Abstract
In Benin, yam production continues to face numerous production constraints, including yield and quality reduction by Scutellonema bradys. Implementation of efficient management techniques against this pest requires an improved understanding, including at the molecular level, of the pest. The current study aimed at identifying the Scutellonema spp. associated with yam in Benin and investigating the phylogenetic relationships between populations. Nematodes of the genus Scutellonema were obtained from tubers exhibiting external dry rot symptoms. DNA was extracted from nematodes belonging to 138 populations collected from 49 fields from 29 villages. For 51 of these populations, both the ITS1 and COI regions could be amplified via PCR, sequenced, compared with available sequences in the NCBI database and were identified as S. bradys. Maximum likelihood was used to construct 60% consensus phylogenetic trees based on 51 sequences. This phylogenetic analysis did not reveal any genetic separation between populations by cultivar, village, cropping system nor by agroecological zone. Neither could any subgroups within S. bradys be separated, indicating that no subspecies were present. An earlier published species-specific primer set was verified with the DNA of the 51 sequences and was considered a reliable and rapid method for S. bradys identification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yam (Dioscorea spp.) is a key starch staple crop contributing to food security and poverty alleviation in Benin (Loko et al. 2015). However, yam faces various constraints to production, especially plant-parasitic nematodes. These organisms induce significant tuber damage in terms of yield suppression and quality of produce (market value), negatively impacting on household income. The most harmful nematodes belong to the genera Scutellonema spp., Meloidogyne spp. and Pratylenchus spp. (Bridge et al. 2005).
The genus Scutellonema contains several species, which are widely distributed across tropical and subtropical regions with more than 60% of the species recorded from Africa (Sher 1964; Siddiqi 2000). Besides the 45 valid species described by Siddiqi (2000), a few new species have been reported by several authors (Kolombia et al. 2017). These nematodes are primarily root ectoparasites, feeding from outside the roots and are associated with a large range of agricultural and horticultural crops (van den Berg et al. 2013). Three species, S. bradys (Steiner and LeHew) Andrássy, S. cavenesi (Sher) and S. brachyurus (Steiner) Andrássy, are considered agricultural pests. However, S. bradys, an important pest of yam, is unusual in that it feeds endoparasitically (Ayala and Acosta 1971; Bridge et al. 2005; Coyne et al. 2006a; Kolombia et al. 2017). It is reported as the most important nematode that affects yam in Benin (Baimey et al. 2009), responsible for dry rot of yam tubers, a degenerative disease which results in significant damage to field production and especially in postharvest losses during storage. Symptoms begin with cream to light yellow lesions developing below the skin of the tuber. They gradually enlarge and darken, sometimes turning dark brown or black. Surfaces may crack and skin parts flake off, exposing dark brown patches on infected tubers (Bridge et al. 2005). According to Coyne et al. (2012), the geographical center of origin for S. bradys is West Africa, in particular Benin and Nigeria. Recently, however, it was also reported infecting locally cultivated yam in East Africa (Coyne et al. 2016).
In West Africa, S. bradys was reported as a potential risk on potato (Solanum tuberosum L.), on which it can also cause substantial damage (Coyne et al. 2011; Mwamula et al. 2015). It has been found parasitising various crops from Africa, the Americas and Asia (Bridge et al. 2005). In the yam belt of West Africa, although the damage caused by S. bradys varied with the yam cultivar/species and agroecological zone (Baimey 2005; Coyne et al. 2006a) as well as with the population (Baimey et al. 2009), no difference in pathogenicity has been detected between S. bradys populations (Coyne et al. 2012). To date, no robust screening process on the wide diversity of yam cultivars in Benin has been undertaken.
