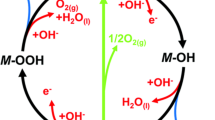
Abstract
Alkaline water electrolysis (AWE) is a prospective method for producing green hydrogen to solve the energy crisis and reduce environmental pollution. However, the hydrogen production efficiency through this process is exceptionally low because the electrodes are expensive and inefficient. In this work, a kind of high-entropy alloy (HEA), bulk AlCoCrFeNi, is used as the efficient electrode for AWE. Results show that the HEA treated by 5-min fast anodization can achieve ultra-high catalytic activities for hydrogen and oxygen evolution reactions with low overpotentials of 880 and 845 mV to reach the current densities of −500 and 500 mA cm−2, respectively. For full water splitting, it only needs 3.00 V and exhibits excellent stability of more than 100 h at 500 mA cm−2. Our study demonstrates that the anodized AlCoCrFeNi HEA has promising applications as a highly efficient catalyst in industrial water electrolysis for hydrogen production, potentially addressing the energy crisis and environmental concerns.

摘要
碱性水电解(AWE)作为一种具有工业应用前景的绿色制氢方法, 能够用来改善能源短缺和环境污染问题. 然而, 由于电极材料昂贵且效率低下, 这种方法生产氢气的效率比较低. 本文采用块状AlCoCrFeNi高熵合金作为碱性电解水的有效电极. 研究发现通过快速阳极氧化(5 min)处理的高熵合金可以同时对析氢和析氧反应(HER和OER)具有超高的催化活性, 只需要8 8 0 和8 4 5 mV的过电位就可以达到−500 mA cm−2 (HER)和500 mA cm−2 (OER)的电流密度. 特别地, 该催化剂只需要3.00 V就可以达到500 mA cm−2的全解水电流密度, 并且在此电流密度下表现出超过100小时的出色稳定性. 我们的研究表明, 阳极氧化的块体AlCoCrFeNi高熵合金作为高效催化剂在工业水电解制氢中具有广阔的应用前景, 有望用于缓解环境问题和能源危机.
Similar content being viewed by others
References
Ye KH, Wang Z, Li H, et al. A novel CoOOH/(Ti,C)−Fe2O3 nanorod photoanode for photoelectrochemical water splitting. Sci China Mater, 2018, 61: 887–894
Yang M, Shang C, Li F, et al. Synergistic electronic and morphological modulation on ternary Co1−xVxP nanoneedle arrays for hydrogen evolution reaction with large current density. Sci China Mater, 2020, 64: 880–891
Liang C, Zou P, Nairan A, et al. Exceptional performance of hierarchical Ni−Fe oxyhydroxide@NiFe alloy nanowire array electrocatalysts for large current density water splitting. Energy Environ Sci, 2020, 13: 86–95
Wang J, Gao Y, Kong H, et al. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem Soc Rev, 2020, 49: 9154–9196
Wang J, Kim SJ, Liu J, et al. Redirecting dynamic surface restructuring of a layered transition metal oxide catalyst for superior water oxidation. Nat Catal, 2021, 4: 212–222
Shang X, Tang JH, Dong B, et al. Recent advances of nonprecious and bifunctional electrocatalysts for overall water splitting. Sustain Energy Fuels, 2020, 4: 3211–3228
Du X, Ai H, Chen M, et al. PLD-fabricated perovskite oxide nanofilm as efficient electrocatalyst with highly enhanced water oxidation performance. Appl Catal B-Environ, 2020, 272: 119046
Mahmood N, Yao Y, Zhang JW, et al. Electrocatalysts for hydrogen evolution in alkaline electrolytes: Mechanisms, challenges, and prospective solutions. Adv Sci, 2018, 5: 1700464
Chen W, Hu Y, Peng P, et al. Multidimensional Ni−Co-sulfide heterojunction electrocatalyst for highly efficient overall water splitting. Sci China Mater, 2022, 65: 2421–2432
Xiao Y, Hu T, Zhao X, et al. Thermo-selenizing to rationally tune surface composition and evolve structure of stainless steel to electrocatalytically boost oxygen evolution reaction. Nano Energy, 2020, 75: 104949
Li C, Hou J, Wu Z, et al. Acid promoted Ni/NiO monolithic electrode for overall water splitting in alkaline medium. Sci China Mater, 2017, 60: 918–928
Zhou Q, Xu C, Li Y, et al. Synergistic coupling of NiFeZn−OH nanosheet network arrays on a hierarchical porous NiZn/Ni heterostructure for highly efficient water splitting. Sci China Mater, 2021, 65: 1207–1216
Zheng X, Chen Y, Bao X, et al. In situ formed bimetallic carbide Ni6Mo6C nanodots and NiMoOx nanosheet array hybrids anchored on carbon cloth: Efficient and flexible self-supported catalysts for hydrogen evolution. ACS Catal, 2020, 10: 11634–11642
