Abstract
Fungicide resistance is an alarming challenge for the Brazilian tropical agricultural systems, with major implications for food safety, human and animal health, as well as for the environment. This review explores strategies to address fungicide resistance within the Brazilian agroecosystem context. We examined historical and current scenarios of fungicide resistance in the Brazilian agroecosystems and the approaches to delay the emergence and mitigate the selection of resistant variants. Our review indicates that the prevalence of resistance in field populations of key plant pathogens in Brazil was due to failures in the implementation of preventive measures. To address this issue, alternative evolutionary-smart strategies against fungicide resistance are proposed, emphasizing institutional actions and public policies. Crucial steps involve strengthening national networks for large-scale foliar and seed fungicide efficacy testing and resistance monitoring, as well as imposing tighter restrictions on the labeling of high-risk single-active formulations. Additionally, the integration of non-chemical disease management strategies and the establishment of a centralized database and information system on fungicide resistance in Brazil are identified as essential for effective resistance monitoring and informed decision-making. To enhance fungicide resistance management, the adoption of a warning system (e.g., based on aerobiology- or on weather-monitoring) for predicting disease epidemics and minimizing fungicide applications is recommended. Increased funding, collaboration, mandatory reporting, and capacity building are required to overcome these challenges. In addition, promoting integrated disease management approaches is vital. By implementing these tailored strategies, Brazil can actively contribute to safeguarding its food safety, protecting human and animal health, and preserving the delicate balance of its unique agroecosystem. The adoption of evolutionary-smart strategies against fungicide resistance will prolong fungicide efficacy, reduce economic costs, and minimize environmental impacts, ensuring sustainable and resilient agriculture in Brazil.
Similar content being viewed by others
Fungicide resistance in the Brazilian tropical agroecosystem
Fungicide resistance in the agroecosystem is considered one of the most serious threats to food security (Fisher et al. 2012, 2018). Since the 1970s, resistance to the major classes of modern site-specific selective fungicides in several plant pathogenic fungi species has compromised the management of plant diseases worldwide, limiting fungicide options or even making them unavailable for agriculture (Brent and Hollomon 2007; Thind 2012; Lucas et al. 2015). As fungicide resistance becomes more prevalent, the effectiveness of fungicides decreases, leading to increased crop losses (Thind 2012; Valarmathi 2018; Steinberg and Gurr 2020). Therefore, fungicide resistance can have substantial economic impacts on farmers profits and the country’s trading revenues from agricultural gross domestic product (Corkley et al. 2022). An increase in the number of sprays due to resistance can lead to excessive fungicides use resulting in adverse effects on the environment. Fungicides can contaminate soil, water, and affect non-target organisms, disrupting ecosystems and causing ecological imbalances (Zubrod et al. 2019). Furthermore, continued reliance on ineffective fungicides, by increasing doses and spraying frequency to compensate for resistance, can contribute to pesticide resistance in other non-target organisms, including human and animal fungi pathogens (Fraaije et al. 2021). For instance, public health concerns have been raised on an environmental route of azole resistance selection through an unintended exposure of the human pathogen Aspergillus fumigatus, one of the most ubiquitous species in the environment (Stensvold et al. 2012; Arastehfar et al. 2021; Burks et al. 2021). Agricultural azole fungicides are chemically close to antifungal medicines (Fisher et al. 2018; Burks et al. 2021; Fraaije et al. 2021). In summary, fungicide resistance undermines the long-term sustainability of agricultural systems, food safety and security, human and animal health.
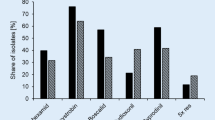
Although fungicide resistance in plant pathogenic fungi is a global threat to food production and security, surprisingly little is known on the prevalence and the evolutionary processes underlying the emergence and spread of fungicide resistance in most of the tropical agroecosystems worldwide (Fisher et al. 2018). Particularly in Brazil, research contributions on the scope and importance of fungicide resistance for most of the locally important pathosystems were scarce and limited to literature reviews until recently (Forcelini et al. 2001; Ghini and Kimati 2002). This scenario has changed in early 2000, as information on fungicide resistance for several plant pathogenic fungi species has started to increase with many cases reported (Table 1, Figs. 1 and 2).
Prevalence of fungicide resistance in populations of 11 plant pathogenic fungi from major crops in distinct Brazilian agroecosystems from 2013 up to 2023.*,**,***. * Comprehensive data on fungicide resistance prevalence in Brazil were compilled for the following plant pathogenic fungi: B. cinerea (Baggio et al. 2018; Maia et al. 2021), C. accutatum (Gama et al. 2020; Moreira et al. 2019), C. musae (Leite et al. 2020; Vieira et al. 2017), C. cassiicola (Mello et al. 2022), Lasiodiplodia spp. (Santos et al. 2019), L. theobromae (Chen et al. 2020; Li et al. 2020), M. fructicola (Dutra et al. 2019, 2020; Fischer et al. 2023; Lichtemberg et al. 2017; Pereira et al. 2017, 2020b; ), N. tropicallis and N. meliosmaemyrianthae (Santos et al. 2021), P. oryzae Oryza lineage (Bezerra et al. 2021; D’Ávila et al. 2021), R. pseudoglycinis (Mathioni et al. 2022), and S. sclerotiorum (Lehner et al. 2015)** Brazilian States colored in green indicate the origin and geographical distribution of the fungal plant pathogen sampling. N indicates the sample size range from each fungal species examined. *** Mean prelavence of fungicide sensitivity and/or resistance, for the combined geographical samples, is represented by a pie chart with distinct colors according to the fungicide classes. Resistance is depicted in red. Sequencial pie charts of the same color indicate a time series evolution in the frequency of fungicide sensitivity and/or resistance in populations of the pathogens
Prevalence of resistance to fungicides QoI, DMI and SDHI in populations of the Asian soybean rust (A), banana Sigatoka disease complex (B) and wheat blast (C) pathogens from Brazil indicating an accelerated evolution for resistance over the past 10 years, from 2013 up to 2023.*,**,***. * Comprehensive data on fungicide resistance prevalence in Brazil were compilled for the following plant pathogenic fungi: A. Phakopsora pachyrhizi (Klosowski et al. 2016b; Mello et al. 2021; Müller et al. 2021; Schmitz et al. 2014; Simões et al. 2018; Stilgenbauer et al. 2023), B. Mycosphaerella fijiensis, M. musicola and M. thailandica (A. G. da Silva (personal communication); Brito 2015; Brito et al. 2020; Gomes et al. 2014; Hanada et al. 2015; Malimpensa 2018; Oliveira et al. 2022; Silva 2023), and C. Pyricularia oryzae Triticum lineage (Castroagudín et al. 2015; Cazón et al. 2023; Dorigan et al. 2019; Poloni et al. 2021; Vicentini et al. 2022a, c).** Brazilian States colored in dark gray or in green indicate the origin and geographical distribution of the fungal plant pathogen sampling. N indicates the sample size range from each fungal species examined.*** Mean prelavence of fungicide sensitivity and/or resistance, for the combined geographical samples, is represented by a pie chart with distinct colors according to the fungicide classes. Resistance is depicted in red. Sequencial pie charts of the same color indicate a time series evolution in the frequency of fungicide sensitivity and/or resistance in populations of the pathogens
A summary covering approximately 10 years of reports, from 2013 up to 2023, on fungicide resistance in populations of distinct plant pathogenic fungi species in major field crops of the Brazilian agroecosystems is presented in Table 1 and Figs. 1 and 2. These fungi species include Botrytis cinerea (gray mold on strawberries), Colletotrichum acutatum (bitter rot on apple and post bloom fruit drop on citrus), C. musae (anthracnose on bananas), Corynespora cassiicola (target leaf spot on soybean), Lasiodiplodia theobromae and other Botryosphaeriaceae species (dieback and stem-end rot of papaya and mango), Monilinia fructicola (brown rot on stone fruits), Mycosphaerella fijiensis (black Sigatoka on bananas), M. musicola (yellow Sigatoka on bananas), M. thailandica (leaf spot on bananas), Neophysopella meliosmaerianthae and N. tropicalis (rust on grapes), Phakopsora pachyrhizi (Asian soybean rust), Pyricularia oryzae Oryzae and Triticum lineages (rice and wheat blast), Ramulariopsis glycines (Ramulariopsis leaf spot on cotton), and Sclerotinia sclerotiorum (white mold on common beans). Though this is not an exhaustive list, as there might be many other plant fungi pathogens for which fungicide resistance has been reported in Brazil, it does provide a snapshot of the findings on the prevalence, the levels, and the mechanisms of resistance to a diversity of site-specific fungicide classes. This information will contribute to understanding the extent and impact of fungicide resistance in Brazilian agriculture and point to the needs for developing effective disease management strategies. It should be pointed out that no resistance was detected between 1999 and 2016 in populations of C. acutatum from citrus, probably due to the adoption of appropriate fungicide mixtures, acting as an anti-emergence strategy against the development of fungicide resistance (Gama et al. 2020).
The data compiled in Table 1 and Figs. 1 and 2 suggest that fungicide resistance is indeed a significant issue as resistance has become pervasive in populations of the major plant pathogenic fungi for the Brazilian agroecosystems. Here, there are some key points that support this assertion: a) High prevalence of fungicide resistance detected in several populations of plant pathogenic fungi, including B. cinerea, C. acutatum, C. musae, C. cassiicola, L. theobromae, M. fructicola, M. fijiensis, M. musicola and M. thailandica, N. meliosmaemyrianthae, P. packyrhizi, P. oryzae Oryzae and Triticum lineages, and R. glycines; b) Distinct classes of the major systemic site-specific fungicides affected by resistance, including dicarboximides, methyl benzimidazoles (MBC), quinone outside inhibitors (QoI), demethylation inhibitors (DMI), and succinate dehydrogenase inhibitors (SDHI). This indicates that resistance was not limited to a specific fungicide group but is found in fungicides with different modes of action; c) The occurrence of multiple (i.e. simultaneous) fungicide resistance, including dual and triple resistance, suggesting the coexistence of resistance mechanisms in the same pathogen population and even in a single individual genotype. Examples of multiple resistance to distinct fungicide classes were observed in C. acutatum, C. cassiicola, L. theobromae, M. fruticola, Mycosphaerella spp., P. packyrhizi, and P. oryzae Oryza and Triticum lineages; d) Increasing prevalence of resistance over time: The data cover a timeline of approximately 10 years, a period that we detected an increase in the prevalence of fungicide resistance over time leading to the fixation of resistance, both within populations and across geographical regions; e) Positive selection favoring the spread of target-site mutations associated with fungicide resistance: For instance, the emergence and subsequent countrywide spread of the specific mutation F129L mutation in the cytB gene associated with QoI resistance, and more recently the SdhC I86F substitution linked to SDHI resistance, both in P. packyrhizi ; f) High stability of resistance under continuous fungicide selection pressure, despite associated fitness penalties: Some studies indicated that resistant isolates may exhibit fitness penalties compared to sensitive ones, suggesting that resistance mechanisms come at a cost to the pathogen, but despite this, resistance still persisted and evolved in agroecosystems (Claus et al. 2022; Klosowski et al. 2016a); g) Stability of resistance in the absence of continued fungicide selection, when there is no fitness penalties (Dorigan et al. 2022; Klosowski et al. 2016a; Leite et al. 2020; Vieira et al. 2017). Overall, these lines of evidence indicate the importance of fungicide resistance in the Brazilian agroecosystems, and its accelerated evolution.
An example of accelerated evolution for fungicide resistance in agriculture can be depicted from the timeline of events spanning the historical evolution for resistance to the three major classes of medium to high risk fungicides in populations of the wheat blast pathogen, Pyricularia oryzae Triticum lineage (PoTl) (Castroagudín et al. 2015; Poloni et al. 2021; Vicentini et al. 2022a) based on several studies (Figs. 2 and 3, Table 1). This timeline virtually began in 1978 with the labeling of the first DMI fungicide for the management of wheat diseases. The first epidemics of wheat head blast disease was reported a few years later with the historic outbreak in Londrina, Paraná State, in 1985 (Ceresini et al. 2018). DMI fungicides were the choice for management of wheat blast due to their systemic prophylactic properties. In subsequent years, a few other DMI fungicides were labeled for controlling wheat diseases, which included propiconazole in 1985, tebuconazole and cyproconazole in the early 1990s, and epoxiconazole in the early 2000s. Despite the reports that fungicides for wheat blast, including the DMIs, were not fully effective on controlling the disease, resistance to DMI fungicides was not explored as a plausible explanation, until 2020 (Poloni et al. 2021). Even so, DMIs were recognized as a medium-risk fungicide for the emergence of resistance (Kuck and Russell 2006; Fungicide Resistance Action Committee (FRAC) 2022b). They were sprayed intensively as single active formulations, probably with no precautions of adopting anti-resistance strategies, at least not on-label until 2012 (Ministry of Agriculture, Livestock and Supply (MAPA) / Coordination of Pesticides and Related Products 2012). Only in 2012 pesticide labeling policies requiring risk assessment of resistance for pesticides and commercial labels containing resistance management recommendations were introduced in Brazil. Furthermore, DMIs were intensively used in calendar-based sprays programs, up to five sprays, on-label, per cropping season, targeting several wheat diseases, including rusts, necrotic leaf spots, Fusarium head blight, and wheat blast (Ministry of Agriculture, Livestock and Supply (MAPA) 2023). With basically non-stop selection pressure for up to 30 years of DMI fungicides sprays (totalling approximately 150 sprays), the evolutionary outcome was the emergence, selection and widespread distribution of a highly resistant PoTl population across all wheat producing areas in Central-southern Brazil (Poloni et al. 2021; Figs. 2 and 3, Table 1).
Timeline depicting the emergence of the wheat blast pathogen Pyricularia oryzae Triticum lineage in South America in the mid 1980’s and the events leading to the accelerated evolution and the widespread distribution of fungicide resistance in Brazil.*,**. *Colored shappes indicate the dates of the events (ribbons) and the order in the timeline (numbered circles). Green colored ribbons indicate events associated with the labelling, deployment of DMI fungicides and reports of resistance. Blue colored ribbons indicate events associated with the labelling, deployment and reports of resistance to QoI fungicides. The red colored ribon indicates the date MAPA began requiring labels with anti-resistance strategies on agricultural fungicides. Bege colored ribbons indicate events associated with the labelling, deployment and reports of resistance to the recent labeled SDHI fungicides. ** Source: Castroagudín et al. 2015; Cazón et al. 2023; Ceresini et al. 2018, 2019; Dorigan et al. 2019; Poloni et al. 2021; Vicentini et al. 2022a, c
The evolutionary outcome of resistance to the high risk QoI fungicides in PoTl populations was somewhat distinct, as it happened quicker. Azoxystrobin was the first QoI fungicide labeled in Brazil in early 2000s (Fig. 3). Although the first report of QoI resistance was in 2015, the study included resistant PoTl isolates sampled in 2005, only five years after the labeling for management of wheat diseases (Castroagudín et al. 2015). QoI resistance is now widespread in all wheat cropping regions from Brazil (Castroagudín et al. 2015; Vicentini et al. 2022a) (Figs. 2 and 3, Table 1). Similarly to the scenario that resulted in resistance to DMIs, the high risk QoI fungicides were sprayed intensively on calendar-based schedules as a single active formulation, exerting a high selection pressure on populations of PoTl (Castroagudín et al. 2015; Vicentini et al. 2022a).
The adoption of the official on-label recommendations of anti-resistance strategies described in Figs. 4 and 5 (such as rotating fungicides with distinct modes of action, carry out frequent disease monitoring on the cropping area, adopt integrated disease management strategies) has failed to delay the emergence and spread of fungicide resistance in PoTl populations since they lacked three key strategies: a) Fungicides that had lost efficacy due to resistance should be withdrawn from the spraying portifolio, specially when supported by large scale monitoring of fungal population across the country; b) The lack of an official chanel to report the inicial emergence of fungicide resistance at local (field) scale, before it becomes widespread; c) The lack of an alternative system to prevent unnecessary calendar-based fungicide spraying hindered the on-label anti-resistance strategy primary goal of reducing the selection pressure on the pathogen’s populations. (Vicentini et al. 2022a)., Additionaly, they did not emphasize the recommendation of spraying multisite fungicides as an important anti-resistance IPM component (Fig. 5) and often one of the few fungicides that will work when resistance to the single-sites becomes widespread (Brent and Hollomon 2007; Brent 2012; Thind 2012).
Description of the effects and major characteristics of (A) anti-emergence and (B) anti-resistance strategies against fungicide resistance in populations of plant pathogens in the agroecosystem.**.