Morphological identification of Scutellonema species is not simple because a number of species share very similar morphological characters (Kolombia et al. 2017). Phylogenetic and sequence analysis of rRNA and other genes were reported to provide attractive solutions to validate morphology-based identifications (van den Berg et al. 2013). Previous molecular studies, based on the ITS1–2 regions of the rDNA and PCR-RFLPs of 14 S. bradys populations collected throughout the West-African region, revealed a relatively high degree of genetic polymorphism both within and between populations (Coyne et al. 2006b). Genetic diversity was reported also by Kolombia et al. (2017) who studied the D2-D3 expansion segments of the 28 s rDNA and the COI sequence of the mtDNA of Scutellonema populations retrieved from yam fields in Nigeria and Ghana. Recently, S. bradys-specific primers have been developed and demonstrated to work with S. bradys populations from Costa Rica (Humphreys-Pereira et al. 2014). Its applicability for identification of Benin’s populations is unknown. This study reports on: i) the species identification of Scutellonema populations obtained from yam tubers collected from various localities in Benin, using ITS1 (rRNA) and COI (mtDNA) sequences; ii) the genetic relationships between Benin S. bradys populations using these sequences as phylogenetic markers; iii) the applicability of an available S. bradys-specific primer set onS. bradys populations from Benin.
Materials and methods
Scutellonema populations
Populations of Scutellonema used in this study were obtained from yam tubers collected during the harvest period (December 2014 to February 2015) from 49 fields in 29 villages, distributed across four yam diversity zones in Benin (Fig. 1). According to Dansi et al. (1999), yam diversity zones in Benin are: (1) Bariba zone (North East) that includes Bariba cultural area; (2) Donga zone (North West) gathering the cultural areas Ani, Yom, Lokpa, Kotokoli; (3) Atacora zone (extreme North West) grouping the cultural areas Ditamari, Wama, Berba, Natimba, M’bermin; (4) Center-South zone with cultural areas Fè, Fon, Idatcha, Mahi and Tchabè. Each zone is characterized by different yam cultivars and cropping systems. One to three fields per village were sampled. Fields with different cultivars were selected and tubers with visual dry rot symptoms were selected. A total of 138 samples (populations) were collected.
For extraction of nematodes from tubers, the centrifugal flotation technique was used. Each tuber was first washed and then peeled using a kitchen vegetable peeler, before removing a 25 g subsample of peels (outer cortex) from the bulked peels (Coyne et al. 2006a; Affokpon et al. 2011). For samples analyzed in Benin, peels were macerated in a blender and the mixture passed through nested sieves (200 μm and 100 μm). The suspension, containing nematodes smaller than 100 μm, was then distributed into 50 ml test tubes and thoroughly mixed with kaolin. The amount of kaolin was 8% of the weight of the suspension, adjusted for each tube. The test tubes containing the suspension-kaolin mixture were subject to a first centrifugation at 3500 rpm for 7 min. After that, the supernatant was discarded and the deposit was mixed with sucrose (250 g/l) and centrifuged again at 3500 rpm for 4 min. The supernatant suspension (containing nematodes and sucrose) was collected on a 10-μm sieve and thoroughly rinsed with tap water to remove the sucrose. Peel subsamples sent to Belgium were analyzed according to the same procedure, except that the peel mixture was poured over an 850-μm sieve and centrifuging was performed with the automated zonal centrifuge (Hendrickx 1995) using MgSO4, instead of manually and using sucrose.
Nematodes were identified to genus level using a stereo microscope (Wild M5). For each sample, a maximum of 30 individuals of Scutellonema specimens were hand-picked and transferred into staining glasses containing 25 μl of milliQ water to rinse the nematode body. Specimens were then individually transferred into labeled eppendorf tubes containing 25 μl sterile water. Five tubes containing one individual nematode specimen and one tube containing 5 individuals were prepared for each of the 138 samples.
DNA extraction
DNA extraction was performed following Holterman et al. (2006). An equal volume (25 μl) of Lysis Buffer (0.2 M NaCl, 0.2 M Tris-HCl pH 8.0, 1% beta-mercaptoethanol and 800 μg/ml proteinase-K), made shortly before DNA extraction, was added to each tube. Tubes were then incubated for 1.5 h at 65 °C followed by 5 min at 99 °C in a thermocycler. After incubation, tubes were stored at −20 °C.