Wang H, Zhang X, Wang J, et al. Puffing quaternary FexCoyNi1−x−yP nanoarray via kinetically controlled alkaline etching for robust overall water splitting. Sci China Mater, 2020, 63: 1054–1064
Liu D, Ai H, Li J, et al. Surface reconstruction and phase transition on vanadium-cobalt-iron trimetal nitrides to form active oxyhydroxide for enhanced electrocatalytic water oxidation. Adv Energy Mater, 2020, 10: 2002464
Guo F, Wu Y, Chen H, et al. High-performance oxygen evolution electrocatalysis by boronized metal sheets with self-functionalized surfaces. Energy Environ Sci, 2019, 12: 684–692
Song C, Liu Y, Wang Y, et al. Highly efficient oxygen evolution and stable water splitting by coupling NiFe LDH with metal phosphides. Sci China Mater, 2021, 64: 1662–1670
Guan J, Li C, Zhao J, et al. FeOOH-enhanced bifunctionality in Ni3N nanotube arrays for water splitting. Appl Catal B-Environ, 2020, 269: 118600
Hu Y, Luo G, Wang L, et al. Single Ru atoms stabilized by hybrid amorphous/crystalline FeCoNi layered double hydroxide for ultraefficient oxygen evolution. Adv Energy Mater, 2021, 11: 2002816
Li YK, Zhang G, Lu WT, et al. Amorphous Ni−Fe−Mo suboxides coupled with Ni network as porous nanoplate array on nickel foam: A highly efficient and durable bifunctional electrode for overall water splitting. Adv Sci, 2020, 7: 1902034
Zhou PF, Xiao DH, Yuan TC. Microstructure, mechanical and corrosion properties of AlCoCrFeNi high-entropy alloy prepared by spark plasma sintering. Acta Metall Sin (Engl Lett), 2019, 33: 937–946
Zhou PF, Xiao DH, Wu Z, et al. Al0.5FeCoCrNi high entropy alloy prepared by selective laser melting with gas-atomized pre-alloy powders. Mater Sci Eng-A, 2019, 739: 86–89
Gao J, Jin Y, Fan Y, et al. Fabricating antibacterial CoCrCuFeNi high-entropy alloy via selective laser melting and in-situ alloying. J Mater Sci Tech, 2022, 102: 159–165
Zhou P, Liu D, Chen Y, et al. Corrosion engineering boosting bulk Fe50Mn30Co10Cr10 high-entropy alloy as high-efficient alkaline oxygen evolution reaction electrocatalyst. J Mater Sci Tech, 2022, 109: 267–275
Batchelor TAA, Pedersen JK, Winther SH, et al. High-entropy alloys as a discovery platform for electrocatalysis. Joule, 2019, 3: 834–845
Huang J, Wang P, Li P, et al. Regulating electrolytic Fe0.5CoNiCuZnx high entropy alloy electrodes for oxygen evolution reactions in alkaline solution. J Mater Sci Tech, 2021, 93: 110–118
Ma P, Zhang S, Zhang M, et al. Hydroxylated high-entropy alloy as highly efficient catalyst for electrochemical oxygen evolution reaction. Sci China Mater, 2020, 63: 2613–2619
Feng G, Ning F, Song J, et al. Sub-2 nm ultrasmall high-entropy alloy nanoparticles for extremely superior electrocatalytic hydrogen evolution. J Am Chem Soc, 2021, 143: 17117–17127
Cui M, Yang C, Li B, et al. High-entropy metal sulfide nanoparticles promise high-performance oxygen evolution reaction. Adv Energy Mater, 2020, 11: 2002887
Huang X, Chang S, Lee WSV, et al. Three-dimensional printed cellular stainless steel as a high-activity catalytic electrode for oxygen evolution. J Mater Chem A, 2017, 5: 18176–18182
Tang J, Xu JL, Ye ZG, et al. Microwave sintered porous CoCrFeNiMo high entropy alloy as an efficient electrocatalyst for alkaline oxygen evolution reaction. J Mater Sci Tech, 2021, 79: 171–177
Li W, Hu Q, Liu Y, et al. Powder metallurgy synthesis of porous Ni-Fe alloy for oxygen evolution reaction and overall water splitting. J Mater Sci Tech, 2020, 37: 154–160
Jia Z, Yang T, Sun L, et al. A novel multinary intermetallic as an active electrocatalyst for hydrogen evolution. Adv Mater, 2020, 32: 2000385
Fang G, Gao J, Lv J, et al. Multi-component nanoporous alloy/(oxy)-hydroxide for bifunctional oxygen electrocatalysis and rechargeable Zn-air batteries. Appl Catal B-Environ, 2020, 268: 118431
