Abstract
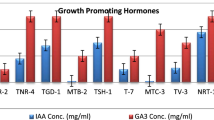
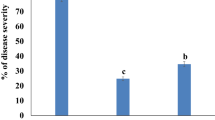
Fusarium wilt of tomato (FW) caused by Fusarium oxysporum f. sp. lycopersici (FOL) is a major challenge for tomato production worldwide. For sustainable management of FW, the potential of five strains of Trichoderma asperellum was evaluated under greenhouse conditions. The results indicated that FOL infected plants treated with T. asperellum strains significantly reduced disease incidence and severity compared with FOL-only infected plants. The reduction of wilt disease on plants treated with T. asperellum strains was accompanied by a significant reduction in FOL populations in tomato stems and rhizosphere. Moreover, the application of T. asperellum promoted tomato plant growth irrespective of the presence or absence of FOL. Two strains of T. asperellum (TS-12 and TS-39) that showed the best performance in minimizing disease development and increases in plant growth parameters were selected for elucidating their ability in triggering tomato defense mechanisms. The expression levels of defense-related genes, chitinase (SlChi3), β-1,3-glucanase (SlGluA) and PR-1 (SlPR-1a) were significantly increased in the stems and roots of Trichoderma treated, FOL infected plants, compared with FOL-only infected ones. These results indicate that the application of T. asperellum strains TS-12 and TS-39 can be used as an alternative strategy to manage FW through their antagonistic activities and abilities to induce systemic resistance.





Similar content being viewed by others
References
Abdallah NA, Shah D, Abbas D, Madkour M (2012) Stable integration and expression of a plant defensin in tomato confers resistance to fusarium wilt. GM Crops 1:344–350
Abu Yaman IK, Abu Blan HA (1972) Major diseases of cultivated crops in the Central Province of Saudi Arabia. 2. diseases of vegetables. Zeitschrift für Pflanzenkrankheiten und Pflanzenschatz 79:227–231
Aimé S, Cordier C, Alabouvette C, Olivain C (2008) Comparative analysis of PR gene expression in tomato inoculated with virulent Fusarium oxysporum f. sp. lycopersici and the biocontrol strain F. oxysporum Fo47. Physiol Mol Plant Pathol 73:9–15
Aleandri MP, Chilosi G, Bruni N, Tomassini A, Vettraino AM, Vannini A (2015) Use of nursery potting mixes amended with local Trichoderma strains with multiple complementary mechanisms to control soil-borne diseases. Crop Prot 67:269–278
Alizadeh H, Behboudi K, Ahmadzadeh M, Javan-Nikkhah M, Zamioudis C, Pieterse CMJ, Bakker PAHM (2013) Induced systemic resistance in cucumber and Arabidopsis thaliana by the combination of Trichoderma harzianum Tr6 and Pseudomonas sp. Ps14. Biol Control 65:14–23
Avis TJ, Hamelin RC, Bélanger RR (2001) Approaches to molecular characterization of fungal biocontrol agents: some case studies. Can J Plant Pathol 23:8–12
Barakat RM, Al-Masri MI (2009) Trichoderma harzianum in combination with sheep manure amendment enhances soil suppressiveness of Fusarium wilt of tomato. Phytopathol Mediterr 48:385–395
Beckman CH (1987) The nature of wilt disease of plants. APS Press, St. Paul
Benhamou N, Bélanger R (1998) Benzothiadiazole-mediated induced resistance to Fusarium oxysporum f. sp. radicis-lycopersici in tomato. Plant Physiol 118:1203–1212
Benítez T, Ana M, Rincón AM, Limón MC, Codón AC (2004) Biocontrol mechanisms of Trichoderma strains. Int Microbiol 7:249–260
Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O, Junge H, Voigt B, Jungblut PR, Vater J, Süssmuth R, Liesegang H, Strittmatter A, Gottschalk G, Borriss R (2007) Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol 25:1007–1014
Chet I, Inbar J (1994) Biological control of fungal pathogens. Appl Biochem Biotechnol 48:37–43