Amplification via polymerase chain reaction (PCR)
An amount of DNA recovered during the DNA extraction was amplified by the Polymerase Chain Reaction (PCR) (Mullis et al. 1986). Two different regions were examined. The first focused on the Internal Transcribed Spacer (ITS1) of the rDNA (ribosomal DNA) using VrainILVO18Sf (forward) and rRNASM5rev (reverse) primers (Vrain et al. 1992). Only samples for which the ITS1 region could be amplified were used for a second PCR, amplifying the cytochrome oxidase subunit I (COI) region of the mtDNA (mitochondrial DNA) using COI-JB3 (forward) and COI-JB5 (reverse) as primers (Derycke et al. 2010). PCR was carried out using a volume of 50 μl (1 μl of each crude DNA extract +49 μl of Master Mix). The Master Mix was prepared as following: 42 μl of sterile water; 5 μl of 10X Pfu Buffer with MgSO4; 1 μl of dNTPs (10 mM each); 0.3 μl of the forward and reverse primers (50 μM) mentioned above; 0.4 μl of Pfu DNA Polymerase (Fermentas). A DNA-polymerase with proof-reading activity was used to obtain a PCR-product with very low or no nucleotide incorporation errors. For some templates, the PCR-mix holding Pfu DNA polymerase was replaced by BIO-X-act short mix (Bioline) containing a ‘normal’ DNA polymerase. The BIO-X-act short mix is designed to work on problematic templates. Using this kit, the Master Mix was prepared as follows: 23.4 μl of sterile water; 25 μl of BIO-X-act short mix (Bioline); 0.3 μl of the same forward and reverse primers. The PCR program comprises an initial DNA denaturation during 3 min at 95 °C (Pfu) or 96 °C (BIO-X-act short mix), 35 cycles including DNA denaturation during 30 s at 95 °C (Pfu) or 96 °C (BIO-X-act short mix), primer annealing during 30 s at 60 °C (for ITS1 primer set) and 41 °C (for CO1 primer set), Extension during 2 min (Pfu) or 1 min (BIO-X-act short mix) at 72 °C and final extension during 10 min at 72 °C. After PCR, 5 μl of loading dye were added to each tube and 5 μl of each mixture was loaded on a 1.5% of agarose gel during 25 min at 100 V for electrophoresis. Then, the gel was stained with ethidium bromide (EtBr) solution (100 μl of EtBr in 1 l of water). After at least 10 min of soaking time, the gel was washed to remove excess of EtBr, visualized on a UV transilluminator and photographed.
Reamplification
After electrophoresis, weak bands representing low yield of PCR-product, were reamplified using the band-stab method (Bjourson and Cooper 1992) to obtain a stronger band for purification. To do so, at least 10 μl of each PCR product was reloaded on 1.5% of agarose gel and stained as described above. Under UV light, each band was stabbed with a pipette tip and the tip was soaked in 49 μl of master mix. PCR followed by electrophoresis were repeated as described above to check if the bands were strong enough, representing good yield, for purification.
PCR products purification, quantification and sequencing
The PCR products of the samples which showed strong bands for ITS1 and COI were purified using the Promega kit Wizard® SV Gel and PCR Clean-Up System. After purification, an amount of 1.5 μl of each purified PCR product was used to check the nucleic acid concentration using a spectrophotometer (Nanodrop). For the samples with sufficient concentration (minimum of 15 ng/μl), purified PCR product (5 μl) were mixed separately with 5 μl of 5 μM forward or reverse primer solution to sequence both strands of the PCR-product. Each total of 10 μl was sent for sequencing (Macrogen). The results were compared with sequences available in the National Center of Biotechnology Information (NCBI, USA) database for identification.
