Abstract
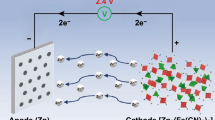
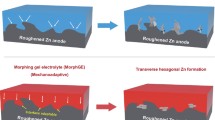
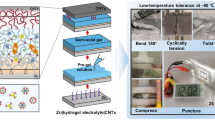
Aqueous zinc-ion batteries (ZIBs) have attracted immense attention for flexible energy storage devices due to their high safety and low cost. However, conventional flexible aqueous ZIBs will undergo severe capacity loss at subzero temperature due to the inevitably freeze of electrolytes. In addition, under large bending or stretching strains, the encapsulation of devices would be damaged, which causes the evaporation of water in electrolytes and results in device failure. Herein, an anti-freezing and anti-drying gel electrolyte based on polyacrylamide (PAM) and glycerol (Gly) is developed. The strong hydrogen-bonding interactions between PAM or Gly and water molecules not only avoid the crystallization of the gel electrolyte at low temperatures, but also constrain the free water and restrict its evaporation. Therefore, such gel electrolyte displays a high ionic conductivity of 9.65 × 10−5 S cm−1 at −40°C. Furthermore, it can restrict the dehydration process when the electrolyte is exposed to ambient environment. The flexible ZIBs based on such gel electrolyte exhibit excellent electrochemical performance at −40°C and the devices without encapsulation retain 98% of their initial capacity in ambient condition after 30 days. This work provides a route to design anti-freezing and anti-drying gel electrolytes for aqueous energy storage devices.

摘要
水系锌离子电池由于具有安全性高、 成本低的特点, 在柔性储能领域得到广泛关注. 然而, 传统柔性水系锌离子电池处于零下温度时, 由于其电解质的凝固, 电池容量会发生严重衰减. 此外, 当电池承受大幅度的形变后, 其外包装易发生破损, 造成电解质中水分的挥发, 最终导致器件失效. 在本文中, 我们开发了一种基于聚丙烯酰胺(PAM)和甘油(Gly)的耐低温、 保湿凝胶电解质. PAM和Gly与水分子之间的强氢键作用不仅抑制了凝胶电解质在低温时的凝固, 而且限制了电解质中自由水的挥发. 基于该电解质的柔性水系锌离子电池在−40°C时仍具有优良的电化学性能, 并且无封装的电池在30天后仍保持了初始容量的98%. 该工作提供了一种设计水系储能器件用耐低温、 保湿凝胶电解质的新思路.
Similar content being viewed by others
References
Zhong C, Deng Y, Hu W, et al. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem Soc Rev, 2015, 44: 7484–7539
Huang S, Zhu J, Tian J, et al. Recent progress in the electrolytes of aqueous zinc-ion batteries. Chem Eur J, 2019, 25: 14480–14494
Wang Z, Li H, Tang Z, et al. Hydrogel electrolytes for flexible aqueous energy storage devices. Adv Funct Mater, 2018, 28: 1804560
Cheng XB, Zhang R, Zhao CZ, et al. A review of solid electrolyte interphases on lithium metal anode. Adv Sci, 2016, 3: 1500213
Xiao Y, Wang Y, Bo SH, et al. Understanding interface stability in solid-state batteries. Nat Rev Mater, 2019, 5: 105–126
Chuai M, Yang J, Wang M, et al. High-performance Zn battery with transition metal ions co-regulated electrolytic MnO2. eScience, 2021, doi: https://doi.org/10.1016/j.esci.2021.11.002
Wan F, Wang X, Bi S, et al. Freestanding reduced graphene oxide/sodium vanadate composite films for flexible aqueous zinc-ion batteries. Sci China Chem, 2019, 62: 609–615
Huang J, Zhou J, Liang S. Guest pre-intercalation strategy to boost the electrochemical performance of aqueous zinc-ion battery cathodes. Acta Phys-Chim Sin, 2021, 37: 2005020
Li P, Kim H, Ming J, et al. Quasi-compensatory effect in emerging anode-free lithium batteries. eScience, 2021, 1: 3–12
Su L, Liu L, Wang Y, et al. Synergetic ternary metal oxide nanodotsgraphene cathode for high performance zinc energy storage. Chin Chem Lett, 2020, 31: 2358–2364
Zhao M, Li XY, Chen X, et al. Promoting the sulfur redox kinetics by mixed organodiselenides in high-energy-density lithium-sulfur batteries. eScience, 2021, 1: 44–52
Zhao Y, Zhu Y, Zhang X. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries. InfoMat, 2020, 2: 237–260
Zhang M, Liang R, Or T, et al. Recent progress on high-performance cathode materials for zinc-ion batteries. Small Struct, 2021, 2: 2000064
Fan X, Liu B, Liu J, et al. Battery technologies for grid-level large-scale electrical energy storage. Trans Tianjin Univ, 2020, 26: 92–103
Zhu YF, Xiao Y, Dou SX, et al. Spinel/post-spinel engineering on layered oxide cathodes for sodium-ion batteries. eScience, 2021, 1: 13–27
Ngai KS, Ramesh S, Ramesh K, et al. A review of polymer electrolytes: Fundamental, approaches and applications. Ionics, 2016, 22: 1259–1279
Liu Z, Liang G, Zhan Y, et al. A soft yet device-level dynamically super-tough supercapacitor enabled by an energy-dissipative dual-crosslinked hydrogel electrolyte. Nano Energy, 2019, 58: 732–742
Niu Z, Zhou W, Chen X, et al. Highly compressible and all-solid-state supercapacitors based on nanostructured composite sponge. Adv Mater, 2015, 27: 6002–6008
