenada and El Sauzal, two harbors in Baja California, Mexico. Marine Environ Res 66:345–358
Kehrig HA, Costa M, Moreira I, Malm O (2002) Total and methylmercury in a Brazilian estuary, Rio de Janeiro. Marine Pollut Bull 44:1018–1023
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manage 19:81–97
Machado W, Santelli RE, Carvalho MF, Molisani MM, Barreto RC, Lacerda LD (2008) Relation of reactive sulfides with organic carbon, iron, and manganese in anaerobic mangrove sediments: implications for sediment suitability to trap trace metals. J Coastal Res 24:25–32
Machado W, Rodrigues APC, Bidone ED, Sella SM, Santelli RE (2011) Evaluation of Cu potential bioavailability changes upon coastal sediment resuspension: an example on how to improve the assessment of sediment dredging environmental risks. Environ Sci Pollut Res 18:1033–1036
Maddock JEL, Carvalho MF, Santelli RE, Machado W (2007) Contaminant metal behaviour during re-suspension of sulphidic estuarine sediments. Water Air Soil Pollut 181:193–200
Marques AN Jr, Monna F, Silva-Filho EV, Fernex FE, Simões-Filho FL (2006) Apparent discrepancy in contamination history of a sub-tropical estuary evaluated through 210Pb profile and chronostratigraphical markers. Mar Pollut Bull 52:532–539
Molisani MM, Marins RV, Machado W, Paraquetti HHM, Bidone ED, Lacerda LD (2004) Environmental changes in Sepetiba Bay, SE Brazil. Reg Environ Change 4:17–27
Morse JW (1994) Interactions of trace metals with authigenic sulfide minerals: implications for their bioavailability. Mar Chem 46:1–6
Ribeiro AP, Figueiredo AMG, Santos JO, Dantas E, Contrim MEB, Figueira RCL, Silva-Filho EV, Wasserman JC, Santos JO (2013) Combined SEM/AVS and attenuation of concentration models for the assessment of bioavailability and mobility of metals in sediments of Sepetiba Bay (SE Brazil). Mar Pollut Bull 68:55–63
Roberts DA (2012) Causes and ecological effects of resuspended contaminated sediments (RCS) in marine environments. Environ Int 40:230–243
Rodrigues APC, Maciel PO, da Silva LCCP, Almosny NRP, Andreata JV, Bidone ED, Castilhos ZC (2011) Relationship between mercury concentrations in the blood with that in the muscle of four estuarine tropical fish species, Rio de Janeiro State, Brazil. Bull Environm Contam Toxicol 86:357–362
Saulnier I, Mucci A (2000) Trace metal remobilization following the resuspension of estuarine sediments: saguenay Fjord, Canada. Appl Geochem 15:191–210
Simpson SL, Apte SC, Batley GE (2000) Effect of short-term resuspension events on the oxidation of cadmium, lead and zinc sulfide phases in anoxic estuarine sediments. Environm Sci Technol 34:4533–4537
Torres RJ, Abessa DMS, Santos FC, Maranho LA, Davanso MB, Nascimento MRL, Mozeto AA (2009) Effects of dredging operations on sediment quality: contaminant mobilization in dredged sediments from the Port of Santos, SP, Brazil. J Soils Sedim 9:420–432
USEPA (1994) Microwave assisted acid digestion of sediments, sludges, soils and oils. USEPA Method 3051
Van den Berg GA, Meijers GGA, Van der Heijdt LM, Zwolsman JJG (2001) Dredging-related mobilization of trace metals: a case study in the Netherlands. Water Res 35:1979–1986
Wasserman JC, Barros SR, Lima GBA (2013) Planning dredging services in contaminated sediments for balanced environmental and investment costs. J Environ Manage 121:48–56
Acknowledgments
The authors would like to thank to the Rio de Janeiro State Research Foundation (FAPERJ) and the Brazilian Research Council (CNPq) for the financial support and R. C Gonçalves for support in this paper. A.P.C. Rodrigues thanks a grant from PNPD/CAPES
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Rio de Janeiro State Research Foundation (FAPERJ) and the Brazilian Research Council (CNPq) for the financial support.
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Monte, C.N., Rodrigues, A.P.C., Cordeiro, R.C. et al. Changes in Cd and Zn bioavailability upon an experimental resuspension of highly contaminated coastal sediments from a tropical estuary. Sustain. Water Resour. Manag. 1, 335–342 (2015). https://doi.org/10.1007/s40899-015-0034-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40899-015-0034-3