Phylogenetic analysis
All sequences were analysed using the software program BioNumerics 7.5 (Applied Maths). This program automatically combines both sequences of the same sample (forward and reverse sequence) into one sequence called a contig. In cases when a contig could not be made due to the lack of an overlap between both sequences, the contig was created manually using the programs Chromas Lite 2.1 (Technelysium, Australia) and BioEdit version 7.1.3.0 (Hall 1999). The sequences of the samples where both regions (ITS1 and COI) could be amplified successfully were loaded into the Mega 6 software program (Tamura et al. 2013) to create a phylogenetic tree (Maximum Likelihood) to visualize possible subgroups within the species S. bradys. In addition, GenBank’s ITS1and COI sequences of S. bradys (7 sequences for ITS1 and 1 for COI) andS. brachyurus (3 sequences for each of ITS1 and COI) were added as references. During the phylogenetic analysis the topography of the trees were tested by bootstrapping (500 repeats). Finally, a 60% consensus tree was constructed.
Assessing the efficiency of Scutellonema bradys-specific primers
The DNA of the samples for which sequences revealed the presence ofS. bradys was used for PCR using the forward and reverse S. bradys-specific primers, SBVF1 (5′- CCTCTCCATGTGTCCCACTT-3′) and SBLR (5’TGCACAAGGCACACATCT-3′) developed by Humphreys-Pereira et al. (2014). PCR was carried out in a volume of 25 μl comprising 2 μl of DNA with 23 μl of Master Mix prepared following the Fermentas protocol: 19.5 μl of water; 2.5 μl of 10X Pfu buffer with MgSO4; 0.5 μl of dNTPs; 0.15 μl of the forward and reverse primers as mentioned above; 0.2 μl of Pfu DNA polymerase. Amplification conditions were as described by Humphreys-Pereira et al. (2014): 94 °C for 5 min, then 35 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 60 s. The final extension was at 72 °C for 7 min. PCR products were separated by electrophoresis and stained as for analysis of the ITS or COI region.
Results
Sequences comparison of ITS1 and COI regions and phylogenetic analysis
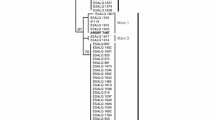
Not all 138 samples produced sequences with sufficient quality for analysis, although Scutellonema sp. were obtained from all samples. Finally, a total of 51 sequences (Table 1) for both ITS1 and COI derived from the same samples were adequate for phylogenetic analysis and were compared with sequences available in the NCBI database. All were identified as belonging to S. bradys. The sizes of our sequences varied among samples and can be consulted in the NCBI databank where all sequences have been deposited (Table 1). A 60% consensus tree was generated. In the ITS1-based ML-tree (Maximum Likelihood) (Fig. 2), two separate groups were visible. The first including all of the 51 sequences and seven S. bradys sequences from GenBank (AY274816-S.bradys, KC999098-S.bradys, KC999096-S.bradys, AY274818-S.bradys, KC999099-S.bradys, KC999091-S.bradys, KC999097-S.bradys). In this group and with decreasing consensus percentage, smaller subgroups were visible but without any specificity in terms of origin: yam host, village, diversity or agroecological zone. The three sequences of S. brachyurus (JX472076-S.brachyurus, JX472077-S.brachyurus and JX472085-S.brachyurus) were gathered in the second group.
Phylogenetic 60% consensus ML-tree of the ITS1-based sequences of 51 populations of S. bradys from Benin (see Table 1 for code) and 10 reference sequences from GenBank. Numbers between 60 and 100 are bootstrap values and those smaller than 1 are the distance values which were representative for the number of substitutions. Distance values lower than 0.01 are not mentioned
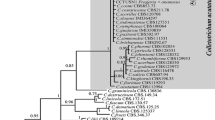
In the COI-based ML-tree (Maximum Likelihood) (Fig. 3), three separate groups were visible. The first comprised 44 sequences and one S. bradys sequence from GenBank (JX472088-S.bradys). These 44 sequences belong to S. bradys extracted from different cultivars collected from different villages belonging to different agroecological and diversity zones. Three subgroups were visible in this group, with decreasing consensus percentages, but without practical meaning or specific properties. The second group assembled seven sequences of which six belong to the same agroecological (sub-humid Savannah) and diversity (Center-South) zones. The third group arranged the sequences of S. brachyurus (JX472098-S.brachyurus, JX472093-S.brachyurus and JX472092-S.brachyurus).