*MAPA, together with the Ministry of Environment and the Brazilian Health Regulatory Agency (Anvisa), as regulatory bodies, oversight the labiling of fungicides formulations for controlling crop diseases, including fungicides co-formulations. For this reason, co-formulations attend the standards of agronomical efficacy, public health safety and reduced environmental impact. MAPA’s pesticide databank on currently labeled fungicides registered for agricultural use in Brazil (https://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons) contains a total of 410 fungicide formulations, from which 172 are co-formulations. The co-formulations of single-site at-risk fungicides with multisite low risk fungicides totalled 26 products, including double or triple co-formulations with chlorothalonil, copper oxychloride, or mancozeb and: azoxystrobin; azoxystrobin + cyproconazole; azoxystrobin + difenoconazole; azoxystrobin + prothioconazole; azoxystrobin + tebuconazole; benalaxil; cymoxanil; cyproconazole + picoxystrobin; difenoconazole; difenoconazole + trifloxystrobin; fluxapyroxad; fluxapyroxad + prothioconazole; imipifloxam + metominostrobin; tebuconazole; tebuconazole + trifloxystrobin; and thiophanate-methyl. The other 146 co-formulations are mixtures of multisite fungicides only, or mixtures of single site at-risk fungicides.
**These anti-resistance strategies indicated in bold are similar to ones proposed in the Brazilian official fungicide labels described in Fig. 5, except for the contents in brackets
(A) General and (B) specific recommendations of fungicide resistance management strategies included in the Brazilian official label leaflets of commercial fungicide formulations (Ministry of Agriculture, Livestock and Supply (MAPA) 2023).* *Contents in brackets were included by the authors for better explaining the information quoted from the original label leaflet. Text in bold emphasizes the strategy. According to the definitions proposed in Fig. 4, these are mainly anti-resistance strategies, except for the strategies indicated in red, which contradicts the principle of minimizing unnecessary fungicide sprays to reduce selection pressure against the pathogen populations
In recent years, new fungicide formulations have been labeled and introduced for the management of wheat diseases in Brazil. These fungicides include the second-generation carboxamide fluxapyroxad, a SDHI that is considered at medium risk for fungicide resistance emergence (FRAC 2022a). Unexpectedly from the point of view of anti-resistance strategies, since 2017 these fungicides have been co-formulated with high risk QoI or moderate risk DMI molecules (pyraclostrobin or epoxiconazole), for which resistance has been reported for PoTl populations (Vicentini et al. 2022a; MAPA – Ministério da Agricultura Pecuária e Abastecimento - Brazil 2023). This labeling strategy represented a continuing selection pressure favoring the survival of DMI and QoI resistant strains of PoTl, that could have contributed to the onset of resistance to SDHIs (Figs. 2 and 3, Table 1) because of the co-formulation with fungicides molecules that had lost the efficacy (Vicentini et al. 2022a). In fact, SDHI-resistant isolates were detected in both older pre-SDHI (2012) and newer post-SDHI (2018) populations of the wheat blast pathogen (Vicentini et al. 2022a).
Evolutionary drivers and trajectory of fungicide resistance in the agroecosystem
Fungicides resistance can emerge in populations of target plant pathogenic fungi soon after exposure to a fungicide (Deising et al. 2008; Lucas et al. 2015; Yin et al. 2023). Fungicide resistance can be defined as “the stable new and heritable trait associated with reduction in the sensitivity of an individual fungus to a specific fungicide” (Delp and Dekker 1985; McGrath 2004).
The emergence of fungicide resistance in populations of plant pathogens in the agroecosystem is a dynamic evolutionary process by which the frequency of resistance alleles changes over time, which is likely to affect the field efficacy of fungicides (Yin et al. 2023). These evolutionary changes usually result either in advantages or disadvantages for the pathogen’s survival, growth, and reproduction under fungicide selection stress or not (Hawkins and Fraaije 2018).
Fungicide resistance appears to evolve from new point mutations (de novo mutations) in genes encoding the target site. In the simplest cases, such as the QoI resistance, fungicide resistance evolves when a single mutation conferring a high level of resistance with negligible adaptive costs emerges and is selected for in a population of a plant pathogen (Lucas et al. 2015). However, the resistance to some fungicides, such as the DMIs, is generally more complex, as this simpler scenario of single point mutation determining resistance usually does not occur. For instance, a total of nine mutations detected in the CYP51 (F120L, V130A, Y131F/H, K142R, I145V/F, F154Y, I475T) in the ASR pathogen P. pachyrhizi, and present in different combinations, determines the level of DMI sensitivity (Stilgenbauer et al. 2023). Furthermore, the limited knowledge on mutations for fungicide resistance outside the range of known target genes is still challenging for understanding the pathways to resistance to particular fungicides, such as the DMIs. As whole-genome sequencing data in combination with association mapping become more accessible, these more comprehensive analytic tools will help to reveal the multilocus genetic architecture of fungicide resistance in populations of important fungal pathogens (Pereira et al. 2020a). The unique application of these combined analytic tools revealed significant differences in azole resistance among global field populations of the important wheat pathogen Parastagonospora nodorum. These populations evolved distinctive combinations of azole resistance alleles, including polymorphisms in major facilitator superfamily transporters, which could interact when co-occurring in the same fungal genetic background (Pereira et al. 2020a).
The evolution towards fungicide resistance in populations of plant pathogens can be split into an emergence phase and a selection phase (Hobbelen et al. 2014). In the emergence phase, the resistant strain arises by mutation and subsequently invades the pathogen population. At this stage, the number of lesions derived from fungicide-resistant strains is very small and resistant strains may become extinct as a result of a simple stochastic variation, although fungicide sprays can provide higher adaptability to resistant strains than to sensitive ones. In the selection phase, a resistant strain is already present in a pathogen´s population and is positively selected by the spray of specific fungicides (Hobbelen et al. 2014).
Under the effect of single-site fungicides, where a single mutation in the target protein can confer a high level of resistance, a qualitative phenotypic change in the pathogen population usually results in two populations with a bimodal distribution for sensitivity. With multi-site fungicides, or with some single-site fungicides where more than one allele contributes to resistance, a unimodal distribution with quantitative changes is observed (Georgopoulos and Skylakakis 1986; Deising et al. 2008; Lucas et al. 2015). In both cases, directional selection is observed towards lower sensitivity acting on discrete variation, in the case of qualitative resistance. In contrast, a continuous distribution for quantitative resistance is observed by gradual changes towards resistance over time (Georgopoulos and Skylakakis 1986; Deising et al. 2008; Lucas et al. 2015).
The agroecosystem under conventional disease management is considered a hotspot for the emergence of fungicide resistance. Five main factors associated with conventional disease management contribute to the emergence and spread of fungicide resistance in the agroecosystem: a) Monoculture farming. While this approach simplifies management and harvesting, the highly homogenous and non-fragmented tropical agricultural landscape favors the emergence and spread of fungicide resistance (Papaïx et al. 2015). In fact, the extensive use of fungicides with a similar mode of action on a homogeneous and highly connected cropping area over multiple seasons provides strong selection pressure that favors the survival and proliferation of resistant strains (Pretty and Bharucha 2014; Valarmathi 2018). b) Dependence on a limited number of fungicides mode of action: Conventional disease management in agriculture often relies heavily on a small number of at risk fungicides. When these fungicides are used repeatedly and exclusively, populations of plant pathogenic fungi with emerging de novo mutations or natural standing genetic variation for fungicide resistance can rise by selection followed by adaption. Limited fungicide options also limit the possibility to rotate or mix different fungicides, which can help mitigate resistance development (van den Bosch et al. 2014b). c) Improper fungicide application: Incorrect application techniques, such as inadequate dosages or improper timing, can contribute to the emergence of resistance. Stress from exposure to sub-lethal doses of fungicides can cause genomic instability in fungi, accelerating the emergence of fungicide resistance or other adaptive traits in populations of fungal plant pathogens (Gambhir et al. 2021). Subletal doses of fungicides can also increase selection in pathogen populations, allowing resistant strains to survive and proliferate. In addition, inadequate coverage of crops during application can leave certain areas untreated, favoring resistant lineages (Brent and Hollomon 2007; Hollomon 2015a, b). d) Lack of diversity in disease management strategies: Over reliance on fungicides as the primary means of disease control, without integrating other management practices, can contribute to the emergence of resistance. Integrated Pest Management (IPM) approaches, which combine several strategies such as crop rotation, resistant varieties, cultural practices, and biological control, can help reduce the dependance on chemical fungicides and minimize the risk of resistance development (McDonald and Linde 2002; Stukenbrock and McDonald 2008; Lucas et al. 2015). e) Limited research and development of new fungicides: The slow pace to develop new fungicides, coupled with the rapid evolution of resistance in populations of plant pathogenic fungi, further exacerbates the problem. The limited availability of novel fungicides hampers the ability to effectively control resistant strains and restricts the options for disease management (Corkley et al. 2022; FRAC 2022b).
Three major drivers can determine the rate of evolution and the fate of fungicide resistance in populations of plant pathogens (Fisher et al. 2018). The first major driver is the heritable genetic variation for fungicide resistance, either as naturally occurring genetic variation or de novo fungicide-driven mutations that subsequently sweeps through the fungal population. The accelerated emergence of de novo alleles confering fungicide resistance in fungi has been associated with the use of sublethal doses of fungicides, which can act as a genomic stressor and promote mutagenesis (Boyce et al. 2017; dos Reis et al. 2019; Gambhir et al. 2021; Healey et al. 2016). The levels of heritable genetic variation for fungicide resistance are dependent on the pathogen effective population size, derived primarily from both historical and current genetic variation processes, including sexual recombination and mutation rates. This also depends on the potential for gene flow, ranging from a regional to a global dispersal of fungicide resistant strains by trade in infected seeds, by which new genes and genotypic diversity are introduced (Fisher et al. 2018).
The second major evolutionary driver for fungicide resistance is the high reproductive rate of fungal pathogens in the agroecosystem (Fisher et al. 2018), which is usually rapid in the agriculture environments as a result of the high genetic homogeneity of host plants in extensive monocultures of susceptible varieties and intensive fungicide use (McDonald and Linde 2002; Croll and McDonald 2017).
The third major evolutionary driver is the differential survival of resistant lineages under strong selection pressure by fungicide sprays lacking chemical diversity, over a long course of prophylactic and/or empirical, and repeated, treatments with fungicides with the same mode of action. Predictions regarding the fate of evolution towards fungicide resistance in field populations of plant pathogens depend on the adaptive cost of mutations associated with resistance (Fisher et al. 2018). To persist in the field, resistant mutants must be pathogenic in planta and competitive against other strains of the pathogen and other microorganisms (Brasseur et al. 1996). As a consequence, mutations that confer high levels of resistance, with lower adaptive cost, are positively selected and tend to persist and become prevalent in the field over time (Hobbelen et al. 2014; Lucas et al. 2015; Hawkins and Fraaije 2018). If there is an adaptive cost, discontinuance of fungicide spray in the field could restore the sensitivity of populations of plant pathogenic fungi. This was detected for M. fructicola in stone fruit orchards from Brazil after discontinuing the use of the MBC thiophanate-methyl for seven years (Fischer et al. 2023) or the DMI tebuconazole for at least three years (Pereira et al. 2020b).
Therefore, fungicide resistance often provides an adaptive advantage to plant pathogen populations under fungicide selection pressure, but mutations that confer resistance can also result in adaptive penalties, such as an evolutionary trade-off (Hobbelen et al. 2014; Lucas et al. 2015; Hawkins and Fraaije 2018). These penalties come from functional restrictions arising from the evolution of a target site or from the costs of reallocating cellular resources through gene overexpression or active transport of the fungicide (Hawkins and Fraaije 2018). The distinct complement of resistance mutations present in populations of plant pathogenic fungi can be complex and challenging in predicting the evolutionary trajectory of fungicide adaptation (Pereira et al. 2020a). The analysis of adaptive landscapes from an evolutionary point of view, combined with genomic-functional tools to investigate the effects of mutations individually and in different combinations, allows a better understanding of the evolutionary trajectories of plant pathogen populations under fungicide selection pressure (Hawkins and Fraaije 2018; Pereira et al. 2020a).
Risks of resistance based on the fungicide chemical mode of action
It has been widely accepted that the chemical mode of action of fungicides is a key factor determining the risks for the development of resistance in populations of plant pathogens (Brent and Hollomon 2007; Brent 2012; Thind 2012). Based on this criterion, there are systemic site-specific fungicides, which interrupt particular cellular processes and bind to specific target proteins such as the MBCs, e.g. thiophanate-methyl and carbendazim; the DMIs, e.g. the azoles epoxiconazole, propiconazole, tebuconazole and others; the QoIs, e.g. azoxystrobin, pyraclostrobin and trifloxystrobin; the SDHIs, e.g. bixafen, boscalid, fluxapyroxad (Brent 2012; Thind 2012). These fungicides are considered to possess medium to high risk for resistance because selection within a population of a plant pathogenic fungus favoring mutants with a single mutation in the target gene could result in loss of fungicide efficacy (Brent and Hollomon 2007; Grimmer et al. 2014, 2015). Therefore, high risk is associated with the high selection pressure exerted by the single-site fungicides as a consequence of their specific mode of action, their high efficacy (i.e., high activity at low doses) and their intensive (and extensive) usage, thus accelerating the evolution for resistance in populations of plant pathogens (Grimmer et al. 2014, 2015). These fungicides contrast with the class of multisite fungicides [the copper-based fungicides, e.g. copper oxychloride; the dithiocarbamates, e.g. mancozeb; and the tetra phthalonitriles, e.g. chlorothalonil] targeting and interfering with a range of cellular and metabolic processes, and that are considered to have lower risk for resistance. Unlike single-gene mutations that confer resistance to single-site fungicides, the occurrence of mutants with simultaneous mutations in multiple target genes would be needed to confer resistance to multisite fungicides (Brent and Hollomon 2007; Brent 2012; Thind 2012). Hence, resistance to multisite fungicides is rare and its mechanisms are not known.
However, the chemical mode of action of a fungicide cannot be considered as the single parameter defining the risk of resistance. For instance, a risk assessment model proposed by the FRAC considers a matrix to calculate the risk of resistance to fungicides, initially based on three criteria derived from practical experience: a) the risk associated with the chemical mode of action of the fungicide; b) the risk attributed to the plant pathogen; and c) the agronomic risk, an indication of the favorability of the agroecosystem (Kuck and Russell 2006; Brent and Hollomon 2007). However, this risk matrix model for fungicides proposed by FRAC showed no correlation with the observed number of years prior to the emergence of resistance (rs = -0.06, p = 0.6474) (McDonald and Linde 2002), and has limited predictive value within the dominant category of high risk fungicides (Grimmer et al. 2014).
In contrast, a more thorough risk assessment model proposed by Grimmer et al. (2015) included the identification of key characteristics as important determinants of resistance risk. This model includes the pathogen’s latent periods in the year [a measure of the duration of the disease epidemic divided by the time between infection and pathogen reproduction], the number of plant species and cultivars infected by the pathogen [narrow versus wide host range, with more intensive fungicide selection active for the latter], production under protected versus open field cropping systems [with higher selection of resistant strains indoors] and the complexity of the fungicide molecule [where high-complexity molecules exhibiting higher target-site binding specificity are more likely to be compromised by small changes in efficacy]. The model combining these key features explained 61% of the temporal variation for the emergence of resistance to high-risk site-specific fungicides. Risk assessment based on these key characteristics could then be used to determine resistance risk for fungicides with novel modes of action, for which there is no prior information on resistance behavior (Grimmer et al. 2015).
Risk for fungicide resistance based on the evolutionary adaptive potential of plant pathogens
An evolutionary risk model based on population biology parameters, such as population size, reproductive rate, reproductive mode, gene flow and long-distance dispersal capacity was a better predictor of evolution of plant pathogenic fungi towards fungicide resistance (rs for pathogen migration parameters = -0.72; p = 0.0001) than the former one based on a risk matrix (McDonald and Linde 2002). In conclusion, though risk matrix-based models can offer a general guide to risk, they cannot predict when or where fungicide resistance will occur, or how quickly it may spread and compromise plant disease management decisions. Such predictions require precise measurements of the adaptive potential for fungicide resistance, which can be derived from estimates of genetic variance for a fitness-related trait (van den Bosch and Gilligan 2008; Willi et al. 2011; Ferro et al. 2020), the population size and mutation rates, the predominant reproductive mode [from sexual to clonal, outcrossing to selfing, or mixed), the extent of gene flow (McDonald and Linde 2002), and the selection coefficient [determined by the difference in adaptability of sensitive strains in relation to resistant to a given fungicide (van den Bosch et al. 2014a)], as well as on other factors influencing the survival and invasion of resistant strains (Gubbins and Gilligan 1999).
