Abstract
Background
Hyaline cartilage damage is an urgent problem for millions of people all over the world. A promising emerging area in the recovery of the hyaline cartilage is the transplantation a modified cell culture in combination with a biodegradable matrix.
Methods
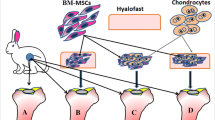
In the present study, chondrogenic differentiation of bone marrow mesenchymal stem cells (MSCs) was achieved by adding 10 ng/mL of recombinant Tgfβ3 protein to the cells during culturing. The resulting cell culture was seeded on a poly(lactic acid) scaffold and transplanted into an experimentally model surface defect of rat hyaline cartilage.
Results
The applying of cellular engineering construct (CEC) with Tgfβ3 protein has led to significant recovery of damaged hyaline cartilage, in contrast to the control group, which has been confirmed by means of histology and scanning electron microscopy. Prolonged observation of the experimental groups treated with CEC has revealed that the initial injury is filled with the newly formed regenerate upon 90 days.
Conclusion
The designed CEC with recombinant Tgfβ3 leads to the formation of a regenerate resistant to external loads with a synthesized extracellular matrix of hyaline cartilage.
Lay Summary
Damages of hyaline cartilage is an urgent problem for millions of people around the world. Current methods of its treatment have not full solved the problem of recovery of the articular surface. At the present time, one of the most promising technique is to use tissue engineering. In our work, we have created a cell-engineered construct (CEC) based on a biodegradable polymer (polylactide) with a culture of mesenchymal stem cells (MSCs) modified with recombinant protein (transforming growth factor beta 3). Further, we transplanted this CEC to an experimental group of rats with a preliminarily created defect of the hyaline cartilage and observed the damage recovery for a long time. We found a decrease the damage size in the experimental group, which indicates the efficiency of this approach. In our future work, we plan to continue with larger animals (rabbits) and to produce out the CEC modification using both a recombinant protein and a lentivirus with increased expression of key genes for chondrogenesis tgfβ3 and sox9.





Similar content being viewed by others
Abbreviations
- CEC:
-
cell-engineered construct
- MSC:
-
mesenchymal stem cells
- Tgfβ3:
-
transforming growth factor beta-3 cytokine
- SEM:
-
scanning electron microscopy
- RNA:
-
ribonucleic acid
- ICRS:
-
International Cartilage Research Society
- DNA:
-
deoxyribonucleic acid
- cDNA:
-
coding DNA
- iPSC:
-
induced pluripotent stem cells
- PCR:
-
polymerase chain reaction
- ECM:
-
extracellular matrix
- SARA:
-
Smad Anchor for Receptor Activation
References
Sakata R, Iwakura T, Reddi AH. Regeneration of articular cartilage surface: morphogens, cells, and extracellular matrix scaffolds. Tissue Eng - Part B Rev. 2015;21(5):461–73. https://doi.org/10.1089/ten.teb.2014.0661.
Rath B, Eschweiler J, Betsch M, Gruber G. Cartilage repair of the knee joint. Orthopade. 2017;46(11):919–27. https://doi.org/10.1007/s00132-017-3463-x.
Ferguson RJ, Palmer AJ, Taylor A, Porter ML, Malchau H, Glyn-Jones S. Hip replacement. Lancet. 2018;392(10158):1662–71. https://doi.org/10.1016/S0140-6736(18)31777-X.
Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage.Nat Rev. Rheumatol. 2015;11(1):21–34. https://doi.org/10.1038/nrrheum.2014.157.
Capeci CM, Turchiano M, Strauss EJ, Youm T. Osteochondral allografts: applications in treating articular cartilage defects in the knee. Bull Hosp Jt Dis. 2013;71(1):60–7.
Bozhokin MS, Bozhkova SA, Netylko GI, Rumakin VP, Nakonechnyi DG, Chepurnenko MN. Morfo-funktsional characteristic of hondroregeneratorny process for articular cartilage injuries. Int J Appl Basic Res. 2017;8(2):302–6. https://doi.org/10.1126/science.139.3549.32.
Krishnan Y, Grodzinsky AJ. Cartilage Diseases. Matrix Biol. 2018;71-72:51–69. https://doi.org/10.1016/j.matbio.2018.05.005.
Bozhokin MS, Bozhkova SA, Netylko GI. Possibilities of current cellular technologies for articular cartilage repair (analytical review). Traumatol Orthop Russia. 2016;22(3):122–34.
Richter DL, Schenck RC, Wascher DC, Treme G. Knee articular cartilage repair and restoration techniques: a review of the literature. Sports Health. 2016;8(2):153–60. https://doi.org/10.1177/1941738115611350.
Erggelet C, Vavken P. Microfracture for the treatment of cartilage defects in the knee joint – A golden standard? J Clin Orthop Trauma. 2016;7(3):145–52. https://doi.org/10.1016/j.jcot.2016.06.015.
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–95. https://doi.org/10.1056/NEJM199410063311401.
Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38(6):1259–71. https://doi.org/10.1177/0363546509346395.
Wakitani S, Nawata M, Tensho K, Okabe T, Machida H. OhgushiH.. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1(1):74–9. https://doi.org/10.1002/term.8.
Derynck R, Budi EH. Specificity, versatility, and control of TGF-b family signaling. Sci Signal. 2019;12(570):eaav5183. https://doi.org/10.1126/scisignal.aav5183.
Hata A, Chen YG. TGF-β signaling from receptors to smads. Cold Spring Harb Perspect Biol. 2016;8(9):a022061. https://doi.org/10.1101/cshperspect.a022061.