Phylogenetic 60% consensus ML-tree of the COI-based sequences of 51 populations of S. bradys from Benin (see Table 1 for code) and four reference sequences from GenBank. Numbers between 60 and 100 are bootstrap values and those smaller than 1 are the distance values which were representative for the number of substitutions. Distance values lower than 0.01 are not mentioned
The subgroups in the COI-based ML-tree are not visible in the ITS-based ML-tree, and vice versa, small subgroups in the ITS-based ML-tree are not visible in the COI ML-tree.
Scutellonema bradys-specific primers efficiency assessment
All the 51 samples positive for S. bradys based on sequence analysis were also positive with the species-specific primers (Fig. 4).
Discussion
Of the 138 nematode samples, only 51 (36.95%) yielded sequences of sufficient quality. Probably deterioration of the nematodes during transportation of nematode suspensions to Belgium led to problems with DNA extraction, yielding insufficient amounts of DNA. It is known that the efficiency of DNA extraction from a sample depends on the quality of nematodes (Perry et al. 2007). There was a difference between the quality of DNA extraction from nematodes extracted in Benin and transported in water suspension to Belgium and DNA extraction from nematodes freshly extracted from peels. This indicates that nematodes freshly extracted from peels are more efficient for molecular diagnostics than those in a water suspension. Perry et al. (2007) reported that if the period between nematode extraction and molecular analyses is several days or weeks, nematodes should be kept at low temperatures before use. According to the authors, the best approach is to use live nematodes or to kill them by heating briefly but leave the DNA undamaged for diagnostics. During extended field sampling visits, fixation in 75–90% alcohol, glycerol or simply drying the nematodes in a plastic tube are alternative methods to preserve nematode DNA for further molecular study.
The 51 populations for which it was possible to study the ITS1 and COI regions can be considered as representative for the yam growing regions of Benin. They originated from 28 villages and each of the four agroecological and diversity zones.
Sequence comparison of ITS1 and COI regions and phylogenetic analysis enabled a distinctive separation between the S. brachyurus sequences and the group with the sequences of S. bradys. This indicates that S. bradys populations from Benin are monophyletic and clearly distinct from other Scutellonema species. Within the S. bradys sequences, no genetic separation was visible, neither by yam cultivar from which nematodes were extracted nor by the corresponding villages, their diversity zone or the agroecological zone. This can possibly be explained by the extensive exchange of planting material between yam farmers. Yam exchange flow, including planting material, between producers in Benin has been reported previously (Loko et al. 2013; Baco et al. 2014). Dissemination of nematodes can occur from one region to another, when a population of S. bradys originating from one place can be present in low densities in an apparently healthy tuber. Comparatively low densities of nematodes occurring in tubers without any external symptoms of damage have been reported in Nigeria (Bridge 1973; Kolombia et al. 2017). Similar results were obtained withS. bradys populations in Costa Rica, where no separation by locality or by yam host were noticed (Humphreys-Pereira et al. 2014). Our finding corroborates those of Coyne et al. (2006a) and Bridge et al. (2005), who reported that, as yam is a vegetative propagated crop, untreated infected material used for planting perpetuates the disease cycle.
Subgroups observed with both ITS1 and COI DNA regions can be attributed to the molecular polymorphism and the genetic diversity within S. bradys populations. However, they are few in number and not well supported phylogenetically. In this way, our results do not support partly the conclusions of Coyne et al. (2006b, 2012) and Kolombia et al. (2017) who reported the relatively high degree of polymorphism both within and between West AfricanS. bradys populations, indicating genetic diversity within as well as between populations. As S. bradys was spread rapidly through intensive exchange of planting material and the polymorphisms cannot be linked to any biological factor, there is no reason to consider them as separate subspecies.