Therefore, fungicide resistance cases provide fascinating evidence of accelerated evolution under strong selection pressure derived from intensive fungicide sprays. This can be used to address fundamental questions regarding the evolutionary origins of resistance and the adaptive potential of populations of plant pathogens to new molecules (Lucas et al. 2015). For instance, (a) whether this adaptive potential stems primarily from new mutations or pre-existing variation; (b) which pre-existing traits in plant pathogen populations could form the basis of resistance adaptations; and (c) whether the recurrence of common resistance mechanisms among plant pathogen species results from outcrossing and horizontal gene transfer or from independent parallel evolution (Hawkins and Fraaije 2018).
Major fungicide resistance mechanisms
To understand the emergence of fungicide resistance in field populations of plant pathogens, the mechanisms that result in reduced sensitivity and the genetic basis of resistance should be determined. Eight mechanisms associated with the development of resistance to fungicides in populations of plant pathogenic fungi have been described so far (Hu and Chen 2021). These mechanisms can be: a) Conformational changes in the protein target site due to mutations in the gene´s coding region, a major mechanism particularly for specific single-site fungicides such as MBC, DMI, QoI and SDHI (Lucas et al. 2015); b) Overexpression of the target site protein, which results in an increase in the fungicide inhibitory concentration (Cools et al. 2012); c) Non-target site mechanism, such as alternative respiration pathways triggering the synthesis of alternative oxidase (AOX) thus providing a QoI-insensitive pathway for oxidation of NADH (Wood and Hollomon 2003); d) Efflux of fungicides, usually involved in multidrug resistance and often associated with overexpression of efflux transporters with broad substrate specificity; these efflux transporters are members of the ATP-binding cassette (ABC) or major facilitator superfamily (MFS) transporter proteins (Rajendran et al. 2011; Perlin et al. 2014); e) Paralog re-emergence as an adaptive pathway, by which a historically contingent dispensable paralogous gene that determine fungicide resistance present at low levels in natural populations of plant pathogens at the point when selection pressures changed its frequency due to anthropogenic fungicide sprays (Hawkins et al. 2014; Mair et al. 2016b; Steinhauer et al. 2019); f) Regulation of environmental stress response in fungi, such as the osmosensors in the high osmolarity glycerol (HOG) pathway, interfered by phenylpyrroles, including fludioxonil and fenpiclonil (Kilani and Fillinger 2016), and mutations in the same osmotic sensitivity loci oftentimes lead to resistance to phenylpyrroles (Zhang et al. 2002); and g) Fungicide degradation by detoxification via metabolic enzymes, which is less common in fungi (Sang et al. 2018), although reported for herbicide resistance in grasses (Cummins et al. 2013) and is also common for insecticide resistance (Nardini et al. 2012). We will discuss in more details the mechanisms associated with fungicide resistance in three major pathosystems for the Brazilian tropical agroecosystem.
Fungicide resistance scenario in three major Brazilian pathosystems
Asian soybean rust
Since its first introduction in Brazil in 2001 and emergence as a major crop disease, the Asian soybean rust (ASR) caused by the obligate parasite Phakopsora pachyrhizi (Pp) has spread widely and became the most important soybean disease in the country (Yorinori 2021a). Further, the disease has caused yield losses of up to 90% on susceptible varieties under favorable weather conditions when fungicides were not applied (Juliatti and Zambolim 2021). Yield losses have been recurrent along the years, ranging from a minimum of 363.5 thousand tons in 2011/2012 (≅ 0,6%) up to a historical 4.6 million tons in 2003/2004 (≅ 9,6%), heavily impacting the Brazilian economy which is based mainly on the export of commodities (CONAB 2004, 2013; Godoy et al. 2016; Juliatti and Zambolim 2021). With the crop distributed throughout the country, epidemics of the disease are very common in different agroecosystems where the fungus can survive all year round on soybean volunteer plants (Fanaro et al. 2011; Garcés Fiallos 2011a, b; Yorinori 2021b). Mandatory regulation enforcing fallow cropping (a soybean-free period of 60 to 90 days in the off-season) was adopted to restrict the occurrence of late-season soybean volunteer plants and consequently reducing the survival of inoculum between growing seasons (MAPA / Secretariat of Agricultural Defense (SDA) 2021a, 2021b, 2022). Despite these measures, 573 ASR infected field sites were reported in the 2021/22 cropping season (≅ 23% of the country’s soybean fields), which was 41.5% higher than 2020/21 and the highest in the past decade (Consórcio Antiferrugem 2023).
Major host resistance genes have been mapped and incorporated in the soybean cultivars (Li et al. 2012; Childs et al. 2018; Lin et al. 2022). However, this resistance has not been durable and stable due to the rapid breakdown of resistance genes by the emergence and selection of new compatible virulent fungus genotypes (Hartman et al. 2005; Yorinori et al. 2005; Akamatsu et al. 2013, 2017; Yorinori 2021c). Therefore, disease management has relied mainly on chemical control with systemic fungicides, but the fungicide efficacy has decreased steadily over the past two decades in Brazil from complete control to only around 20% efficacy (Godoy et al. 2016; Dalla Lana et al. 2018; Barro et al. 2021). This is probably due to the emergence of resistance to the two major classes of fungicides, i.e. QoIs and DMIs (Schmitz et al. 2014; Müller et al. 2021). The emergence of fungicide resistance can be attributed to high selection pressure on the ASR pathogen populations, as a response to large-scale use of calendar-based prophylactic fungicide spray programs (Godoy et al. 2016; Yorinori 2021d). Due to problems faced by the current fungicide-dependency and the lack of ASR control, the Brazilian MAPA, followed by the Paraná Agricultural Defense Agency (ADAPAR), suspended the recommendation of 63 mixtures of commercial fungicides to control the disease (ADAPAR 2015; MAPA / Secretariat of Agricultural Defense (SDA) 2016). Despite this, the chemical control of ASR with fungicides still represents a cost of up to US$ 2,2 billion per year for the Brazilian soybean industry (Godoy et al. 2016; Yorinori 2021a, e; Ishikawa-Ishiwata and Furuya 2021).
As odd as it seems, it was only after resistance to the systemic single-site DMI and QoI fungicides became widespread in Brazil (Schmitz et al. 2014; Müller et al. 2021) that the chemical management of ASR has began to rely on old broad-spectrum protectant multisite fungicides, such as copper and dithiocarbamates, with some acceptable efficacy (Godoy et al. 2016; Juliatti et al. 2017; Netto et al. 2020). On the other hand, the SDHI fungicides, the latest group of systemic fungicides introduced in the Brazilian market in 2013 are available for management of soybean diseases. However, considered as medium to high-risk fungicide group for selecting resistance in exposed populations (Simões et al. 2018; Borba 2020), they were introduced in mixtures with QoI and DMI fungicides, for which shifts in sensitivity had already been detected in Pp populations (Klosowski et al. 2016a, b, 2018; Müller et al. 2021). Perhaps not surprisingly, insensitivity to SDHI had already been reported in Pp isolates from Brazil shortly after the labeling of these fungicides (Simões et al. 2018; Müller et al. 2021).
Fungicide resistance mechanisms in the ASR pathogen
Resistance to QoI and DMI fungicides is widespread in Brazil, and has been reported in the states of Goiás (GO), Mato Grosso (MT), Mato Grosso do Sul (MS), Minas Gerais (MG), São Paulo (SP), Paraná (PR) and Rio Grande do Sul (RS) (Klosowski et al. 2016b, 2018; Müller et al. 2021) (Fig. 2A, Table 1). This distribution and prevalence of resistance is probably associated with a countrywide concerted pattern of fungicide recommendation from similar mode of action over two decades (MAPA 2023), or perhaps due to the pathogen efficient long distance dispersal (Twizeyimana et al. 2011). For the QoI-resistant strains, a cytB mutation resulting in the cytB b F129L substitution was detected as prevalent in Brazil (Klosowski et al. 2016b) (Table 2). Multiple target site mutations detected in the CYP51 gene of the DMI-resistant strains (F120L, V130A, Y131F/H, K142R, I145V/F, F154Y, I475T, totalling nine mutations), as single or in combinations of double or triple mutations, determine the specificity and the levels of sensitivity to DMIs (Klosowski et al. 2016a, 2018; Müller et al. 2021; Stilgenbauer et al. 2023) (Table 2). CYP51 overexpression can also reduce the sensitivity to DMIs, as reported for other rust fungi (Stammler et al. 2009). For the first time since the recent deployment of SDHIs, reduced sensitivity to SDHIs was detected in Pp isolates sampled during the 2015/2016 cropping season (Müller et al. 2021). The insensitive isolates carried a mutation in the SdhC gene, resulting in the SdhC-I86F target alteration (Müller et al. 2021) (Table 2). A selective advantage of this mutation is probably responsible for the accumulation of this allele and its fast spread in soybean fields across Brazil from 2015 through 2019, under fungicide pressure (Mello et al. 2021). The mutation in the SdhC gene reached similar relevance as the mutations for QoI and DMI resistance.
Banana Sigatoka disease complex
The Banana Sigatoka disease complex (BSDC) includes the Black and Yellow Sigatokas. In Brazil, Black Sigatoka [caused by Mycosphaerella fijiensis (Mf) (syn. Pseudocercospora fijiensis)] was first reported in 1998 in the Amazon region (Gasparotto et al. 2000; Brito et al. 2015). Since then, the disease has been detected in 19 states (Uchôa et al. 2021), including the two most important banana producing states: São Paulo (Ferrari et al. 2005) and Bahia (Ramos et al. 2018). Black Sigatoka is regarded as the major constraint to banana production, reducing yield up to 100% (Brito et al. 2015; Nomura et al. 2020). However, some reports indicate that the Black Sigatoka may have been misdiagnosed as the less devastating Yellow Sigatoka [caused by M. musicola (Mm) (syn: P. musae)] (Gomes et al. 2013). Yellow Sigatoka, first reported in the Amazon region in 1944, is more widespread and known to be present in all banana-growing regions from Brazil (Gomes et al. 2018). Yield losses of up to 50% have been reported for yellow Sigatoka (Brito et al. 2015; Nomura et al. 2020). In addition, M. thailandica (Mt) (syn. Parapallidocercospora thailandica) (Crous et al. 2004; Arzanlou et al. 2008), highly prevalent in Ribeira Valley, SP (Malimpensa 2018), and the less frequent eumusae leaf spot pathogen M. eumusae (syn. P. eumusae) (Carlier et al. 2000; Brito et al. 2015, 2020) can also cause leaf spots on bananas and are present in Brazilian banana producing regions. Black and Yellow Sigatoka are still considered the two most relevant diseases of the BSDC in Brazil, though (Santos 2005; Rocha et al. 2012; Nomura et al. 2020; Oliveira et al. 2022).
BSDC are polycyclic diseases and the pathogens Mf and Mm have a mixed reproductive system, with a predominant clonal epidemic dispersal via conidia and a cyclic sexual reproduction followed by the release of ascospores (Burt 1994; Beltrán-García et al. 2014). Populations of Mf and Mm from Brazil, Mexico, and the Philippines have high genotypic variation arising both from sexual reproduction and gene flow originating from distant migration of the pathogens (Brito et al. 2015; Gomes et al. 2018; Manzo-Sánchez et al. 2019; Mendoza and Ardales 2019). Genetic resistance to BSDC is absent or partial in most of the commercial banana cultivars (Churchill 2011). Therefore, disease control strategies are mainly based on programmed calendar-based systemic or protectant fungicides sprays (Brito et al. 2015). Up to 52 sprays of protectant or 26 sprays of systemic fungicides can be applied per year under high disease pressure, particularly in Costa Rica and Ecuador (Malimpensa 2018; Uchôa et al. 2021; Brito et al. 2015). Contrastingly, in commercial banana plantations from the Ribeira Valley, Brazil, the control of Black Sigatoka is made by weekly monitoring of the disease, which results in as much as 15–20 fungicide sprays per year (Uchôa et al. 2021). Sigatoka control is highly dependent on frequent use of the systemic site-specific QoI and DMI fungicide applications (Churchill 2011). The consequences of the excessive use of fungicides are increased production costs, a negative impact on the environment, and a high selective pressure on pathogen populations, which can lead to emergence, selection and spread of fungicide resistant strains (Cañas-Gutiérrez et al. 2009; Grice et al. 2013; Diaz-Trujillo et al. 2018; Brito et al. 2020; Oliveira et al. 2022).
Fungicide resistance in BSDC pathogens
In the last decade, only a limited number of studies have been published on fungicide resistance in Mf , Mm, and Mt, mostly for QoI and DMI fungicides, besides a single contemporary study with reduced sensitivity to SDHIs (Fig. 2B, Tables 1 and 2). QoI resistance based on cytB G143A has developed rapidly in Mf populations in several countries since 2000 (Sierotzki et al. 2000; Amil et al. 2007), while the first report for Mm populations is from 2012 in Australia (Grice et al. 2013). In Brazil, no QoI resistance was detected in populations of Mf from the Amazon (Northern Brazil) and from Ribeira Valley in São Paulo (Southeastern Brazil, SP), as well as in populations of Mm from the Federal District (Central Western) and São Paulo states sampled as early as 2008 and as recently as 2018 (Gomes et al. 2014; Brito 2015; Hanada et al. 2015). In a more recent survey, a total of 10.0%, 9.4% and 85% of all isolates of Mf, Mm and Mt, respectively, sampled from banana fields under different fungicide spray regimes at four distinct locations in São Paulo and Minas Gerais states (Southeastern Brazil) were QoI resistant carrying the G143A substitution in cytB (Oliveira et al. 2022). Pathogens populations from the field where conventional or intensive use of fungicides was done had a higher frequency of resistant isolates than populations from no fungicide input. The species M. thailandica, in particular, was highly prevalent in the populations from Ribeira Valley, representing more than 45% of the isolates sampled independently in 2018 and in 2021 from leaves with Black Sigatoka like symptoms, and most of these isolates were QoI-R (Malimpensa 2018; Oliveira et al. 2022).
Although studies on DMI fungicides resistance phenotypes linked to mutations in the corresponding target genes were largely scarce in Brazil until 2015, there are evidences of reduced sensitivity in the same Mf populations from the Amazon back to 2008 - 2009 (Gomes et al. 2014) and 2015 (Hanada et al. 2015) (Table 3). Concerning the mechanisms of resistance to DMI fungicides in Mf and Mm, CYP51 changes have been reported elsewhere for both species (Cañas-Gutiérrez et al. 2009; Brito et al. 2020), while CYP51 overexpression associated with different tandem repeats in CYP51 promoter sequences has also been reported for Mf (Diaz-Trujillo et al. 2018). In Brazil, the target site alterations CYP51 G462D and Y463H (Malimpensa 2018), and CYP51 T18I, V106D, Y461D and Y463D (A. G. da Silva, personal communication) in Mf, CYP51 A381G, Y461N and Y463H (Brito 2015; Malimpensa 2018; Brito et al. 2020), and CYP51 V106D, Y136F, A446S, Y461H, Y461N, and Y463D (A. G. da Silva, personal communication) in Mm have been associated with resistance to DMIs in insensitive strains of the pathogens (N=2—10 Mf and 1—44 Mm) from the Federal District (Central Western), Northern Minas Gerais, Western and Ribeira Valley regions in São Paulo (Southeastern Brazil). As DMI fungicide sprays are very frequent in banana plantations (Gasparotto et al. 2000; Martínez-Bolaños et al. 2012; Chong-Aguirre 2016; Moraes and Nomura 2020), constant monitoring of Mf and Mm populations and detailed investigation regarding the evolution, emergence, spread and persistence of resistance to DMIs in distinctively favorable agroecosystems for each of the pathogens is urgently needed. Without up-to-date information on optimal fungicide risk and disease management strategies, a more sustainable control cannot be devised (Cools et al. 2013; Corkley et al. 2022).
Concerning the site-specific SDHI fungicides, they were labeled for BSDC management in 2014, and present a medium to high risk for the emergence of resistance if deployed intensively and singly (Sierotzki and Scalliet 2013). Currently, the SDHI fungicides labeled for the management of the BSDC pathogens in banana plantations elsewhere include boscalid, fluopyram, fluxapyroxad, and isopyrazam (FRAC 2022c). Particularly in Brazil, only a single co-formulation fungicide (Collis™, from BASF) containing the SDHI boscalid and the QoI kresoxim-methyl has been labeled (MAPA 2023). In vitro SDHI sensitivity testing of Mf and Mm populations sampled from banana plantations in different geographical regions of Southeastern Brazil in 2021 revealed that resistance was already present (Silva 2023) (Fig. 2B, Table 1). Further research is needed to translate the results from the in vitro tests into efficacy loss of practical disease control under field conditions. Among the 10 Mf and 57 Mm isolates for which the sdhB, sdhC and sdhD genes were examined, only one (Mf SdhC N55D) and two (Mm SdhB E196Q and SdhD K66N) Sdh target site alterations, respectively, were detected (Silva 2023) (Table 3). We highlight that, to our knowledge, none of these substitutions has been associated with resistance to SDHI fungicides in the BSDC pathogens according to the most recent survey conducted by the SDHI Working Group in 2022 (FRAC 2022a). Further monitoring for Sdh target mutations is important, but other resistance mechanisms such as the presence of multiple Sdh paralogs (Yamashita and Fraaije 2018; Steinhauer et al. 2019), and multidrug resistance (MDR) associated with efflux pump mechanism cannot be ruled out (Silva 2023).