Todoroki N, Wadayama T. Heterolayered Ni−Fe hydroxide/oxide nanostructures generated on a stainless-steel substrate for efficient alkaline water splitting. ACS Appl Mater Interfaces, 2019, 11: 44161–44169
Zhou P, Niu P, Liu J, et al. Anodized steel: The most promising bifunctional electrocatalyst for alkaline water electrolysis in industry. Adv Funct Mater, 2022, 32: 2202068
Abrashev MV, Ivanov VG, Stefanov BS, et al. Raman spectroscopy of alpha-FeOOH (goethite) near antiferromagnetic to paramagnetic phase transition. J Appl Phys, 2020, 127: 205108
Yang JJ, Martens WN, Frost RL. Transition of chromium oxyhydroxide nanomaterials to chromium oxide: A hot-stage Raman spectroscopic study. J Raman Spectrosc, 2011, 42: 1142–1146
Meng T, Li Q, Yan M, et al. Electrochemically induced in-situ surface self-reconstruction on Ni, Fe, Zn ternary-metal hydroxides towards the oxygen-evolution performance. Chem Eng J, 2021, 410: 128331
Masikhwa TM, Madito MJ, Momodu D, et al. High electrochemical performance of hybrid cobalt oxyhydroxide/nickel foam graphene. J Colloid Interface Sci, 2016, 484: 77–85
Li Y, Wang H, Xie L, et al. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J Am Chem Soc, 2011, 133: 7296–7299
Du X, Guo J, Chen M, et al. Surface reconstruction on silver nanoparticles decorated trimetallic hydroxide nanosheets to generate highly active oxygen-deficient (oxy)hydroxide layer for high-efficient water oxidation. Chem Eng J, 2021, 425: 131662
Zhou Q, Chen Y, Zhao G, et al. Active-site-enriched iron-doped nickel/cobalt hydroxide nanosheets for enhanced oxygen evolution reaction. ACS Catal, 2018, 8: 5382–5390
Hardcastle FD, Wachs IE. Raman spectroscopy of chromium oxide supported on Al2O3, TiO2 and SiO2: A comparative study. J Mol Catal, 1988, 46: 173–186
Meng F, Wang Q, Li H, et al. Corrosion behavior for 3Cr steel under oil-water two-phase laminar flow conditions. Chin J Eng, 2020, 42: 1029–1039
Gao ZW, Ma T, Chen XM, et al. Strongly coupled CoO nanoclusters/CoFe LDHs hybrid as a synergistic catalyst for electrochemical water oxidation. Small, 2018, 14: 1800195
Ling T, Zhang T, Ge B, et al. Well-dispersed nickel- and zinc-tailored electronic structure of a transition metal oxide for highly active alkaline hydrogen evolution reaction. Adv Mater, 2019, 31: 1807771
Tsai FT, Deng YT, Pao CW, et al. The HER/OER mechanistic study of an FeCoNi-based electrocatalyst for alkaline water splitting. J Mater Chem A, 2020, 8: 9939–9950
Sun H, Chen L, Lian Y, et al. Topotactically transformed polygonal mesopores on ternary layered double hydroxides exposing under-coordinated metal centers for accelerated water dissociation. Adv Mater, 2020, 32: 2006784
Huang J, Sheng H, Ross RD, et al. Modifying redox properties and local bonding of Co3O4 by CeO2 enhances oxygen evolution catalysis in acid. Nat Commun, 2021, 12: 3036
Xu CY, Zhang PX, Yan L. Blue shift of Raman peak from coated TiO2 nanoparticles. J Raman Spectrosc, 2001, 32: 862–865
Iqbal MW, Shahzad K, Akbar R, et al. A review on Raman finger prints of doping and strain effect in TMDCs. MicroElectron Eng, 2020, 219: 111152
Bo X, Li Y, Chen X, et al. Operando Raman spectroscopy reveals Cr-induced-phase reconstruction of NiFe and CoFe oxyhydroxides for enhanced electrocatalytic water oxidation. Chem Mater, 2020, 32: 4303–4311
Qin F, Zhao Z, Alam MK, et al. Trimetallic NiFeMo for overall electrochemical water splitting with a low cell voltage. ACS Energy Lett, 2018, 3: 546–554
Wang K, Du H, He S, et al. Kinetically controlled, scalable synthesis of γ-FeOOH nanosheet arrays on nickel foam toward efficient oxygen evolution: The key role of in-situ-generated γ-NiOOH. Adv Mater, 2021, 33: 2005587
Wang Y, Zhu Y, Zhao S, et al. Anion etching for accessing rapid and deep self-reconstruction of precatalysts for water oxidation. Matter, 2020, 3: 2124–2137