Maximum likelihood (ML) is generally considered to make the most efficient use of data to provide the most accurate estimates of phylogeny. The basic idea is to compute the probability of the observed data assuming it has evolved under a particular evolutionary tree and a given probabilistic model of substitution (Subbotin et al. 2013). Both the 60% consensus COI-based ML-tree and the ITS1-based ML-tree show that the visible subgroups were not very well supported due to the lower bootstrap value (less than 70). Also, the visible subgroups in the COI-based ML-tree were not confirmed in the ITS-based ML-tree. Similarly, the smaller subgroups in the ITS-based ML-tree were not visible in the COI ML-tree. This supports our conclusion that there is no indication of subspecies in the S. bradys populations in Benin. Consequently, one given population can be used to screen all yam cultivars for resistance to S. bradys. Moreover, Coyne et al. (2012) reported that greater variability of S. bradys damage may occur because of environmental effects and host differences, than due to differences in pathogenicity of these populations. Therefore, on-farm screening in the different agroecological zones where different environmental conditions prevail is preferred over screening with different populations at one location. This is the first extensive molecular phylogenetic analysis of S. bradys populations from Benin.
In addition, the current study confirms that the species-specific primer set described by Humphreys-Pereira et al. (2014) is a reliable and rapid method for S. bradys identification. This primer set could be used for high throughput analysis of samples from yam and yam fields to detect S. bradys.
Change history
09 January 2020
This paper should have published with open access. It has been corrected online.
09 January 2020
This paper should have published with open access. It has been corrected online.
References
Affokpon A, Coyne DL, Htay CC, Dossou Agbèdè R, Lawouin L, Coosemans J (2011) Biocontrol potential of native Trichoderma isolates against root-knot nematodes in West African vegetable production systems. Soil Biology & Biochemistry 43:600–608
Ayala A, Acosta N (1971) Observations on yam (Dioscorea alata) nematodes. Nematropica 1:39–40
Baco MN, Moumouni IM, Idrissou L, Egah J, Tossou RC (2014) Réseaux sociaux et système semencier igname au nord Bénin. Agronomie Africaine 26:181–192
Baimey H (2005) Scutellonema bradys as a pathogen of yam in Benin. PhD Thesis. University of Pretoria. South Africa
Baimey H, Coyne D, Labuschagne N (2009) Pathogenicity of Scutellonema bradys populations from different geographical areas in Benin on yam (Dioscorea spp.). Crop Protection 28:715–721
Bjourson AJ, Cooper JE (1992) Band-stab PCR: a simple technique for the purification of individual PCR products. Nucleic Acids Research 20:4675
Bridge J (1973) Nematodes as pests of yams in Nigeria. Mededelingen Faculteit Landbouwwetenschappen Gent 38:841–852
Bridge J, Coyne DL, Kwoseh CK (2005) Nematode parasites of tropical root and tuber crops. In: Luc M, Sikora RA, Bridge J (eds) Plant parasitic nematodes in subtropical and tropical agriculture. CAB International, Wallingford, pp 221–258
Coyne DL, Tchabi A, Baimey H, Labuschagne N, Rotifa I (2006a) Distribution and prevalence of nematodes (Scutellonema bradys and Meloidogyne spp.) on marketed yam (Dioscorea spp.) in West Africa. Field Crops Research 96:142–150
Coyne DL, Williamson VM, Banna AB, Tchabi A, Baimey H, Labuschagne N, Rotifa I (2006b) Analysis of the pathogenic, morphological and genetic variability of S. bradys on yam in West Africa. African Plant Protection 12:1
Coyne DL, Akpheokhai LI, Adeniran AF (2011) The yam nematode (Scutellonema bradys), a potential threat to potato (Solanum tuberosum) production in West Africa. Plant Pathology 60:992–997
Coyne DL, Williamson V, Tchabi A, Baimey H, Rotifa I (2012) Comparison of pathogenicity of geographically separate populations of Scutellonema bradys on yam (Dioscorea spp.) in West Africa. Nematropica 42:181–190