Bozhokin MS, Bozhkova SA, Volkov AA. Patent. Device for the formation of standardized defects of the cartilage surface of the joints in the experiment. RU175628U1. https://patents.google.com/patent/RU175628U1/ru.
van den Borne MPJ, Raijmakers NJH, Vanlauwe J. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. OsteoarthrCartil. 2007;15(12):1397–402. https://doi.org/10.1016/j.joca.2007.05.005.
Ruvinov E, TavorRe’em T, Witte F, Cohen S. Articular cartilage regeneration using acellular bioactive affinity-binding alginate hydrogel: a 6-month study in a mini-pig model of osteochondral defects. J Orthop Translat. 2018;16:40–52. https://doi.org/10.1016/j.jot.2018.08.003.
Bozhokin MS, Bozhkova SA, Netyl’ko GI, Nakonechnyj DG, Blinova MI, Nashchekina Yu A. Experimental results of rat hyaline cartilage surface defect replacement with a cell engineering structure. Proc Karelian Res Cent Russ Acad Sci. 2018;4:13. https://doi.org/10.17076/them815.
Haaparanta AM, Järvinen E, Cengiz IF. Preparation and characterization of collagen/PLA, chitosan/PLA, and collagen/chitosan/PLA hybrid scaffolds for cartilage tissue engineering. J Mater Sci Mater Med. 2014;25(4):1129–36. https://doi.org/10.1007/s10856-013-5129-5.
Reed S, Wu BM. Biological and mechanical characterization of chitosan-alginate scaffolds for growth factor delivery and chondrogenesis. J Biomed Mater Res B Appl Biomater. 2017;105(2):272–82. https://doi.org/10.1002/jbm.b.33544.
Daly AC, Critchley SE, Rencsok EM, Kelly DJ. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication. 2016;8(4):045002. https://doi.org/10.1088/1758-5090/8/4/045002.
Gonzalez-Fernandez T, Tierney EG, Cunniffe GM, O’Brien FJ, Kelly DJ. Gene delivery of TGF-β3 and BMP2 in an MSC-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering. Tissue Eng - Part A. 2016;22(9-10):776–87. https://doi.org/10.1089/ten.tea.2015.0576.
Dahlin RL, Meretoja V, Kasper FK, Mikos AG. TGF-β3-induced chondrogenesis in co-cultures of chondrocytes and mesenchymal stem cells on biodegradable scaffolds. Biomaterials. 2014;35(1):123–32. https://doi.org/10.1016/j.biomaterials.2013.09.086.
Ude CC, Shamsul BS, Ng MH. Long-term evaluation of osteoarthritis sheep knee, treated with TGF-β3 and BMP-6 induced multipotent stem cells. ExpGerontol. 2018;104:43–51. https://doi.org/10.1016/j.exger.2018.01.020.
Krstic J, Trivanovic D, Obradovic H, Kukolj T, Bugarski D, Santibanez JF. Regulation of mesenchymal stem cell differentiation by transforming growth factor beta superfamily. Curr Protein Pept Sci. 2017;19(12):1138–54. https://doi.org/10.2174/1389203718666171117103418.
Neybecker P, Henrionnet C, Pape E. In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells. Stem Cell Res Ther. 2018;9(1):329. https://doi.org/10.1186/s13287-018-1071-2.
Zhang X, Wu S, Naccarato T. Regeneration of hyaline-like cartilage in situ with SOX9 stimulation of bone marrow-derived mesenchymal stem cells. PLoS One. 2017;12(6):e0180138. https://doi.org/10.1371/journal.pone.0180138.
Wang M, Yuan Z, Ma N. Advances and prospects in stem cells for cartilage regeneration. Stem Cells Int. 2017;4130607:1–16. https://doi.org/10.1155/2017/4130607.
Ha CW, Noh MJ, Choi KB, Lee KH. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy. 2012;14(2):247–56. https://doi.org/10.3109/14653249.2011.629645.
Ji X, Lei Z, Yuan M, Zhu H, Yuan X, Liu W, et al. Cartilage repair mediated by thermosensitive photocrosslinkable TGFβ1-loaded GM-HPCH via immunomodulating macrophages, recruiting MSCs and promoting chondrogenesis. Theranostics. 2020;10(6):2872–87. https://doi.org/10.7150/thno.41622 eCollection 2020.
He C-X, Zhang T-Y, Miao P-H, Hu Z-J, Han M, Tabata Y, et al. TGF-β1 gene-engineered mesenchymal stem cells induce rat cartilage regeneration using nonviral gene vector. Biotechnol Appl Biochem. 2012;59(3):163–9. https://doi.org/10.1002/bab.1001.
Zhou T, Li X, Guo L, Tian T, Lin S, Shi S, et al. Injectable and thermosensitive TGF-β1-loaded PCEC hydrogel system for in vivo cartilage repair. Sci Rep. 2017;5(7):10553. https://doi.org/10.1038/s41598-017-11322-w.
Ho ST, Hutmacher DW, et al. Rev Biomater. 2006;27(8):1362–76. https://doi.org/10.1016/j.biomaterials.2005.08.035.
Acknowledgements
The authors thank Dmitriy Veryaskin and Tatyana Lievina for their help in writing this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
The protocol of the animal experiments was approved by the Bioethical Committee for conducting biomedical research using animals
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bozhokin, M.S., Bozhkova, S.A., Netylko, G.I. et al. Experimental Replacement of the Surface Defect of Rat Hyaline Cartilage by a Cell-Engineered Construct. Regen. Eng. Transl. Med. 7, 184–193 (2021). https://doi.org/10.1007/s40883-021-00205-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-021-00205-2