Qiu Z, Ma Y, Edvinsson T. In operando Raman investigation of Fe doping influence on catalytic NiO intermediates for enhanced overall water splitting. Nano Energy, 2019, 66: 104118
Chen J, Li H, Chen S, et al. Co−Fe−Cr (oxy)hydroxides as efficient oxygen evolution reaction catalysts. Adv Energy Mater, 2021, 11: 2003412
Zhao S, Tan C, He CT, et al. Structural transformation of highly active metal-organic framework electrocatalysts during the oxygen evolution reaction. Nat Energy, 2020, 5: 881–890
Zhang B, Wang L, Cao Z, et al. High-valence metals improve oxygen evolution reaction performance by modulating 3d metal oxidation cycle energetics. Nat Catal, 2020, 3: 985–992
Acknowledgements
This work was supported by the Multi-Year Research Grants (MYRG2020-00207-IAPME) from the University of Macau, the Science and Technology Development Fund from Macao SAR (FDCT) (0125/2018/A3, 0081/2019/AMJ, 0033/2019/AMJ, 0102/2019/A2, and 0154/2019/A3), the Nature Science Foundation of Shandong Province (ZR2020ZD04), and Hunan Science Fund for Distinguished Young Scholars (2020JJ2046). UM Macao PhD Scholarship is appreciated by Zhou P for the support.
Author information
Authors and Affiliations
Contributions
Author contributions Pan H conceived the idea. Zhou P did the electrochemical experiments and wrote the manuscript. Wong PK did the SEM and TEM measurements. Niu P and Li R prepared the AlCoCrFeNi HEA and did the analysis. Chen M conducted the in-situ Raman. Zhou P performed the XPS. Kwok CT revised the manuscript. Wang S and Tang Y analyzed the data and helped write the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Experimental details and supporting data are available in the online version of the paper.
Pengfei Zhou is currently a PhD student in Prof. Hui Pan’s group at the Institute of Applied Physics and Materials Engineering, University of Macau, Macao. His main research interests include the synthesis of bulk alloys and their applications in energy conversion.
Yuxin Tang is a professor at the College of Chemical Engineering, Fuzhou University. He obtained his PhD degree in materials science from Nanyang Technological University (NTU) in 2013. After postdoctoral training at NTU, he worked at the University of Macau as an assistant professor in 2018–2020. His research interest is the development of advanced electrolytes and electrode materials towards high-performance energy storage devices.
Ruidi Li is a professor at the State Key Laboratory of Powder Metallurgy, Central South University. He obtained his PhD degree from Huangzhong University of Science and Tehnology (2010). His research mainly focuses on high-entropy alloys, stainless steels, Al alloys, and additive manufacturing.
Shuangpeng Wang is an assistant professor at the Institute of Applied Physics and Materials Engineering, University of Macau. He obtained his PhD degree from the State Key Laboratory of Luminescence and Applications, Changchun Institute of Optics, Chinese Academy of Sciences (2011). His research interests include low-dimensional materials and their applications in optoelectronics and surface/interface states.
Hui Pan is a professor at the Institute of Applied Physics and Materials Engineering, University of Macau. He obtained his PhD degree from the National University of Singapore in 2006. He was a scientist at the Institute of High Performance Computing (Singapore) from 2009 to 2013. His research mainly focuses on energy harvesting and storage (photocatalysis, electrocatalysis, biomass, CO2 and N2 reductions, battery, supercapacitor, and hydrogen production/storage).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhou, P., Wong, P.K., Niu, P. et al. Anodized AlCoCrFeNi high-entropy alloy for alkaline water electrolysis with ultra-high performance. Sci. China Mater. 66, 1033–1041 (2023). https://doi.org/10.1007/s40843-022-2234-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-022-2234-5