Wheat blast disease
Wheat blast (WB), caused by the hemibiotroph ascomycete Pyricularia oryzae Triticum lineage (PoTl) (Castroagudín et al. 2016; Gladieux et al. 2018), has been a major disease across Central and Southern Brazil since it was first reported in Paraná state in 1985 (Igarashi et al. 1986). Following the emergence of wheat blast in Bangladesh in 2016 (Islam et al. 2016) and its further spread into Zambia, East Africa, in 2017 (Tembo et al. 2020), PoTl came to the attention of Asian and African governments and the international research community, bringing to light an urgent need to develop plans to contain the spread of this destructive pathogen in Asia and Africa (Islam et al. 2016; Ceresini et al. 2018, 2019; Tembo et al. 2020). Strategies for WB management must be based on information on PoTl biology and epidemiology, including the pathogen’s life cycle, survival, spread, host range and reproductive mode(s) and environmental conditions triggering disease (Ceresini et al. 2018, 2019). The two most common disease management strategies, use of resistant varieties and fungicide sprays, are likely to fail if applied individually. Fungicides are considered only partially effective, for reasons detailed in the next topic (Pagani et al. 2014; Rocha et al. 2014; Sharma 2017; Cruz and Valent 2017; Cruz et al. 2019; Ascari et al. 2021). Although sources of durable resistance to PoTl have been identified (Cruz et al. 2010; Wang et al. 2018; Cruppe et al. 2020; Dianese et al. 2021), major host resistance genes are likely to be overcome by the emergence of virulent races from the highly diverse pathogen population (Maciel et al. 2014; Ceresini et al. 2018). Integrated disease management (IDM) strategies are needed to reduce crop losses without impacting the environment (Maciel 2011; Mehta 2014; Cruz and Valent 2017; Ceresini et al. 2019). The implementation of IDM strategies should be coordinated locally, taking into account the particular circumstances of each country or region (Ceresini et al. 2018).
Low efficacy of fungicides to control wheat blast in Brazil
Fungicides are regularly used to manage WB and ear-associated diseases. However, the field efficacy of fungicides is considered low, resulting in only small decreases in blast severity on symptomatic spikes. A meta-analysis from 42 field trials over a nine year period, from 2012 through 2020, pointed to an average control efficacy of QoIs and azoles (DMIs) fungicides ranging from 43% up to 58% (Ascari et al. 2021). Disease control of no more than 50%, in comparison to untreated plots (Maciel 2011), and reduced crop losses were only achieved when mixtures of DMIs and QoIs were applied early on moderately resistant wheat varieties under low or moderate disease pressure (Rios et al. 2016). The effectiveness of applications at early heading and early grain-filling stages seemed to be associated with a reduction in PoTl inoculum produced on the lower leaves, leading to a reduction in ear infections (Cruz et al. 2015). The limited efficacy of fungicide treatments is likely to be due to several factors, including the difficulty of reaching the infection sites on spikelets, the high diversity of PoTl strains, the highly favorable weather conditions coupled with high levels of varietal susceptibility, and the low intrinsic efficacy of some fungicides, such as the methyl benzimidazole carbamates (Maciel 2011; Ceresini et al. 2018; Torres et al. 2022). In addition, PoTl has a broad host range, including several invasive grass species present in or near wheat fields, which do not receive fungicides sprays, thus providing a continuous external source of new inoculum (Ceresini et al. 2018).
A total of 49 fungicides have been labeled for management of WB disease in Brazil, comprising 17 azoles and seven co-formulations of azoles and QoIs (MAPA 2023). These two fungicide groups have been used extensively for management of rusts and other wheat leaf and head diseases for one to three decades (Poloni et al. 2021; Torres et al. 2022; Vicentini et al. 2022a). Their poor performance against wheat blast and lower profitability may have resulted from the emergence of fungicide resistance (Castroagudín et al. 2015; Poloni et al. 2021; Vicentini et al. 2022a; Cazón et al. 2023). New fungicide formulations labeled locally since 2017 for wheat diseases are mixtures of the second-generation carboxamide fluxapyroxad, a SDHI, combined with the QoI pyraclostrobin or the azole epoxiconazole, two active ingredients to which populations of PoTl were already found to be resistant (Dorigan et al. 2019; Poloni et al. 2021; Vicentini et al. 2022a; Cazón et al. 2023). The efficacy of melanin biosynthesis inhibitors targeting polyhydroxynaphthalene reductase (MBI-R fungicides, such as tryciclazole) and plant defense activators (PDA, such as acibenzolar-S-methyl) controlling wheat blast is yet unknown, though they are labeled either for rice blast control or for controlling other foliar diseases on wheat, respectively (MAPA 2023).
Fungicide resistance mechanisms in PoTl field populations
In recent years, Brazilian PoTl populations sampled in 2012 and 2018, across the major wheat growing areas of Central and Southern Brazil, showed moderate to high levels of resistance to all the three major groups of medium to high-risk systemic site-specific fungicides labeled for management of WB in Brazil (Castroagudín et al. 2015; Poloni et al. 2021; Vicentini et al. 2022a; Cazón et al. 2023) (Fig. 2C, Tables 1 and 4). These fungicides included the DMIs (Dorigan et al. 2019; Poloni et al. 2021; Vicentini et al. 2022a; Cazón et al. 2023), QoIs (Castroagudín et al. 2015; Vicentini et al. 2022a; Cazón et al. 2023), and the SDHI fluxapyroxad (Vicentini et al. 2022a) (Table 4). The in vitro sensitivity tests indicated that resistance to azoles and QoI fungicides was widespread in the country, with prevalence higher than 89% (Castroagudín et al. 2015; Dorigan et al. 2019; Poloni et al. 2021). For SDHI sensitivity, moderate resistance to fluxapyroxad (EC50 > 20 μg.mL-1) were detected in isolates from five out of the six field populations sampled in 2012 (4.5% of the total sample) and in 47.6% of the PoTl strains isolated in 2018 (Vicentini et al. 2022a) (Fig. 2C, Table 4). In addition, in vivo fungicide sensitivity tests for PoTl under controlled environment conditions has also indicated moderate to high levels of resistance to multiple fungicides of these three groups, with blast control efficacy as low as 3.3% for the QoI azoxystrobin, 31.3% for the QoI pyrachlostrobin, 31.0% for fluxapyroxad, and 51.0% for the epoxiconazole (Cazón et al. 2023).
QoI resistance is linked to cytB G143A (Castroagudín et al. 2015; Vicentini et al. 2022a; Cazón et al. 2023), a well-known target site alteration conferring high levels of resistance in other pathogens, including Mf and Mm (Oliveira et al. 2022). For the azole resistance mechanism, the CYP51A and B haplotypes are not predictive of phenotype. For instance, isolates carrying a prevalent CYP51A R158K substitution were not more resistant than those expressing R158 (Dorigan et al. 2019; Poloni et al. 2021). Similarly, no association was found between target site mutations in the sdhB, sdhC, and sdhD genes and the levels of SDHI resistance, indicating that a pre-existing resistance mechanism not associated with target site mutations is probably present in the Brazilian wheat blast populations. However, under additional selection of SDHI fungicides, it is plausible that populations of PoTl will evolve target-site mutations as resistance mechanisms similar to the ones already reported for other cereal fungal pathogens such as Zymoseptoria tritici, Pyrenophora teres, or Ramularia collo-cygni (Mair et al. 2016a). In addition, the multiple fungicide resistance, or multidrug resistance (MDR), detected both in vitro and in vivo (Cazón et al. 2023) was probably due to enhanced efflux pump activity in PoTl populations (Vicentini et al. 2022c).
Strategies for fighting against fungicide resistance in the agroecosystem
We will discuss the practical management of resistance to fungicides, looking to answer the following questions: (i) Is it possible to prevent resistance to a fungicide from occurring?, and (ii) Is it possible to manage resistance to fungicides once its emergence has been identified?
In our Review the term ’emergence’ is employed to encompass a range of scenarios where resistant individuals, within a pathogen population, become established. This includes the natural occurrence of rare mutations that confer resistance as well as mutations that may arise due to the direct effect of fungicides. Given the widespread and extensive use of fungicides in agriculture, it is conceivable that de novo mutations for fungicide resistance induced directly by the fungicide’s effects on the fungal genome may be more prevalent in certain cases. This phenomenon occurs when sublethal doses of fungicides exert selection pressure on the pathogen population, leading to the survival and reproduction of individuals with resistance-conferring mutations (Boyce et al. 2017; dos Reis et al. 2019; Gambhir et al. 2021; Healey et al. 2016). In practical terms, distinguishing between these sources of resistance mutations can be challenging. Therefore, we have chosen to use the term ’emergence’ broadly to acknowledge that resistance can arise through various mechanisms, whether driven by natural genetic variability or induced by fungicide exposure.
Therefore, we postulate that, in general, the overall objective of resistance management strategies is twofold: first, to delay the emergence of variants of plant pathogens that can resist a fungicidal treatment (in the emergence phase of resistance development), and second, to reduce the selection of such variants (in the selection phase) (van den Bosch et al. 2014a).
For pathogens whom resistance to a specific fungicide mode of action has never been detected, the basic anti-emergence strategies should be to prevent resistant lineages from emerging in the agroecosystem (Hobbelen et al. 2014; Corkley et al. 2022). As simple as it seems, a systemic single-site high-risk agricultural fungicide molecule should not be released in the market as a solo active, but only in co-formulations with low risk fungicide (i.e., a protectant multisite fungicide) (Fig. 4A). It may be very simple to implement, indeed, as the choice of a fungicide mixture is already an embedded anti-emergence strategy against the development of resistance that requires no other complex strategy, such as the decision on rotating fungicide with distinct modes of action. The lifespan of the high-risk agricultural fungicides is prolonged as their efficacy and profitability are kept along the years. Consequently, no environmental spread of fungicide resistant lineages of plant pathogenic fungi occurs (Fig. 4A). The relative effect of the doses of the multisite in mixture with the single-site fungicide on reducing the rate of selection for resistance should be determined (van den Bosch et al. 2014b). Unfortunately, anti-emergence strategies against fungicide resistance are not a choice any longer for the relevant plant pathogens of Brazilian agriculture (Figs. 1 and 2, Table 1).
The recurrent scenario of fungicide resistance becoming prevalent in field populations of several important plant pathogens for Brazilian tropical agriculture (Table 1) is rather serious and should be addressed as a general strategic failure in developing and deploying high-risk single-site agricultural fungicides properly. This also indicates that the application of the anti-resistance strategies (Fig. 5, A and B), legally included in fungicide labels since 2012 (MAPA / Coordination of Pesticides and Related Products 2012), have failed to delay the emergence and spread of resistance. It is particularly true for new fungicide molecules released thereafter (Mello et al. 2021; Müller et al. 2021; Vicentini et al. 2022a; Silva 2023). This has occurred despite the large amount of information derived from historical cases reported on fungicide resistance in populations of local key pathogens such as the ones of ASR, BSDC and WB (Schmitz et al. 2014; Klosowski et al. 2016b, 2018; Brito et al. 2020; Oliveira et al. 2022).
The anti-resistance strategies described in Fig. 5 (MAPA / Coordination of Pesticides and Related Products 2012) include general recommendations based on individual actions (Fig. 5A), for most of the plant pathogenic fungi, and specific recommendations for the ASR pathogen (Fig. 5B). Among them is the general recommendation to rotate sprays of fungicides formulated in mixtures, alternating distinct modes of action and never spray a commercial fungicide from a single mode of action alone. The objective is to avoid successive sprays of fungicides with the same mode of action as that could lead to an increased selection pressure for resistance in the pathogen populations. As an anti-resistance strategy, we would expect to lower the prevalence of recently emerged or established fungicide resistant lineages by reducing or removing the selection pressure from the extensive spraying of high-risk fungicides in the country [Fig. 4B; (Mikaberidze et al. 2014; van den Bosch et al. 2014a)].
Individual decision-based strategies on fungicide choice may be easily applicable. However, the effectiveness is heavily dependent on fitness cost associated with resistance, under the absence of the fungicide sprays (Hawkins and Fraaije 2018). These strategies usually do not include a key recommendation to prevent the spray of a particular high-risk fungicide that lost efficacy due to resistance, or for which there is cross-resistance among actives from the same mode of action. Consequently, the resistant lineages tend to persist in the environment and to increase in frequency as the fungicide selection pressure persists (Hawkins and Fraaije 2018). In addition, some plant pathogenic fungi, such as the ASR, the BSDC and the WB pathogens, as well several others, are easily spread at long distance, overcoming non-concerted regional efforts to block the dispersal and re-introduction of resistance lineages (Twizeyimana et al. 2011; Maciel et al. 2014; Gomes et al. 2018; Brito et al. 2020).
Particularly for ASR, four of the anti-resistance strategies recommended contradict the principle of minimizing unnecessary fungicide sprays to reduce selection pressure for resistance, i.e., adoption of preventive spray tactics; including spray of the [respective] commercial fungicide only at the recommended timing and spray intervals; to follow a maximum interval of 14 days between spraying; carry out, at most, the number of sprays of the commercial fungicide as described in the label. Rather, growers and extension plant pathologists should be advised to avoid unnecessary preventive fungicide sprays, but to follow disease epidemics forecast systems based on weather favorability and risks for disease development to guide spray decisions (Fig. 4 B). Paradoxically, the disease epidemics forecast systems are virtually non-existent for most of the Brazilian states, with the exception of the Epagri/Ciram Agroconnect from Santa Catarina (available at the URL: https://ciram.epagri.sc.gov.br/agroconnect/). Alternatively, growers, technical assistants and agricultural extensionists are recommended to carry out frequent disease monitoring on the cropping area to detect any shift towards an increase in disease incidence. As plant disease epidemics are hard to predict, either institutionally or individually, calendar-based excessive fungicide sprays persist.
Validation of the effects of each of these anti-resistance strategies, and support for further decisions based on fungicides mode of action at risk, requires a massive effort for detection and monitoring shifts in fungicide resistance prevalence, in loco. This also requires highly skilled scientific and lab support for resistance detection and monitoring, at regional or countrywide scale, which demand appropriate and steady funding (Fig. 4B).
Alternatively, we have proposed evolutionary-smart anti-resistance strategies based on the reduction of the fungicide selection pressure on the pathogen populations, which are warranted to prolong the efficacy lifespan of agricultural fungicides (Mikaberidze et al. 2014; Corkley et al. 2022). Although they are not really new strategies, they differ from the former anti-resistance strategies as they are not focused on individual actions, but rather are focused on institutional actions, either from the public or private sectors involved, and also include public policies.
The evolutionary-smart anti-resistance strategies aimed to prolong the efficacy of fungicides in agriculture by reducing the rate of evolution of plant pathogenic fungi towards fungicide resistance are:
-
a)
Strengthening of the existing Tropical Plant Health Network (URL https://www.fitossanidadetropical.org.br/informacoes-tecnicas/areas-de-atuacao). This is an official national network on foliar and seed fungicide testing to provide consistent, large-scale, and meta-analytic evaluation of fungicide efficacy, as well as crop yield response under disease-conducive environments over time and space. The network also aimed to monitor early negative trends in crop yield, pointing to the emergence of fungicide resistance in the field, and granting rapid and open access to the relevant information (Dalla Lana et al. 2018; Custódio et al. 2020, 2022; Ascari et al. 2021).