Chowdappa P, Mohan Kumar SP, Jyothi Lakshmi M, Upreti KK (2013) Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol Control 65:109–117
Conrath U, Pieterse CMJ, Mauch-Mani B (2002) Priming in plant-pathogen interactions. Trends Plant Sci 7:209–216
De Cal A, Pascual S, Larena I, Melgarejo P (1995) Biological control of Fusarium oxysporum f.sp. lycopersici. Plant Pathol 44:909–917
De Palma M, D’Agostino N, Proietti S, Bertini L, Loritoe M, Ruocco M, Caruso C, Chiusano ML, Tucci M (2016) Suppression subtractive hybridization analysis provides new insights into the tomato (Solanum lycopersicum L.) response to the plant probiotic microorganism Trichoderma longibrachiatum MK1. J. Plant Physiol 190:79–94
Dekker J (1981) Resistance to fungicides in plant pathogens: abstracts of papers. Neth J Plant Pathol 87:233–255
Dubey SC, Suresh M, Singh B (2007) Evaluation of Trichoderma species against Fusarium oxysporum f.sp. ciceris for integrated management of chickpea wilt. Biol Control 40:118–127
El_Komy MH, Saleh AA, Ernthodi A, Molan YY (2015) Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato Fusarium wilt. Plant Pathol J 30:50–60
Elad Y, Chet I (1983) Improved selective media for isolation of Trichoderma spp. or Fusarium spp. Phytoparasitica 11:55–58
Gams W, Bissett J (1998) Morphology and identification of Trichoderma. In: Kubicek CP, Harman GE (eds) Trichoderma and Gliocladium, vol. 1. basic biology, taxonomy, and genetics. Taylor and Francis, London, pp 3–34
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research, 2nd edn. Wiley, New York
Gravel V, Antoun H, Tweddell RJ (2007) Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem 39:1968–1977
Hammerschmidt R, Kuc JJ (1995) Induced resistance to disease in plants. Kluwer Academic Publishers, Dordsecht
Harman GE (2000) Myths and dogmas of biocontrol: changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis 84:377–393
Harman GE (2006) Overview of mechanisms and uses of Trichoderma spp. Phytopathology 96:190–194
Harman GE (2011) Multifunctional fungal plant symbionts: new tools to enhance plant growth and productivity. New Phytol 189:647–649
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev 2:43–56
Hermosa R, Rubio MB, Cardoza RE, Nicolás C, Monte E, Gutiérrez S (2013) The contribution of Trichoderma to balancing the costs of plant growth and defense. Int Microbiol 16:69–80
Hibar K, Daami-Remadi M, Hamada W, El Mahjoub M (2006) Bio-fungicides as an alternative for tomato Fusarium crown and root rot control. Tunis J Plant Prot 1:19–29
Horinouchi H, Katsuyama N, Taguchi Y, Hyakumachi M (2008) Control of Fusarium crown and root rot of tomato in a soil system by combination of a plant growth-promoting fungus, Fusarium equiseti, and biodegradable pots. Crop Prot 27:859–864
Howell CR (2003) Mechanisms employed by Trichoderma species in the biological control of plant diseases, the history and evolution of current concepts. Plant Dis 87:4–10
Huang X, Chen L, Ran W, Shen Q, Yang X (2011) Trichoderma harzianum strain SQR-T37 and its bio-organic fertilizer could control Rhizoctonia solani damping-off disease in cucumber seedlings mainly by the mycoparasitism. Appl Microbiol Biotechnol 91:741–755
Jones JB, Jones JP, Stall RE, Zitter TA (1991) Compendium of tomato diseases. APS Press, St. Paul
Khan J, Ooka JJ, Miller SA, Madden LV, Hoitink HAJ (2004) Systemic resistance induced by 573 Trichoderma hamatum 382 in cucumber against Phytophthora crown rot and leaf blight. Plant Dis 88:280–286
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Komada H (1975) Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Rev Plant Prot Res 8:114–123
Kubicek CP, Mach RL, Peterbauer CK, Lorito M (2001) Trichoderma: from genes to biocontrol. J Plant Pathol 83:11–23