Coyne DL, Kolombia YA, Luambano N, Bert W (2016) First report of dry rot disease of yam caused by Scutellonema bradys in East Africa. Plant Disease 100:1794–1794
Dansi A, Mignouna HD, Zoundjihekpon J, Sangare A, Asiedu R, Quin FM (1999) Morphological diversity, cultivar groups and possible descent in the cultivated yams (Dioscorea cayenensis-Dioscorea rotundata complex) of Benin Republic. Genetic Resources and Crop Evolution 46:371–388
Derycke S, Vanaverbeke J, Rigaux A, Backeljau T, Moens T (2010) Exploring the use of cytochrome oxidase c subunit 1 (COI) for DNA barcoding of free-living nematodes. PLoS One 5:e13716
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium. Series 41:95–98
Hendrickx G (1995) An automatic apparatus for extracting free-living nematode stages from soil. Nematologica 41:308
Holterman M, van der Wurff A, van den Elsen S, van Megen H, Bongers T, Holovachov O, Bakker J, Helder J (2006) Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Molecular Biology and Evolution 23:1792–1800
Humphreys-Pereira DA, Williamson VM, Lee S, Coyne DL, Salazar L, Gomez-Alpizar L (2014) Molecular and morphological characterization of Scutellonema bradys from yam in Costa Rica and development of specific primers for its detection. Nematology 16:137–147
Kolombia YA, Karssen G, Viaene N, Kumar PL, Joos L, Coyne DL, Bert W (2017) Morphological and molecular characterization of Scutellonema species from yam (Dioscorea spp.) and a key to the species of the genus. Nematology 19:751–787
Loko YL, Dansi A, Linsoussi C, Tamo M, Vodouhè R, Akoegninou A, Sanni A (2013) Current status and spatial analysis of Guinea yam (Dioscorea cayenensis Lam. -D. rotundata Poir. complex) diversity in Benin. International Research Journal of Agricultural Science and Soil Science 3:219–238
Loko YL, Adjatin A, Dansi A, Vodouhe R, Sanni A (2015) Participatory evaluation of Guinea yam (Dioscorea cayenensis Lam.–D. rotundata Poir. complex) landraces from Benin and agro-morphological characterization of cultivars tolerant to drought, high soil moisture and chips storage insects. Genetic Resources and Crop Evolution 62:1181–1192
Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H (1986) Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symposia on Quantitative Biology. 51:263–273
Mwamula AO, Waeyenberge L, Viaene N (2015) The yam nematode,Scutellonema bradys, a new threat to potato. Potato Research 58:189–203
Perry RN, Subbotin SA, Moens M (2007) Molecular diagnostics of plant-parasitic nematodes. In: Punja ZK, De Boer SH, Sanfaçon H (2007) Biotechnology and plant disease management. CAB International. pp. 195-226
Sher SA (1964) Revision of the Hoplolaiminae (Nematoda) III.Scutellonema Andrássy, 1958. Nematologica 9:421–443
Siddiqi MR (2000) Tylenchida parasites of plants and insects, 2nd edn. CAB International, Wallingford 833 p
Subbotin SA, Waeyenberge L, Moens M (2013) Molecular systematics. In: Perry RN, Subbotin SA, Moens M (eds) Plant nematology, 2nd edn. CAB International, Wallingford, pp 41–58
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30:2725–2729
van den Berg E, Tiedt LR, Coyne DL, Ploeg AT, Navas-Cortés JA, Roberts PA, Yeates GW, Subbotin SA (2013) Morphological and molecular characterization and diagnostics of some species of Scutellonema Andrássy, 1958 (Tylenchida: Hoplolaimidae) with a molecular phylogeny of the genus. Nematology 15:719–745
Vrain TC, Wakarchuk DA, Lévesque AC, Hamilton RL (1992) Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundamental and Applied Nematology 15:563–573
Acknowledgements
Funding for this study was provided by the project “Yam Nematodes Benin” (YANEB/BGMF-PEARL). Thanks to Yoika Foucart, Philippe Akodji, Rachid Tahzima and to all YANEB team for their diverse contributions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Diana Fernandez
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Etchiha Afoha, S.A.P., Affokpon, A., Waeyenberge, L. et al. Molecular diversity of Scutellonema bradys populations from Benin, based on ITS1 rDNA and COI mtDNA. Trop. plant pathol. 43, 323–332 (2018). https://doi.org/10.1007/s40858-018-0221-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-018-0221-5