-
b)
Capacity building and establishment of a network for early detection of fungicide resistance emergence and increased dispersal by continual large scale monitoring of pathogen populations for fungicide sensitivity, analyses and compilation of phenotypic metadata, interpretation and consistent public release of the relevant information;
-
c)
Limiting the labeling of solo-active formulations of the high-risk single-site fungicides such as the SDHIs, or premixtures with other high risk fungicides for which resistance has been prevalent, such as QoIs or DMIs (Mikaberidze et al. 2014);
-
d)
Preferential labeling of premixtures of single-site at-risk fungicides with multi-site fungicides such as mancozeb (FRAC group M03), chlorothalonil (FRAC group M05) (Godoy et al. 2016; Netto et al. 2020) or copper-based actives (FRAC group M01) (Juliatti et al. 2017), as they have low-risk for fungicide resistance emergence; though all three fungicides are facing scrutiny due to environmental and health concerns in Canada, European Union, and United States (Jones et al. 2020), they remain labeled in Brazil (MAPA 2023);
-
e)
Constant reassessment of fungicides labeled for controlling ASR, BSDC, WB, and several other plant pathogens, for which a decrease in field efficacy as well as resistance has been detected, using the facilities of the network described previously in (a), and allowing prompt actions from the pesticide regulating agencies at federal and state levels (MAPA / Secretariat of Agricultural Defense (SDA) 2017);
-
f)
Technical recommendation, included in the fungicide labels, and information-transfer by the agricultural extension services, discouraging prophylactic, calendar-based, sequential sprays of active ingredients from medium to high-risk, single-site fungicide groups (SDHI, as case example), particularly in disease-conducive environments; recommending the choice of pre-mixtures of new SDHIs with multi-site fungicides, instead (Brent and Hollomon 2007; Fraaije et al. 2012);
-
g)
Advising, in the fungicide label and reinforced by the agricultural extension services, the need for integration of diverse disease management strategies other than fungicide sprays only, which include, as specific example, the full adherence to any mandatory off-season crop-free policy (MAPA / Secretariat of Agricultural Defense (SDA) 2022, p. 607), early sowing to escape favorable conditions for disease incidence (Koga et al. 2014; Dias et al. 2014), choice and deployment of resistant cultivars, particularly those with complete resistance, so fungicides sprays are not required (Hartman et al. 2005; Childs et al. 2018; Yorinori 2021c), and others, such as biological control with biofungicides.
Prospects and challenges
Establishment of a smart disease surveillance and fungicide resistance monitoring network to rationalize fungicide application
Lessons learned from the accelerated evolution and prevalence of fungicide resistance in populations of plant pathogenic fungi from Brazilian tropical agriculture should be applied to implement new disease management strategies to minimize fungicide sprays.
Smart surveillance and monitoring tools are needed in order to rationalize fungicide applications, e.g. choice of products, dose rate, frequency and timing of sprays and mixing or alternating fungicides. These tools will enable the quantification of inoculum levels of plant pathogens and the detection of alleles that confer resistance to fungicides. All these in combination with systems to predict the occurrence of diseases, in real time, by using automated spore capture and molecular detection of specific DNA markers of plant pathogens. These tools will be also important to test the efficacy of anti-resistance strategies aimed at reducing disease inocula, such as the implementation of cropping-free periods, to delay the evolution and the spread of resistance to current and new fungicides.
As a result, the molecular mechanisms underlying resistance, i.e. functional characterization of the alleles that confer resistance, to the main fungicidal chemical groups, must be described and characterized for a considerably larger range of plant pathogens important to Brazilian agriculture. High-throughput molecular diagnostics for real-time monitoring of resistance to fungicides in the agroecosystem must also be developed.
Therefore, we propose, as a smart anti-resistance strategy, the test and implementation of an aerobiology-based warning system to predict fungal diseases epidemics and to help minimize fungicide sprays (Fig. 6). Fundamentally, this smart and improved system implies the direct and early automated capture of airborne inoculum levels, in combination with molecular detection of the pathogen and fungicide resistance alleles. Further, the system can be useful in providing more accurate predictions of the risks of severe plant disease epidemics (Del Ponte et al. 2006; do Nascimento et al. 2012; Danelli and Reis 2016; Minchio et al. 2018; Beruski et al. 2019). It can also provide predictions of the spread of fungicide resistance alleles, preferentially before it occurs, as already reported for few important pathosystems (Luo et al. 2007; Deising et al. 2008; van der Heyden et al. 2021; Vicentini et al. 2022b, 2023).
Novel real-time disease surveillance and fungicide resistance automatized monitoring network to foster a smart and sustainable crop protection platform in Brazil*. * Model description, rationale, fundamentals, and the association of state of the art technology and open information sharing are discussed in detail in this item of the review
Such a smart and improved disease forecasting system optimized for monitoring the fungicide resistance, and individualized for each crop, would benefit the growers by reducing the cost of production and prolonging the effectiveness of the fungicides, and the consumers by increasing food safety and security and reducing fungicide residues in foods. The environment will also benefit due to reduction in the applications of pesticides, by avoiding unnecessary fungicide sprays or the use of inefficient ones due to the occurrence of high levels of resistance.
Establishment of a centralized database and information system on fungicide resistance in Brazil
Several stakeholders and segments of the Brazilian tropical agroecosystem may have interest in cataloging, reporting and sharing data on fungicide resistance in Brazil. These segments typically include:
-
a.
Agricultural research institutions: Government and private Institutions involved in research, development, and innovation activities related to agriculture are often interested in sharing data on fungicide resistance to contribute to the scientific information and to give support to evidence-based decisions in disease management.
-
b.
Governmental agricultural agencies: Agencies responsible for agricultural policies and regulations, such as the Brazilian Ministry of Agriculture, Livestock and Supply (MAPA), have an interest in disseminating data on fungicide resistance to establish policies, to develop guidelines, and to promote sustainable agricultural practices.
-
c.
Industry associations and agricultural manufacturers: Associations representing agricultural industries, including fungicide manufacturers and distributors, may have an interest in publicizing the data to raise awareness on resistance issues and to promote responsible fungicide use.
Notwithstanding, the permanent cataloging and reporting of data on fungicide resistance in Brazil faces limitations and challenges that include inadequate funding and the absence of specific public policies mandatory for the comprehensive monitoring of fungicide efficacy and resistance. Efforts to monitor fungicide resistance in Brazil in the last decade have been made independently by several research groups from quite a few institutions and agricultural organizations. However, the coverage and extent of these efforts were limited and varied across regions and crops. Some of the factors contributing to these limitations include:
-
a.
Funding constraints: Adequate funding is crucial to carry out comprehensive monitoring programs, research projects, and data collection related to fungicide resistance. Limited financial resources can hamper the scale and scope of the monitoring efforts, making it difficult to gather large amounts of data on fungicide resistance for different crops and regions.
-
b.
Lack of specific public policies: The absence of explicit public policies mandating comprehensive monitoring of fungicide efficacy and resistance can impact the priority given to these activities. In the absence of clear directives, there may be variations on the commitment and resources allocated to monitor fungicide resistance at a national or regional level.
-
c.
Emphasis on immediate yield quality protection: In agricultural systems focused primarily on maximizing immediate yields, fungicide efficacy is often prioritized over long-term resistance management. This emphasis on short-term gains can result in a reduced emphasis on monitoring and documenting fungicide resistance.
-
d.
Limited coordination and infrastructure: Coordinated efforts and infrastructure for collecting, analyzing, and disseminating data on fungicide resistance may be lacking or underdeveloped. The absence of centralized data repositories or standardized protocols for monitoring fungicide resistance can make it challenging to gather and share comprehensive information.
To overcome the lack of an official public open access data repository to accommodate permanent fungicide resistance information in Brazil, several key strategies can be implemented:
-
a.
Strengthening efforts on research and monitoring fungicide resistance: Increase in investments on research and monitoring programs on fungicide resistance is essential. This involves allocating adequate funding and resources to support comprehensive studies on different crops, regions, and pathogens. Research institutions, agricultural organizations, and governmental agencies should collaborate to establish robust monitoring systems and standardized protocols for data collection.
-
b.
Enhancing collaboration and information sharing: Encouraging collaboration among researchers, institutions, and stakeholders is crucial for sharing expertise, data, and best practices. This can be facilitated through partnerships, research networks, and platforms for information exchange. Encouraging open communication and collaboration can help overcome barriers and ensure a more comprehensive understanding of fungicide resistance in Brazil.
-
c.
Implementation of mandatory reporting and surveillance: Introduction of regulations or policies that enforce reports on fungicide resistance cases can improve the availability of data. This can be achieved through partnerships between governmental agencies, research institutions, and agricultural industry stakeholders. Mandatory reports would ensure a more accurate and comprehensive assessment of the prevalence and distribution of fungicide resistance on different regions and crops.
-
d.
Building capacity and awareness: Investments in training programs and capacity-building initiatives for researchers, extension agents, and farmers is essential. These initiatives should focus on enhancing information on fungicide resistance, promoting best management practices, and raising awareness on the importance of monitoring and management of resistance. Building capacity at all levels of the agricultural system will contribute to a more informed and proactive approach in regard to fungicide resistance.
-
e.
Implementations of integrated disease management strategies: Shifting from overconfidence on fungicides to a more integrated disease management approach is crucial. This includes implementation of cultural, biological, and chemical control methods to reduce the selection pressure for fungicide resistance. Crop rotation, diversification of planting materials, use of resistant cultivars, and adoption of best agricultural practices can help minimize the dependence on fungicides and mitigate the development of resistance.
-
f.
Establishment of centralized databases and information systems: Development of centralized databases or information systems dedicated to cataloging and sharing data on fungicide resistance in Brazil would streamline and facilitate access to information. Such systems should be user-friendly, accessible to researchers, extension professionals, and farmers, and regularly updated with new findings. Centralized databases would help to consolidate information, facilitate data analysis, and provide a comprehensive overview on fungicide resistance trends in the country.
A vital alliance: The strategic inclusion of extension service and growers in the fight against fungicide resistance in the Brazilian agriculture
By the end of this review we addressed the importance of relationship between extension personnel and farmers in strategies to fight fungicide resistance in the tropical Brazilian agroecosystem while considering the challenges that can be faced.
In regard to the ongoing efforts to fight against fungicide resistance, it is imperative to recognize the major role of the farmers and the extension service personnel to implement effective strategies. The success of any strategy designed to deal with fungicide resistance relies on the active engagement and collaboration of these two key players. However, we recognize the unique structural challenges presented by the literacy rates of the Brazilian growers, as reported by the 2017 Brazilian Agricultural Census (available at https://censoagro2017.ibge.gov.br/resultados-censo-agro-2017.html), where 17.2% are illiterate, and a significant portion has limited formal education.
The inclusion of farmers and extension service personnel in a policy to fight against fungicide resistance is not just a desirable aspect for the solution; it is an integral part of it. Without an active participation and full commitment, any proposed strategy for fungicide resistance managment would be at risk of faltering and could result in eventual failure. Therefore, we must work collectively by integrating farmers and extension service personnell into the process of fungicide resistance management, to ensure that they not only understand but also accept the strategies proposed here for an effective fungicide resistance management.
Therefore, only individuals with certified technical expertise should play a central role in disease management practices based on fungicide applications. This includes both farmers and extension personnel with the necessary professional expertise. We recognize, though, that until a lasting solution is found for improvements to occur on the basic educational level of the Brazilian growers, their direct involvement in fungicide-related disease management decisions could pose risks to the environment, agricultural productivity, and public health.
Thus, we support a certified technical assistance as a prerequisite for all stages of the responsible choosing, recommending, handling and applying of fungicides. In regard the certified technical assistance, we should ensure that fungicide-related practices are carried out with precision, and responsibility, and in a way that preserve the interests of agriculture, the environment, and public health. This approach protects against unintended consequences arising from insufficient knowledge and expertise. Fortunatelly, the current legislation approved in 2022 (at both Federal and State levels) regulates the registration of companies that provide pesticides application services for agricultural needs and the appointment of extension agents, i.e. certified agronomists, for prescription of pesticides, and technical responsibility concerning their correct and safe use (Secretariat of Agriculture and Supply (SAA) from São Paulo State (2022)).
In conclusion, we are firm in our commitment to a sustainable fungicide resistance management in the Brazilian agriculture. By implementing these strategies, Brazil can improve the understanding of fungicide resistance, enhancing data collection and sharing, and promoting more effective management policies. The collaboration among growers and extension service personnel, investment in research and monitoring, and the adoption of proactive strategies are key to address the lack of consistent information on fungicide resistance and will encourage sustainable disease management practices in the Brazilian tropical agriculture.
Finally, the fight against fungicide resistance today and its increasing threats requires the adoption of the “One Health” as a transdisciplinary and collaborative approach proposed by WHO (World Health Organization) when releasing new treatments or therapies for control of fungal diseases, recognizing the interconnection among plants, people, animals, and their shared environment (Fraaije et al. 2021; Woods et al. 2023)
Data availability
All data generated or analysed during this study are included in this published article.