Larkin RP, Fravel DR (1998) Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Dis 82:1022–1028
Leslie JF, Summerell BA (2006) The Fusarium laboratory manual. Blackwell Publishing, Ames
Lorito M, Woo S (2015) Trichoderma: a multi-purpose tool for integrated pest management. In: Lugtenberg B (ed) Principles of plant-microbe interactions. Springer International Publishing, Zurich, pp 345–353
Meyer SFM, Roberts DP (2002) Combinations of biocontrol agents for management of plant-parasitic nematodes and soilborne plant pathogenic fungi. J Nematol 34:1–8
Miranda MEA, Estrella HA, Cabriales JJP (2006) Colonization of the rhizosphere, rhizoplane and endorhiza of garlic (Allium sativum L.) by strains of Trichoderma harzianum and their capacity to control allium white-rot under field conditions. Soil Biol Biochem 38:1823–1830
Moreno CA, Cotes AM (2007) Survival in the phylloplane of Trichoderma koningii and biocontrol activity against tomato foliar pathogens. IOBC/WPRS Bull 30:557–561
Mukherjee M, Mukherjee PK, Horwitz BA, Zachow C, Berg G, Zeilinger S (2012) Trichoderma-plant pathogen interactions: advances in genetics of biological control. Indian J Microbiol 52:522–529
Nicolás C, Hermosa R, Rubio B, Mukherjee P, Monte E (2014) Trichoderma genes in plants for stress tolerance - status and prospects. Plant Sci 228:71–78
Osuinde M, Aluya E, Emoghene A (2002) Control of Fusarium wilt of tomato (Lycopersicon esculentum Mill) by Trichoderma species. Acta Phytopathol Entomol Hung 37:47–55
Papavizas GC (1985) Trichoderma and Gliocladium: biology, ecology, and potential for biocontrol. Annu Rev Phytopathol 23:23–54
Pfaffi MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:614–645
Pozo MJ, Van loon LC, Pieterse CMJ (2005) Jasmonates-signals in plant–microbe interactions. J Plant Growth Regul 23:211–222
Rifai MA (1969) A revision of the genus Trichoderma. Mycol Papers 116:1–56
SAS Institute Inc (2003) SAS/STATA guide for personal computers version 9.1 edition. SAS Institute, Carry NC, USA
Segarra G, Casanova E, Avilés M, Trillas I (2010) Trichoderma asperellum strain T34 controls Fusarium wilt disease in tomato plants in soilless culture through competition for iron. Microbial Ecol 59:141–149
Shoresh M, Yedidia I, Chet I (2005) Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 95:76–84
Sid Ahmad A, Sanchez CP, Candela ME (2000) Evaluation of induction of systemic resistance in pepper plants (Capsicum annuum) to Phytophthora capsici using Trichoderma harzianum and its relation with capsidiol accumulation. Eur J Plant Pathol 106:817–824
Taheri P, Tarighi S (2012) The role of pathogenesis-related proteins in the tomato-Rhizoctonia solani interaction. J Botany. doi:10.1155/2012/137037
Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, Lorito M (2008) Trichoderma-plant pathogen interactions. Soil Biol Biochem 40:1–10
Zahoor A, Saifulla FR, Hakim K, Muhammad I (2012) Chemical and biological control of root rot of Okra. Pak J Bot 44:453–457
Zang W, Dick WA, Hoitink HAJ (1996) Compost-induced systemic acquired resistance in cucumber to Pythium root rot and anthracnose. Phytopathology 86:1066–1070
Acknowledgments
This research was financially supported by the King Abdulaziz City for Science and Technology (KACST), Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Trazilbo J. de Paula Jr.
Rights and permissions
About this article
Cite this article
El_Komy, M.H., Saleh, A.A., Ibrahim, Y.E. et al. Trichoderma asperellum strains confer tomato protection and induce its defense-related genes against the Fusarium wilt pathogen. Trop. plant pathol. 41, 277–287 (2016). https://doi.org/10.1007/s40858-016-0098-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-016-0098-0