References
Akamatsu H, Yamanaka N, Yamaoka Y, Soares RM, Morel W, Ivancovich AJG, Bogado AN, Kato M, Yorinori JT, Suenaga K (2013) Pathogenic diversity of soybean rust in Argentina, Brazil, and Paraguay. Journal of General Plant Pathology 79:28–40
Akamatsu H, Yamanaka N, Soares RM, Ivancovich AJG, Lavilla MA, Bogado AN, Morel G, Scholz R, Yamaoka Y, Kato M (2017) Pathogenic variation of South American Phakopsora pachyrhizi populations isolated from soybeans from 2010 to 2015. Japan Agricultural Research Quarterly JARQ 51:221–232
Amil AF, Heaney SP, Stanger C, Shaw MW (2007) Dynamics of QoI sensitivity in Mycosphaerella fijiensis in Costa Rica during 2000 to 2003. Phytopathology 97:1451–1457
Arastehfar A, Carvalho A, Houbraken J, Lombardi L, Garcia-Rubio R, Jenks JD, Rivero-Menendez O, Aljohani R, Jacobsen ID, Berman J, Osherov N, Hedayati MT, Ilkit M, Armstrong-James D, Gabaldón T, Meletiadis J, Kostrzewa M, Pan W, Lass-Flörl C, Perlin DS, Hoenigl M (2021) Aspergillus fumigatus and aspergillosis: arom basics to clinics. Studies in Mycology 100:100115
Arzanlou M, Groenewald JZ, Fullerton RA, Abeln ECA, Carlier J, Zapater MF, Buddenhagen IW, Viljoen A, Crous PW (2008) Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia, Molecular Phylogeny and Evolution of Fungi 20:19–37
Ascari JP, Barro JP, Santana FM, Padua JMV, Maciel JLN, Lau D, Torres GAM, Sbalcheiro CC, Seixas CDS, Goulart ACP, Sussel AAB, Schipanski CA, Chagas DF, Coelho MAO, Montecelli TDN, Amara DR, Custódio AAP, Moreira LSO, Utiamada CM, Venâncio WS, Goussain RCS, Alves KS, Del Ponte EM (2021) Sequential post-heading applications for controlling wheat blast: A 9-year summary of fungicide performance in Brazil. Plant Disease 105:4051–4059
Baggio JS, Peres NA, Amorim L (2018) Sensitivity of Botrytis cinerea isolates from conventional and organic strawberry fields in Brazil to azoxystrobin, iprodione, pyrimethanil, and thiophanate-methyl. Plant Disease 102:1803–1810
Barro JP, Alves KS, Godoy CV, Dias AR, Forcelini CA, Utiamada CM, Andrade Júnior ER, Juliatti FC, Grigolli FJ, Feksa HR, Campos HD, Chaves ICPV, Araújo Júnior IP, Roy JMT, Nunes Júnior J, Belufi LMR, Carneiro LC, Silva LHCP, Canteri MG, Goussain Júnior MM, Senger M, Meyer MC, Dias MD, Müller MA, Martins MC, Debortoli MP, Tormen NR, Furlan SH, Konageski TF, Carlin VJ, Venâncio WS, Del Ponte EM (2021) Performance of dual and triple fungicide premixes for managing soybean rust across years and regions in Brazil: A meta analysis. Plant Pathology 70:1920–1935
Beltrán-García MJ, Prado FM, Oliveira MS, Ortiz-Mendoza D, Scalfo AC, Pessoa A Jr, Medeiros MHG, White JF, Di Mascio P (2014) Singlet molecular oxygen generation by light-activated DHN-melanin of the fungal pathogen Mycosphaerella fijiensis in black Sigatoka disease of bananas. PLOS ONE 9:e91616
Beruski GC, Sentelhas PC, Pereira AB, Câmara GMS, Junior IPA, Schiebelbein LM (2019) Soybean rust epidemics as affected by weather conditions in Brazil. Journal of Agricultural Science 12:213
Bezerra GDA, Chaibub AA, de Sousa Oliveira MI, Mizubuti ESG, de Filippi MCC (2021) Evidence of Pyricularia oryzae adaptability to tricyclazole. Journal of Environmental Science and Health Part B 56:869–876
Borba JP (2020) Does the I86F mutation of succinate dehydrogenase subunit c increase fungicide resistance and have a fitness cost in Asian Soybean Rust (Phakopsora pachyrhizi)? Master’s Thesis, University of São Paulo
Boyce KJ, Wang Y, Verma S, Shakya VPS, Xue C, Idnurm A (2017) Mismatch repair of DNA replication errors contributes to microevolution in the pathogenic fungus Cryptococcus neoformans. MBio 8:e00595-e17
Braga K, Fantin LH, Minchio CA, Scolin LB, Paduan FN, Canteri MG (2020) Sensibilidade de populações de Phakopsora pachyrhizi ao fungicida protioconazol. Summa Phytopathologica 46:150–154
Brasseur G, Saribaş AS, Daldal F (1996) A compilation of mutations located in the cytochrome b subunit of the bacterial and mitochondrial bc1 complex Biochimica et biophysica acta. Bioenergetics 1275:61–69
Brent KJ, Hollomon DW (2007) Fungicide resistance in crop pathogens: how can it be managed?, 2nd, revised. Fungicide Resistance Action Comittee, Brussels
Brent KJ (2012) Historical perspectives of fungicide resistance. In: Thind TS (ed) Fungicide resistance in crop protection: Risk and management. CABI, Wallingford, pp 3–18
Brito FSD, Fraaije B, Miller RNG (2015) Sigatoka disease complex of banana in Brazil: Management practices and future directions. Outlooks Pest Management 26:78–81
Brito FSD, Santos JRP, Azevedo VCR, Peixouto YS, de Oliveira SA, Ferreira CF, Haddad F, Amorim EP, Fraaije B, Miller RNG (2020) Genetic diversity and azole fungicide sensitivity in Pseudocercospora musae field populations in Brazil. Frontiers in Microbiology 11:99
Brito FSD (2015) Diversidade genética de populações de Mycosphaerella musicola e caracterização do efetor LysM em Mycosphaerella graminicola. PhD Dissertation, University of Brasilia
Burks C, Darby A, Gómez Londoño L, Momany M, Brewer MT (2021) Azole-resistant Aspergillus fumigatus in the environment: Identifying key reservoirs and hotspots of antifungal resistance. PLOS Pathogens 17:e1009711
Burt PJA (1994) Windborne dispersal of sigatoka leaf spot pathogens. Grana 33:108–111
Cañas-Gutiérrez GP, Angarita-Velásquez MJ, Restrepo-Flórez JM, Rodríguez P, Moreno CX, Arango R (2009) Analysis of the CYP51 gene and encoded protein in propiconazole-resistant isolates of Mycosphaerella fijiensis. Pest Management Science 65:892–899
Carlier J, Zapater M-F, Lapeyre F, Jones DR, Mourichon X (2000) Septoria leaf spot of banana: a newly discovered disease caused by Mycosphaerella eumusae (anamorph Septoria eumusae). Phytopathology 90:884–890
Castroagudín VL, Ceresini PC, de Oliveira SC, Reges JT, Maciel JL, Bonato AL, Dorigan AF, McDonald BA (2015) Resistance to QoI fungicides is widespread in Brazilian populations of the wheat blast pathogen Magnaporthe oryzae. Phytopathology 105:284–294
Castroagudín VL, Moreira SI, Pereira DA, Moreira SS, Brunner PC, Maciel JL, Crous PW, McDonald BA, Alves E, Ceresini PC (2016) Pyricularia graminis-tritici, a new Pyricularia species causing wheat blast. Persoonia-Molecular Phylogeny and Evolution of Fungi 37:199–216
Cazón LI, Ascari JP, dos Santos GB, Del Ponte EM (2023) Differential response to DMI, QoI and SDHI fungicides in wheat and signal grass blast populations from Minas Gerais, Brazil. Plant Pathology 72:449–467
Ceresini PC, Castroagudín VL, Rodrigues FÁ, Rios JA, Aucique-Pérez CE, Moreira SI, Alves E, Croll D, Maciel JLN (2018) Wheat blast: past, present, and future. Annual Review of Phytopathology 56:427–456
Ceresini PC, Castroagudín VL, Rodrigues FÁ, Rios JA, Aucique-Pérez CE, Moreira SI, Croll D, Alves E, de Carvalho G, Maciel JLN, McDonald BA (2019) Wheat blast: from its origins in South America to its emergence as a global threat. Molecular Plant Pathology 20:155–172
Chen F, Tsuji SS, Li Y, Hu M, Bandeira MA, Câmara MPS, Michereff SJ, Schnabel G (2020) Reduced sensitivity of azoxystrobin and thiophanate-methyl resistance in Lasiodiplodia theobromae from papaya. Pesticide Biochemistry and Physiology 162:60–68
Childs SP, Buck JW, Li Z (2018) Breeding soybeans with resistance to soybean rust (Phakopsora pachyrhizi). Plant Breeding 137:250–261
Chong-Aguirre P (2016) The origin, versatility and distribution of azole fungicide resistance in the banana black Sigatoka pathogen Pseudocercospora fijiensis. PhD Dissertation, Wageningen University & Research
Churchill ACL (2011) Mycosphaerella fijiensis, the black leaf streak pathogen of banana: progress towards understanding pathogen biology and detection, disease development, and the challenges of control. Molecular Plant Pathology 12:307–328
Claus A, Simões K, De Mio LLM (2022) Sdh C-I86F mutation in Phakopsora pachyrhizi is stable and can be related to fitness penalties. Phytopathology 112:1413–1421
CONAB – Compania Nacional de Abastecimento (2004) Indicadores da Agropecuária. Indic Agropec 13:1–61
CONAB – Compania Nacional de Abastecimento (2013) Indicadores da Agropecuária. Indic. Agropec 21:1–83
Consórcio Antiferrugem (2023) / Anti-soybean rust Consortium. Dispersion map. In: Anti-Soybean Rust Consort. http://www.consorcioantiferrugem.net/#/main. Accessed 13 Mar 2023
Cools HJ, Bayon C, Atkins S, Lucas JA, Fraaije BA (2012) Overexpression of the sterol 14α-demethylase gene (MgCYP51) in Mycosphaerella graminicola isolates confers a novel azole fungicide sensitivity phenotype. Pest Management Science 68:1034–1040
Cools HJ, Hawkins NJ, Fraaije BA (2013) Constraints on the evolution of azole resistance in plant pathogenic fungi. Plant Pathology 62:36–42
Corkley I, Fraaije B, Hawkins N (2022) Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathology 71:150–169
Croll D, McDonald BA (2017) The genetic basis of local adaptation for pathogenic fungi in agricultural ecosystems. Molecular Ecology 26:2027–2040
Crous PW, Groenewald JZ, Pongpanich K, Himaman W, Arzanlou M, Wingfield MJ (2004) Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology 50:457–469
Cruppe G, Cruz CD, Peterson G, Pedley K, Asif M, Fritz A, Calderon L, Lemes da Silva C, Todd T, Kuhnem P, Singh PK, Singh RP, Braun H-J, Barma NCD, Valent B (2020) Novel sources of wheat head blast resistance in modern breeding lines and wheat wild relatives. Plant Disease 104:35–43
Cruz CD, Valent B (2017) Wheat blast disease: danger on the move. Tropical Plant Pathology 42:210–222
Cruz MFA, Prestes AM, Maciel JL, Scheeren PL (2010) Resistência parcial à brusone de genótipos de trigo comum e sintético nos estádios de planta jovem e de planta adulta. Tropical Plant Pathology 35:024–031
Cruz CD, Kiyuna J, Bockus WW, Todd TC, Stack JP, Valent B (2015) Magnaporthe oryzae conidia on basal wheat leaves as a potential source of wheat blast inoculum. Plant Pathology 64:1491–1498
Cruz CD, Santana FM, Todd TC, Maciel JLN, Kiyuna J, Baldelomar DF, Cruz AP, Lau D, Seixas CS, Goulart ACP, Sussel AA, Schipanski CA, Chagas DF, Coelho M, Montecelli TDN, Utiamada C, Custódio AP, Rivadeneira MG, Bockus WW, Valent B (2019) Multi-environment assessment of fungicide performance for managing wheat head blast (WHB) in Brazil and Bolivia. Tropical Plant Pathology 44:183–191
Cummins I, Wortley DJ, Sabbadin F, He Z, Coxon CR, Straker HE, Sellars JD, Knight K, Edwards L, Hughes D, Kaundun SS, Hutchings S-J, Steel PG, Edwards R (2013) Key role for a glutathione transferase in multiple-herbicide resistance in grass weeds. Proceedings of the National Academy of Sciences 110:5812–5817
Custódio AAP, Utiamada CM, Madalosso T (2020) Eficiência de fungicidas no controle múltiplo de doenças foliares do milho segunda safra 2020, 2nd edn. IAPAR, Londrina
Custódio AAP, Araújo AE, Machado ACZ (2022) The plant diseases epidemiology in the tropical plant health network scope. In: Canale MC, Nesi CN, Bergamim Filho A, Del Ponte E, Amorim L, May De Mio LL, Laranjeira F (eds) Proceedings of the VI EpidemioBrasil: Brazilian Workshop of Plant Disease Epidemiology. Epagri, Chapecó, p 29
D’Ávila LS, De Filippi MCC, Café-Filho AC (2021) Sensitivity of Pyricularia oryzae populations to fungicides over a 26-year time frame in Brazil. Plant Disease 105:1771–1780
Dalla Lana F, Paul PA, Godoy CV, Utiamada CM, da Silva LHCP, Siqueri FV, Forcelini CA, de Souza Jaccoud-Filho D, Miguel-Wruck DS, Borges EP, Juliatti FC, Campos HD, Nunes J, Carneiro LC, Canteri MG, Ito MF, Meyer MC, Martins MC, Balardin RS, Furlan SH, Carlin VJ, Del Ponte EM (2018) Meta-analytic modeling of the decline in performance of fungicides for managing soybean rust after a decade of use in Brazil. Plant Disease 102:807–817
Danelli ALD, Reis EM (2016) Quantification of incubation, latent and infection periods of Phakopsora pachyrhizi in soybean, according to chronological time and degree-days. Summa Phytopathologica 42:11–17
Deising HB, Reimann S, Pascholati SF (2008) Mechanisms and significance of fungicide resistance. Brazilian Journal of Microbiology 39:286–295
Del Ponte EM, Godoy CV, Canteri MG, Reis EM, Yang XB (2006) Models and applications for risk assessment and prediction of Asian soybean rust epidemics. Fitopatologia Brasileira 31:533–544
Delp CJ, Dekker J (1985) Fungicide resistance: definitions and use of terms. EPPO Bulletin 15:333–335
Dianese AC, Zacaroni AB, Souza BCP, da Silva Pagani AP, Pinheiro NO, de Castro Gomes EM, Torres GAM, Consoli L, Café-Filho AC (2021) Evaluation of wheat genotypes for field resistance to wheat blast caused by Magnaporthe oryzae pathotype Triticum (MoT) and correlation between yield loss and disease incidence in the Brazilian Cerrado. Euphytica 217:84
Dias APS, Li X, Yang XB (2014) Modeling the effects of cloudy weather on regional epidemics of soybean rust. Plant Disease 98:811–816
Diaz-Trujillo C, Chong P, Stergiopoulos I, Cordovez V, Guzman M, De Wit PJGM, Meijer HJG, Scalliet G, Sierotzki H, Lilia Peralta E, Arango Isaza RE, Kema GHJ (2018) A new mechanism for reduced sensitivity to demethylation-inhibitor fungicides in the fungal banana black Sigatoka pathogen Pseudocercospora fijiensis. Molecular Plant Pathology 19:1491–1503
do Nascimento JF, Vida JB, Tessmann DJ, Zambolim L, Vieira RA, de Oliveira RR (2012) Progress of Asian soybean rust and airborne urediniospores of Phakopsora pachyrhizi in southern Brazil. Summa Phytopathologica 38:280–287
Dorigan AF, de Carvalho G, Poloni NM, Negrisoli MM, Maciel JLN, Ceresini PC (2019) Resistance to triazole fungicides in Pyricularia species is associated with invasive plants from wheat fields in Brazil. Acta Scientiarum Agronomy 41:e39332
Dorigan AF, Moreira SI, Ceresini PC, Pozza EA, Belan LL, da Silveira PR, Alves E (2022) Higher fitness and competitive advantage of Pyricularia oryzae Triticum lineage resistant to QoI fungicides. Pest Management Science 78:5251–5258
Dos Reis TF, Silva LP, de Castro PA, do Carmo RA, Marini MM, da Silveira JF, Ferreira BH, Rodrigues F, Lind AL, Rokas A, Goldman GH (2019) The Aspergillus fumigatus mismatch repair MSH2 homolog is important for virulence and azole resistance. MSphere 4:e00416–e00419
Dutra PSS, Pereira WV, May De Mio LL (2019) Brazilian isolates of Monilinia fructicola from peach do not present reduced sensitivity to iprodione. European Journal of Plant Pathology 153:1341–1346
Dutra PSS, Lichtemberg PSF, Martinez MB, Michailides TJ, May De Mio LL (2020) Cross-resistance among demethylation inhibitor fungicides with Brazilian Monilinia fructicola isolates as a foundation to discuss brown rot control in stone fruit. Plant Disease 104:2843–2850
Fanaro GB, Villavicencio ALCH, El-Shemy H (2011) The Asian soybean rust in South America. Soybean: Physiology and Biochemistry. IntechOpen, London, pp 475–488
Ferrari JT, Nogueira EMC, Gasparotto L, Hanada RE, Loureira IM (2005) Ocorrência da Sigatoka negra em bananeiras no estado de São Paulo. Arquivos do Instituto Biológico 72:133–134
Ferro CG, Silva TC, Vicentini SNC, Ferraudo GM, Ceresini PC (2020) Levels of regional phenotypic adaptation (QST) indicate that neutrality has shaped the population structure of the soybean-infecting pathogen Rhizoctonia solani AG-1 IA. Revista Caatinga 33:608–618
Fischer JMM, Dutra PSS, Araujo HE, Glienke C, May De Mio LL (2023) Field isolates of Monilinia fructicola change resistance pattern to greater sensitivity to thiophanate-methyl in recent populations. European Journal of Plant Pathology 166:51–64
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186–194
Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ (2018) Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360:739–742
Forcelini CA, Goellner CI, May De Mio LL (2001) Resistência de fungos a fungicidas. Revisão Anual de Patologia de Plantas 9:339–381
Fraaije BA, Bayon C, Atkins S, Cools HJ, Lucas JA, Fraaije MW (2012) Risk assessment studies on succinate dehydrogenase inhibitors, the new weapons in the battle to control Septoria leaf blotch in wheat. Molecular Plant Pathology 13:263–275
Fraaije BA, Atkins SL, Santos RF, Hanley SJ, West JS, Lucas JA (2021) Epidemiological studies of pan-azole resistant Aspergillus fumigatus populations sampled during tulip cultivation show clonal expansion with acquisition of multi-fungicide resistance as potential driver. Microorganisms 9:2379
FRAC – Fungicide Resistance Action Committee (2022a) Recommendations for SDHI fungicides. In: Crop Life Int. Bruss. Belg. https://www.frac.info/frac-teams/working-groups/sdhi-fungicides/recommendations-for-sdhi. Accessed 22 Feb 2023
FRAC – Fungicide Resistance Action Committee (2022b) FRAC Code List ©*2022: Fungal control agents sorted by cross-resistance pattern and mode of action. In: Crop Life Int. Bruss. Belg. https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2022--final.pdf?sfvrsn=b6024e9a_2. Accessed 23 May 2023
FRAC – Fungicide Resistance Action Committee (2022c) Recommendations for Bananas. In: Crop Life Int. Bruss. Belg. https://www.frac.info/frac-teams/working-groups/banana-group/recommendations-for-bananas. Accessed 22 Feb 2023
Gama AB, Baggio JS, Rebello CS, Lourenço SA, Gasparoto MCG, da Silva Junior GJ, Peres NA, Amorim L (2020) Sensitivity of Colletotrichum acutatum Isolates from Citrus to Carbendazim, Difenoconazole, Tebuconazole, and Trifloxystrobin. Plant Disease 104:1621–1628
Gambhir N, Kamvar ZN, Higgins R, Amaradasa BS, Everhart SE (2021) Spontaneous and fungicide-induced genomic variation in Sclerotinia sclerotiorum. Phytopathology 111:160–169
Garcés Fiallos FR (2011a) A ferrugem asiática da soja causada por Phakopsora pachyrhizi Sydow & Sydow. Ciencia y Tecnología 4:45–60
Garcés-Fiallos FR (2011b) Progresso temporal da ferrugem e redução sobre a área foliar e os componentes do rendimento de grãos em soja. Acta Agronomica 60:147–157
Gasparotto L, Pereira JCR, Pereira MCN, Costa MM (2000) Controle químico da Sigatoka negra da bananeira. I - trifloxistrobin, propiconazole e difenoconazole. Comunicado Técnico 7:1
Georgopoulos SG, Skylakakis G (1986) Genetic variability in the fungi and the problem of fungicide resistance. Crop Protection 5:299–305
Ghini R, Kimati H (2002) Resistência de fungos a fungicidas, 2nd edn. Embrapa Meio Ambiente, Jaguariuna
Gladieux P, Condon B, Ravel S, Soanes D, Maciel JLN, Nhani A Jr, Chen L, Terauchi R, Lebrun MH, Tharreau D, Mitchell T, Pedley KF, Valent B, Talbot NJ, Farman M, Fournier E (2018) Gene flow between divergent cereal- and grass-specific lineages of the rice blast fungus Magnaporthe oryzae. mBio 9:e01219-17
Godoy CV, Seixas CDS, Soares RM, Marcelino-Guimarães FC, Meyer MC, Costamilan LM (2016) Asian soybean rust in Brazil: past, present, and future. Pesquisa Agropecuária Brasileira 51:407–421
Gomes LIS, Douhan GW, Bibiano LBJ, Maffia LA, Mizubuti ESG (2013) Mycosphaerella musicola identified as the only pathogen of the Sigatoka disease complex present in Minas Gerais state, Brazil. Plant Disease 97:1537–1543
Gomes LIS, Bibiano LBJ, da Silva GF, Hanada RE, Mizubuti ESG (2014) Baseline sensitivity of Brazilian Mycosphaerella fijiensis isolates to protectant and systemic fungicides. Tropical Plant Pathology 39:172–177
Gomes LIS, Douhan GW, Lehner MS, Bibiano LBJ, Mizubuti ESG (2018) Yellow sigatoka epidemics caused by a panmictic population of Mycosphaerella musicola in Brazil. Plant Pathology 67:295–302
Grice K, Vawdrey LB, Stammler GC, Koch A, Wilson D, Mathew N (2013) Yellow Sigatoka (Mycosphaerella musicola) populations develop resistance to QoI fungicides in Australia. In: Dehne H-W, Deising HB, Fraaije BA, Gisi U, Hermann D, Mehl A, Oerke EC, Russell PE, Kuck KH, Lyr H (eds) Modern Fungicides and Antifungal Compounds. Deutsche Phytomedizinische Gesellschaft, Braunschweig, pp 269–270
Grimmer MK, Van Den Bosch F, Powers SJ, Paveley ND (2014) Evaluation of a matrix to calculate fungicide resistance risk: Evaluation of fungicide resistance risk assessment. Pest Management Science 70:1008–1016
Grimmer MK, Van Den Bosch F, Powers SJ, Paveley ND (2015) Fungicide resistance risk assessment based on traits associated with the rate of pathogen evolution: Fungicide resistance risk assessment. Pest Management Science 71:207–215
Gubbins S, Gilligan CA (1999) Invasion thresholds for fungicide resistance: deterministic and stochastic analyses. Proceedings of the Royal Society B: Biological Sciences 266:2539–2549
Hanada R, Gasparotto L, Moreira A (2015) Avaliação da sensibilidade de Mycosphaerella fijiensis oriundos de plátanos aos fungicidas propiconazole e azoxystrobina. Revista de Ciências Agrárias / Amazonian Journal of Agricultural and Environmental Sciences 58:21–26
Hartman GL, Miles MR, Frederick RD (2005) Breeding for resistance to soybean rust. Plant Disease 89:664–666
Hawkins NJ, Fraaije BA (2018) Fitness penalties in the evolution of fungicide resistance. Annual Review of Phytopathology 56:339–360
Hawkins NJ, Cools HJ, Sierotzki H, Shaw MW, Knogge W, Kelly SL, Kelly DE, Fraaije BA (2014) Paralog re-emergence: A novel, historically contingent mechanism in the evolution of antimicrobial resistance. Molecular Biology and Evolution 31:1793–1802
Healey KR, Zhao Y, Perez WB, Lockhart SR, Sobel JD, Farmakiotis D, Kontoyiannis DP, Sanglard D, Taj-Aldeen SJ, Alexander BD, Jimenez-Ortigosa C, Shor E, Perlin DS (2016) Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nature Communications 7:11128
Hobbelen PHF, Paveley ND, Van Den Bosch F (2014) The emergence of resistance to fungicides. PLoS ONE 9:e91910
Hollomon DW (2015) Fungicide resistance: Facing the challenge - a review. Plant Protection Science 51:170–176
Hollomon DW (2015) Fungicide resistance: 40 Years on and still a major problem. In: Ishii H, Hollomon DW (eds) Fungicide Resistance in Plant Pathogens. Springer Japan, Tokyo, pp 3–11
Hu M, Chen S (2021) Non-target site mechanisms of fungicide resistance in crop pathogens: A review. Microorganisms 9:502
Igarashi S, Utiamada CM, Igaradhi LC, Kazuma AH, Lopes RS (1986) Pyricularia em trigo. 1. Ocorrência de Pyricularia sp. no estado do Paraná. Fitopatologia Brasileira 11:351–352
Ishikawa-Ishiwata Y, Furuya J (2021) Soybean rust and resistant cultivar effects on global soybean supply and demand. Japan Agricultural Research Quarterly JARQ 55:59–67
Islam MT, Croll D, Gladieux P, Soanes DM, Persoons A, Bhattacharjee P, Hossain MS, Gupta DR, Rahman MM, Mahboob MG, Cook N, Salam MU, Surovy MZ, Sancho VB, Maciel JL, Nhani Júnior A, Castroagudín VL, Reges JT, Ceresini PC, Ravel S, Kellner R, Fournier E, Tharreau D, Lebrun MH, McDonald BA, Stitt T, Swan D, Talbot NJ, Saunders DG, Win J, Kamoun S (2016) Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biology 14:84
Jones JG, Korir RC, Walter TL, Everts KL (2020) Reducing chlorothalonil use in fungicide spray programs for powdery mildew, anthracnose, and gummy stem blight in melons. Plant Disease 104:3213–3220
Juliatti FC, Zambolim L (2021) Etiology, epidemiology and management of Asian soybean rust (ASR) in Brazil and vulnerability of chemical control of specific without multisite fungicides. In: Kumar Goyal A (ed) Cereal Grains. IntechOpen, London
Juliatti FC, de Azevedo LAS, Juliatti FC (2017) Strategies of chemical protection for controlling soybean rust. In: Kasai M (ed) Soybean - The Basis of Yield, Biomass and Productivity. IntechOpen, London
Kilani J, Fillinger S (2016) Phenylpyrroles: 30 Years, two molecules and (nearly) no resistance. Frontiers in Microbiology 7:2014
Klosowski AC, Brahm L, Stammler G, De Mio LLM (2016) Competitive fitness of Phakopsora pachyrhizi isolates with mutations in the CYP51 and cytB genes. Phytopathology 106:1278–1284
Klosowski AC, May De Mio LL, Miessner S, Rodrigues R, Stammler G (2016) Detection of the F129L mutation in the cytochrome b gene in Phakopsora pachyrhizi. Pest Management Science 72:1211–1215
Klosowski AC, Castellar C, Stammler G, May De Mio LL (2018) Fungicide sensitivity and monocyclic parameters related to the Phakopsora pachyrhizi - soybean pathosystem from organic and conventional soybean production systems. Plant Pathology 67:1697–1705
Koga LJ, Canteri MG, Calvo ES, Martins DC, Xavier SA, Harada A, Kiihl RAS (2014) Managing soybean rust with fungicides and varieties of the early/semi-early and intermediate maturity groups. Tropical Plant Pathology 39:129–133
Kuck KH, Russell PE (2006) FRAC: combined resistance risk assessment. Aspects of Applied Biology 78:3–10
Lehner MS, Paula Júnior TJ, Silva RA, Vieira RF, Carneiro JES, Schnabel G, Mizubuti ESG (2015) Fungicide sensitivity of Sclerotinia sclerotiorum: A thorough assessment using discriminatory dose, EC50, high-resolution melting analysis, and description of new point mutation associated with thiophanate-methyl resistance. Plant Disease 99:1537–1543
Leite ICHL, Silva RA, Santos JECC, Freitas Lopes RL, Câmara MPS, Michereff SJ, Lopes UP (2020) Analysis of Colletotrichum musae populations from Brazil reveals the presence of isolates with high competitive ability and reduced sensitivity to postharvest fungicides. Plant Pathology 69:1529–1539
Li S, Smith JR, Ray JD, Frederick RD (2012) Identification of a new soybean rust resistance gene in PI 567102B. Theoretical and Applied Genetics 125:133–142
Li Y, Tsuji SS, Hu M, Câmara MPS, Michereff SJ, Schnabel G, Chen F (2020) Characterization of difenoconazole resistance in Lasiodiplodia theobromae from papaya in Brazil. Pest Management Science 76:1344–1352
Lichtemberg PSF, Luo Y, Morales RG, Muehlmann-Fischer JM, Michailides TJ, May De Mio LL (2017) The point mutation G461S in the MfCYP51 gene is associated with tebuconazole resistance in Monilinia fructicola populations in Brazil. Phytopathology 107:1507–1514
Lin F, Chhapekar SS, Vieira CC, Da Silva MP, Rojas A, Lee D, Liu N, Pardo EM, Lee YC, Dong Z, Pinheiro JB, Ploper LD, Rupe J, Chen P, Wang D, Nguyen HT (2022) Breeding for disease resistance in soybean: a global perspective. Theoretical and Applied Genetics 135:3773–3872
Lucas JA, Hawkins NJ, Fraaije BA (2015) The evolution of fungicide resistance. In: Advances in Applied Microbiology. Elsevier, pp 29–92
Luo Y, Ma Z, Reyes HC, Morgan D, Michailides TJ (2007) Quantification of airborne spores of Monilinia fructicola. European Journal of Plant Pathology 118:145–154
Maciel JLN (2011) Magnaporthe oryzae, the blast pathogen: current status and options for its control. CABI Reviews 2011:1–8
Maciel JL, Ceresini PC, Castroagudin VL, Zala M, Kema GH, McDonald BA (2014) Population structure and pathotype diversity of the wheat blast pathogen Magnaporthe oryzae 25 years after its emergence in Brazil. Phytopathology 104:95–107
Maia JN, Beger G, Pereira WV, May De Mio LL, da Silva Silveira Duarte H (2021) Gray mold in strawberries in the Paraná state of Brazil is caused by Botrytis cinerea and its isolates exhibit multiple-fungicide resistance. Crop Protection 140:105415
Mair W, Lopez-Ruiz F, Stammler G, Clark W, Burnett F, Hollomon D, Ishii H, Thind TS, Brown JK, Fraaije B, Cools H, Shaw M, Fillinger S, Walker A-S, Mellado E, Schnabel G, Mehl A, Oliver RP (2016) Proposal for a unified nomenclature for target site mutations associated with resistance to fungicides. Pest Management Science 72:1449–1459
Mair WJ, Deng W, Mullins JGL, West S, Wang P, Besharat N, Ellwood SR, Oliver RP, Lopez-Ruiz FJ (2016) Demethylase inhibitor fungicide resistance in Pyrenophora teres f. sp. teres associated with target site modification and inducible overexpression of CYP51. Frontiers in Microbiology 7:1279
Malimpensa JR (2018) Caracterização da resistência a fungicidas em populações de fungos associados a lesões de Sigatokas em bananais do Vale do Ribeira (SP). Master’s Thesis, Instituto Biológico
Manzo-Sánchez G, Orozco-Santos M, Islas-Flores I, Martínez-Bolaños L, Guzmán-González S, Leopardi-Verde CL, Canto-Canché B (2019) Genetic variability of Pseudocercospora fijiensis, the black Sigatoka pathogen of banana (Musa spp.) in Mexico. Plant Pathology 68:513–522
MAPA – Ministério da Agricultura Pecuária e Abastecimento - Brazil (2023) Agrofit - Sistemas de Agrotóxicos Fitossanitários, Coordenação Geral de Agrotóxicos e Afins. http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Accessed 5 Feb 2023
Martínez-Bolaños L, Téliz-Ortiz D, Rodríguez-Maciel JC, Mora-Aguilera JA, Nieto-Ángel D, Cortés-Flores JI, Mejía-Sánchez D, Nava-Diaz C, Silva-Aguayo G (2012) Resistencia a fungicidas en poblaciones de Mycosphaerella fijiensis del sureste mexicano. Agrociencia 46:707–717
Mathioni SM, Mello FE, Antunes R, Duvaresch DL, Milanesi DF, Brommonschenkel SH, Pinho DB, Rosa DD (2022) Species determination and CytB-G143A monitoring of Ramulariopsis spp. Isolated from cotton in Brazil. Plant Health Progress 23:4–6
McDonald BA, Linde C (2002) Pathogen population genetics, evolutionary potential, and durable resistance. Annual Review of Phytopathology 40:349–379
McGrath MT (2004) What are Fungicides? The Plant Health Instructor
Mehta YR (2014) Wheat diseases and their management, 1st edn. Springer International Publishing, Cham
Mello FE, Mathioni SM, Fantin LH, Rosa DD, Antunes RFD, Filho NRC, Duvaresch DL, Canteri MG (2021) Sensitivity assessment and SDHC-I86F mutation frequency of Phakopsora pachyrhizi populations to benzovindiflupyr and fluxapyroxad fungicides from 2015 to 2019 in Brazil. Pest Management Science 77:4331–4339
Mello FE, Lopes-Caitar VS, Xavier-Valencio SA, da Silva HP, Franzenburg S, Mehl A (2022) Resistance of Corynespora cassiicola from soybean to QoI and MBC fungicides in Brazil. Plant Pathology 71:373–385
Mendoza MJ, Ardales E (2019) Population Structure of the Banana Black Sigatoka Pathogen [Pseudocercospora fijiensis (M. Morelet) Deighton] in Luzon Philippines. The Philippine Agricultural Scientist 102:211–219
Mikaberidze A, McDonald BA, Bonhoeffer S (2014) Can high-risk fungicides be used in mixtures without selecting for fungicide resistance? Phytopathology 104:324–331
Minchio CA, Fantin LH, Caviglione JH, Braga K, Silva MA, Canteri MG (2018) Predicting asian soybean rust epidemics based on off-season occurrence and El Niño southern oscillation phenomenon in Paraná and Mato Grosso states. The Brazilian Journal of Agricultural Sciences 10:562
Ministry of Agriculture, Livestock and Supply (MAPA) (2023) Agrofit Crop Pesticides System, General Coordination of Pesticides and Similar Products. 2023. https://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Accessed 3 May 2023
Ministry of Agriculture Livestock and Supply (MAPA), Coordination of Pesticides and Related Products (2012) Manual of Procedures for Pesticides Labeling. MAPA, Brasília
Ministry of Agriculture, Livestock and Supply (MAPA) / Secretariat of Agricultural Defense (SDA) (2021a) Ordinance 306: Establishes the National Asian Soybean Rust (Phakopsora pachyrhizi) Control Program (ASRCP) under the Ministry of Agriculture, Livestock and Supply. Diário Of. União edition 90-A, section 1 (extra), pg. 1
Ministry of Agriculture, Livestock and Supply (MAPA) / Secretariat of Agricultural Defense (SDA) (2016) Ordinance 84: Establishes the procedures for the agronomic reassessment of pesticides labeled for controlling Phakopsora pachyrhizi on soybean crops. Diário Of. União edition 158, section 1, pgs. 5–6
Ministry of Agriculture, Livestock and Supply (MAPA) / Secretariat of Agricultural Defense (SDA) (2017) Technical Note 8/2017: Establishes the Technical Commission to carry out the agronomic reassessment of pesticides labeled for controlling Phakopsora pachyrhizi on soybean crops
Ministry of Agriculture, Livestock and Supply (MAPA) / Secretariat of Agricultural Defense (SDA) (2021b) Ordinance 388: Amends Ordinance No. 306, of May 13, 2021, which establishes the National Asian Soybean Rust (Phakopsora pachyrhizi) Control Program (PNCFS). Diário Of. União edition 166, section 1, pg. 23
Ministry of Agriculture, Livestock and Supply (MAPA) / Secretariat of Agricultural Defense (SDA) (2022) Ordinance 607: Establishes the soybean sowing calendars for the 2022/2023 cropping season. Diário Of. União edition 120, section 1, pg. 18
Moraes WDS, Nomura ES (2020) Monitoramento da Sigatoka e controle químico. Cultivo da bananeira. Coordenadoria de Assistência Técnica Integral (CATI), Campinas, pp 135–144
Moreira RR, Hamada NA, Peres NA, De Mio LLM (2019) Sensitivity of the Colletotrichum acutatum species complex from apple trees in Brazil to dithiocarbamates, methyl benzimidazole carbamates, and quinone outside inhibitor fungicides. Plant Disease 103:2569–2576
Müller MA, Stammler G, May De Mio LL (2021) Multiple resistance to DMI, QoI and SDHI fungicides in field isolates of Phakopsora pachyrhizi. Crop Protection 145:105618
Nardini L, Christian RN, Coetzer N, Ranson H, Coetzee M, Koekemoer LL (2012) Detoxification enzymes associated with insecticide resistance in laboratory strains of Anopheles arabiensis of different geographic origin. Parasites & Vectors 5:113
Netto A, Sacon D, Gallina A, Fochesatto M, Spitza Stefanski F, Mendes Milanesi P (2020) Use of systemic fungicides combined with multisite to control of Asian rust and soybean yield. Colloquium Agrararie 16:101–108
Nomura ES, Moraes WDS, Kobori RT, Penteado LADC (2020) Doenças. Cultivo da bananeira. Coordenadoria de Assistência Técnica Integral (CATI), Campinas, pp 107–134
Oliveira TYK, Silva TC, Moreira SI, Christiano FS Jr, Gasparoto MCG, Fraaije BA, Ceresini PC (2022) Evidence of resistance to QoI fungicides in contemporary populations of Mycosphaerella fijiensis, M. musicola and M. thailandica from banana plantations in Southeastern Brazil. Agronomy 12:2952
Pagani AP, Dianese AC, Café Filho AC (2014) Management of wheat blast with synthetic fungicides, partial resistance and silicate and phosphite minerals. Phytoparasitica 42:609–617
Papaïx J, Burdon JJ, Zhan J, Thrall PH (2015) Crop pathogen emergence and evolution in agro-ecological landscapes. Evolutionary Applications 8:385–402
Paraná Agribusiness Defense Agency (ADAPAR).(2015) Ordinance 91 (Summary). Diário Of. Estado Paraná edition 9458, pg. 51
Pereira WV, Primiano IV, Morales RGF, Peres NA, Amorim L, May De Mio LL (2017) Reduced sensitivity to azoxystrobin of Monilinia fructicola isolates from Brazilian stone fruits is not associated with previously described mutations in the cytochrome b gene. Plant Disease 101:766–773
Pereira D, McDonald BA, Croll D (2020) The genetic architecture of emerging fungicide resistance in populations of a global wheat pathogen. Genome Biology and Evolution 12:2231–2244
Pereira WV, Morales RGF, Bauer AIG, Kudlawiec K, May-De-Mio LL (2020) Discontinuance of tebuconazole in the field restores sensitivity of Monilinia fructicola in stone fruit orchards. Plant Pathology 69:68–76
Perlin MH, Andrews J, San Toh S (2014) Essential letters in the fungal alphabet: ABC and MFS transporters and their roles in survival and pathogenicity. In: Advances in Genetics. Elsevier, pp 201–253
Poloni NM, Carvalho G, Nunes Campos Vicentini S, Dorigan AF, Nunes Maciel JL, McDonald BA, Intra Moreira S, Hawkins N, Fraaije BA, Kelly DE (2021) Widespread distribution of resistance to triazole fungicides in Brazilian populations of the wheat blast pathogen. Plant Pathology 70:436–448
Pretty J, Bharucha ZP (2014) Sustainable intensification in agricultural systems. Annals of Botany 114:1571–1596
Rajendran R, Mowat E, McCulloch E, Lappin DF, Jones B, Lang S, Majithiya JB, Warn P, Williams C, Ramage G (2011) Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrobial Agents and Chemotherapy 55:2092–2097
Ramos JB, Bragança CAD, Rocha LS, Oliveira ADS, Cordeiro ZJM, Haddad F (2018) First report of black Sigatoka of banana caused by Mycosphaerella fijiensis in Bahia, Brazil. Plant Disease 102:2035–2035
Rios JA, Rios VS, Paul PA, Souza MA, Araujo L, Rodrigues FA (2016) Fungicide and cultivar effects on the development and temporal progress of wheat blast under field conditions. Crop Protection 89:152–160
Rocha FS, Catão HCRM, Muniz MFS (2012) Aspectos diagnósticos entre Mycosphaerella spp. da bananeira, distribuição e manejo no Brasil. Enciclopédia Biosfera 8:64–84
Rocha JRDASDC, Pimentel AJB, Ribeiro G, de Souza MA (2014) Eficiência de fungicidas no controle da brusone em trigo. Summa Phytopathologica 40:347–352
Sang H, Hulvey JP, Green R, Xu H, Im J, Chang T, Jung G (2018) A xenobiotic detoxification pathway through transcriptional regulation in filamentous fungi. mBio 9:e00457-18
Santos AJ (2005) Dominância da Sigatoka-negra em bananais do Vale do Ribeira. Fitopatologia Brasileira 30:193
Santos KM, Tsuji SS, Câmara MPS, Michereff SJ, Lopes UP (2019) Sensitivity to methyl benzimidazole carbamate fungicides of Botryosphaeriaceae species from mango orchards in the Northeast of Brazil. European Journal of Plant Pathology 153:209–222
Santos RF, Amorim L, Wood AKM, Bibiano LBJ, Fraaije BA (2021) Lack of an intron in cytochrome b and overexpression of sterol 14α-demethylase indicate a potential risk for qoi and dmi resistance development in Neophysopella spp. on grapes. Phytopathology 111:1726–1734
Schmitz HK, Medeiros C-A, Craig IR, Stammler G (2014) Sensitivity of Phakopsora pachyrhizi towards quinone-outside-inhibitors and demethylation-inhibitors, and corresponding resistance mechanisms. Pest Management Science 70:378–388
Secretariat of Agriculture and Supply (SAA) from São Paulo State (2022) Resolution SAA - 05, of 01/21/2022: Approves the standards and procedures for the registration of pesticides production chain in agricultural areas. Diário Of. Est. São Paulo edition 132, section 14, pgs. 24
Sharma R (2017) Wheat blast research: Status and imperatives. African Journal of Agricultural Research 12:377–381
Sierotzki H, Scalliet G (2013) A review of current knowledge of resistance aspects for the next-generation succinate dehydrogenase inhibitor fungicides. Phytopathology 103:880–887
Sierotzki H, Parisi S, Steinfeld U, Tenzer I, Poirey S, Gisi U (2000) Mode of resistance to respiration inhibitors at the cytochrome bc1 enzyme complex of Mycosphaerella fijiensis field isolates. Pest Management Science 56:833–841
Silva TC (2023) Prevalence of resistance to SDHI fungicides in populations of the black and yellow Sigatoka pathogens from bananas in Southeastern Brazil. Dissertation (Doctorate degree in Agronomy), São Paulo State University (UNESP)
Simões K, Hawlik A, Rehfus A, Gava F, Stammler G (2018) First detection of a SDH variant with reduced SDHI sensitivity in Phakopsora pachyrhizi. Journal of Plant Diseases and Protection 125:21–26
Stammler G, Cordero J, Koch A, Semar M, Schlehuber S (2009) Role of the Y134F mutation in cyp51 and overexpression of cyp51 in the sensitivity response of Puccinia triticina to epoxiconazole. Crop Protection 28:891–897
Steinberg G, Gurr SJ (2020) Fungi, fungicide discovery and global food security. Fungal Genetics and Biology 144:103476
Steinhauer D, Salat M, Frey R, Mosbach A, Luksch T, Balmer D, Hansen R, Widdison S, Logan G, Dietrich RA, Kema GHJ, Bieri S, Sierotzki H, Torriani SFF, Scalliet G (2019) A dispensable paralog of succinate dehydrogenase subunit C mediates standing resistance towards a subclass of SDHI fungicides in Zymoseptoria tritici. PLOS Pathogens 15:e1007780
Stensvold CR, Jørgensen LN, Arendrup MC (2012) Azole-resistant invasive Aspergillosis: Relationship to agriculture. Current Fungal Infection Reports 6:178–191
Stilgenbauer S, Simões K, Craig IR, Brahm L, Steiner U, Stammler G (2023) New CYP51-genotypes in Phakopsora pachyrhizi have different effects on DMI sensitivity. Journal of Plant Diseases and Protection 130:973–983
Stukenbrock EH, McDonald BA (2008) The origins of plant pathogens in agro-ecosystems. Annual Review of Phytopathology 46:75–100
Tembo B, Mulenga RM, Sichilima S, M’siska KK, Mwale M, Chikoti PC, Singh PK, He X, Pedley KF, Peterson GL, Singh RP, Braun HJ (2020) Detection and characterization of fungus (Magnaporthe oryzae pathotype Triticum) causing wheat blast disease on rain-fed grown wheat (Triticum aestivum L.) in Zambia. PLoS One 15:e0238724
Thind TS (2012) Fungicide resistance in crop protection: risk and management. CABI, Wallingford
Torres GAM, Ferreira JR, Binneck E, Maciel JLN, Consoli L (2022) Blast disease and wheat production in Brazil. Pesquisa Agropecuária Brasileira 57:e02487
Twizeyimana M, Ojiambo PS, Haudenshield JS, Caetano-Anollés G, Pedley KF, Bandyopadhyay R, Hartman GL (2011) Genetic structure and diversity of Phakopsora pachyrhizi isolates from soyabean. Plant Pathology 60:719–729
Uchôa CN, Pozza EA, Moraes WS, Rocha HS, Costa FCL (2021) Modelling black Sigatoka epidemics with seasonal dispersal of Mycosphaerella fijiensis ascospores over a banana plantation in the Ribeira Valley, São Paulo, Brazil. European Journal of Plant Pathology 161:463–474
Valarmathi P (2018) Dynamics of fungicidal resistance in the agro eco-system: a review. Agricultural Reviews 39:272–281
van den Bosch F, Gilligan CA (2008) Models of fungicide resistance dynamics. Annual Review of Phytopathology 46:123–147
van den Bosch F, Oliver R, Berg FVD, Paveley N (2014) Governing principles can guide fungicide-resistance management tactics. Annual Review of Phytopathology 52:175–195
van den Bosch F, Paveley N, Van Den Berg F, Hobbelen P, Oliver R (2014) Mixtures as a fungicide resistance management tactic. Phytopathology 104:1264–1273
van der Heyden H, Dutilleul P, Charron JB, Bilodeau GJ, Carisse O (2021) Monitoring airborne inoculum for improved plant disease management A review. Agronomy for Sustainable Development 41:40
Vicentini SN, Casado PS, de Carvalho G, Moreira SI, Dorigan AF, Silva TC, Silva AG, Custódio AA, Gomes ACS, Nunes Maciel JL, Hawkins N, McDonald BA, Fraaije BA, Ceresini PC (2022) Monitoring of Brazilian wheat blast field populations reveals resistance to QoI, DMI, and SDHI fungicides. Plant Pathology 71:304–321
Vicentini SNC, Krug LD, Ceresini PC, Custódio AAP, Leite RP Jr, West JS, Fraaije BA (2022) Can fungal aerobiology tools help tracking fungicide resistance alleles and block the spread of fungicide resistance in the agroecosystem? In: Canale MC, Nesi CN, Bergamimfilho A, Del Ponte E, Amorim L, May De Mio LL, Laranjeira F (eds) Proceedings of the VI EpidemioBrasil: Brazilian Workshop of Plant Disease Epidemiology. Epagri, Chapecó, pp 25–26
Vicentini SNC, Moreira SI, da Silva AG, de Oliveira TYK, Silva TC, Assis FG Jr, Krug LD, de Paiva Custódio AA, Leite RP Jr, Teodoro PE, Fraaije B, Ceresini PC (2022) Efflux pumps and multidrug-resistance in Pyricularia oryzae Triticum lineage. Agronomy 12:2068
Vicentini SNC, Hawkins NJ, King KM, Moreira SI, Custódio AA, Leite RP Jr, Portalanza D, Garcés-Fiallos FR, Krug LD, West JS, Fraaije BA, Jesus WC Jr, Ceresini PC (2023) Aerobiology of the wheat blast pathogen: inoculum monitoring and detection of fungicide resistant alleles. Agronomy 13:1238
Vieira WADS, Lima WG, Nascimento ES, Michereff SJ, Reis A, Doyle VP, Câmara MPS (2017) Thiophanate-methyl resistance and fitness components of Colletotrichum musae isolates from banana in Brazil. Plant Disease 101:1659–1665
Wang S, Asuke S, Vy TTP, Inoue Y, Chuma I, Win J, Kato K, Tosa Y (2018) A new resistance gene in combination with Rmg8 confers strong resistance against Triticum isolates of Pyricularia oryzae in a common wheat landrace. Phytopathology 108:1299–1306
Willi Y, Frank A, Heinzelmann R, Kälin A, Spalinger L, Ceresini PC (2011) The adaptive potential of a plant pathogenic fungus, Rhizoctonia solani AG-3, under heat and fungicide stress. Genetica 139:903–908
Wood PM, Hollomon DW (2003) A critical evaluation of the role of alternative oxidase in the performance of strobilurin and related fungicides acting at the Qo site of Complex III. Pest Management Science 59:499–511
Woods M, McAlister JA, Geddes-McAlister J (2023) A One Health approach to overcoming fungal disease and antifungal resistance. WIREs Mech Dis e1610
Yamashita M, Fraaije B (2018) Non-target site SDHI resistance is present as standing genetic variation in field populations of Zymoseptoria tritici. Pest Management Science 74:672–681
Yin Y, Miao J, Shao W, Liu X, Zhao Y, Ma Z (2023) Fungicide resistance: Progress in understanding mechanism, monitoring, and management. Phytopathology 113:707–718
Yorinori JT (2021a) Development and economic importance. Soybean rust: Lessons learned from the pandemic in Brazil. The American Phytopathological Society, Saint Paul, pp 15–48
Yorinori JT (2021b) Hosts and sources of inoculum of soybean rust. In: Soybean rust: Lessons learned from the pandemic in Brazil. The American Phytopathological Society, pp 87–91
Yorinori JT (2021c) Breeding for rust resistance. In: Soybean rust: Lessons learned from the pandemic in Brazil. The American Phytopathological Society, pp 109–117
Yorinori JT (2021d) Fungicide management. Soybean rust: Lessons learned from the pandemic in Brazil. The American Phytopathological Society, Saint Paul, pp 93–108
Yorinori JT (2021e) Difficulties controlling soybean rust. Soybean rust: Lessons learned from the pandemic in Brazil. The American Phytopathological Society, Saint Paul, pp 75–86
Yorinori JT, Paiva WM, Frederick RD, Costamilan LM, Bertagnolli PF, Hartman GE, Godoy CV, Nunes J Jr (2005) Epidemics of soybean rust ( Phakopsora pachyrhizi ) in Brazil and Paraguay from 2001 to 2003. Plant Disease 89:675–677
Zhang Y, Lamm R, Pillonel C, Lam S, Xu JR (2002) Osmoregulation and fungicide resistance: the Neurospora crassa os-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Applied and Environmental Microbiology 68:532–538
Zubrod JP, Bundschuh M, Arts G, Brühl CA, Imfeld G, Knäbel A, Payraudeau S, Rasmussen JJ, Rohr J, Scharmüller A, Smalling K, Stehle S, Schulz R, Schäfer RB (2019) Fungicides: An overlooked pesticide class? Environmental Science & Technology 53:3347–3365
Acknowledgements
The authors of this review article acknowledge funding received from the São Paulo Research Foundation, Brazil (FAPESP) through regular research grants to P.C.C. (FAPESP 2020/07611-9; 2021/03402-9; and 2022/12661-0); and in partnership with Fundação Araucária to A.A.P.C. (FPS2020011000057); a FAPESP Grant to P.C.C. (2017/50456-1; 2018/21197-0) and to S.I.M. (postdoctoral scholarship 2019/12509-1) as partner institution in Brazil in partnership with Newton Fund from the Biotechnology and Biological Sciences Research Council (BBSRC) and UK Research and Innovation (UKRI), CNPq (Brazilian National Council for Scientific and Technological Development), grant/award number: CNPq Pq-1D 313825/2018-1, Pq-1C 311895/2022-0 to P.C.C., Pq 1B 306886/2021-9 to L.L.M.M., Pq-2 309805/2019-8 and Pq-2 307193/2022-5 to W.C.J.J.; CAPES (Coordination for the Improvement of Higher Level Personnel), Grant/Award Numbers: CAPES/AUXPE Program 88881.593505/2020-01—UNESP Ilha Solteira Campus, CAPES PrInt/UNESP 41/2017–88887.570719/2020-00. A.G.S., L.D.K., S.N.C.V., and T.C.S were supported by Ph.D. studentships from CAPES studentship Program 001. S.S.M was supported by a PhD studentship from UNESP – CAADI Coordenadoria de Ações Afirmativas, Diversidade e Equidade. L.D.K. was supported by a technical assistance studentship from FAPESP (2022/09787-2).
Author information
Authors and Affiliations
Contributions
Ceresini PC, Conceptualization, Resources, Supervision, Writing – original draft, Writing – review & editing; Silva TC, Vicentini SNC, Leite Júnior RP, Krug LD, de Moura SS, Silva AG, Moreira SI, Castro-Ríos K, Garcés-Fiallos FR, Custódio AAP, May De Mio LL, Gasparoto MCG, Portalanza D & Jesus Júnior WC, Writing – review & editing, Figures design, Portalanza D. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ceresini, P.C., Silva, T.C., Vicentini, S.N.C. et al. Strategies for managing fungicide resistance in the Brazilian tropical agroecosystem: Safeguarding food safety, health, and the environmental quality. Trop. plant pathol. 49, 36–70 (2024). https://doi.org/10.1007/s40858-023-00632-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-023-00632-2