Li H, Lv T, Sun H, et al. Ultrastretchable and superior healable supercapacitors based on a double cross-linked hydrogel electrolyte. Nat Commun, 2019, 10: 536
Dong C, Xu F, Chen L, et al. Design strategies for high-voltage aqueous batteries. Small Struct, 2021, 2: 2100001
Wei J, Wei G, Shang Y, et al. Dissolution-crystallization transition within a polymer hydrogel for a processable ultratough electrolyte. Adv Mater, 2019, 31: 1900248
Zhou D, Chen F, Handschuh-Wang S, et al. Biomimetic extreme-temperature- and environment-adaptable hydrogels. ChemPhysChem, 2019, 20: 2139–2154
Wang Z, Cheng J, Zhou J, et al. All-climate aqueous fiber-shaped supercapacitors with record areal energy density and high safety. Nano Energy, 2018, 50: 106–117
Huang S, Wan F, Bi S, et al. A self-healing integrated all-in-one zinc-ion battery. Angew Chem Int Ed, 2019, 58: 4313–4317
Huang Y, Zhu M, Huang Y, et al. Multifunctional energy storage and conversion devices. Adv Mater, 2016, 28: 8344–8364
Wan F, Zhu J, Huang S, et al. High-voltage electrolytes for aqueous energy storage devices. Batteries Supercaps, 2020, 3: 323–330
Liu J, Xie C, Kretzschmann A, et al. Metallopolymer organohydrogels with photo-controlled coordination crosslinks work properly below 0°C. Adv Mater, 2020, 32: 1908324
Ju M, Wu B, Sun S, et al. Redox-active iron-citrate complex regulated robust coating-free hydrogel microfiber net with high environmental tolerance and sensitivity. Adv Funct Mater, 2020, 30: 1910387
Chen M, Zhou W, Wang A, et al. Anti-freezing flexible aqueous Zn-MnO2 batteries working at −35°C enabled by a borax-crosslinked polyvinyl alcohol/glycerol gel electrolyte. J Mater Chem A, 2020, 8: 6828–6841
Li H, Zhang H, Diemant T, et al. Reversible copper sulfide conversion in nonflammable trimethyl phosphate electrolytes for safe sodium-ion batteries. Small Struct, 2021, 2: 2100035
Peng S, Jiang X, Xiang X, et al. High-performance and flexible solidstate supercapacitors based on high toughness and thermoplastic poly(vinyl alcohol)/NaCl/glycerol supramolecular gel polymer electrolyte. Electrochim Acta, 2019, 324: 134874
Chen F, Zhou D, Wang J, et al. Rational fabrication of anti-freezing, non-drying tough organohydrogels by one-pot solvent displacement. Angew Chem Int Ed, 2018, 57: 6568–6571
Ji X. A perspective of ZnCl2 electrolytes: The physical and electrochemical properties. eScience, 2021, doi: https://doi.org/10.1016/j.esci.2021.10.004
Hou J, Yang M, Wang D, et al. Fundamentals and challenges of lithium ion batteries at temperatures between −40 and 60°C. Adv Energy Mater, 2020, 10: 1904152
Jin X, Song L, Yang H, et al. Stretchable supercapacitor at −30°C. Energy Environ Sci, 2021, 14: 3075–3085
Peng M, Wang L, Li L, et al. Molecular crowding agents engineered to make bioinspired electrolytes for high-voltage aqueous supercapacitors. eScience, 2021, 1: 83–90
Liu L, Dou Q, Sun Y, et al. A moisture absorbing gel electrolyte enables aqueous and flexible supercapacitors operating at high temperatures. J Mater Chem A, 2019, 7: 20398–20404
Sun Y, Ma H, Zhang X, et al. Salty ice electrolyte with superior ionic conductivity towards low-temperature aqueous zinc ion hybrid capacitors. Adv Funct Mater, 2021, 31: 2101277
Mo F, Liang G, Meng Q, et al. A flexible rechargeable aqueous zinc manganese-dioxide battery working at −20°C. Energy Environ Sci, 2019, 12: 706–715
Ma L, Zhao Y, Ji X, et al. A usage scenario independent “air chargeable” flexible zinc ion energy storage device. Adv Energy Mater, 2019, 9: 1900509
Ma L, Chen S, Wang D, et al. Super-stretchable zinc-air batteries based on an alkaline-tolerant dual-network hydrogel electrolyte. Adv Energy Mater, 2019, 9: 1803046
Lou Z, Shen G. Flexible image sensors with semiconducting nanowires for biomimic visual applications. Small Struct, 2021, 2: 2000152
Han L, Liu K, Wang M, et al. Mussel-inspired adhesive and conductive hydrogel with long-lasting moisture and extreme temperature tolerance. Adv Funct Mater, 2018, 28: 1704195
Liu T, Liu M, Dou S, et al. Triboelectric-nanogenerator-based soft energy-harvesting skin enabled by toughly bonded elastomer/hydrogel hybrids. ACS Nano, 2018, 12: 2818–2826
Yuk H, Zhang T, Parada GA, et al. Skin-inspired hydrogel-elastomer hybrids with robust interfaces and functional microstructures. Nat Commun, 2016, 7: 12028
Yang J, Gao L, Liu M, et al. Advanced biotechnology for cell cryopreservation. Trans Tianjin Univ, 2020, 26: 409–423
Wang R, Yao M, Huang S, et al. Sustainable dough-based gel electrolytes for aqueous energy storage devices. Adv Funct Mater, 2021, 31: 2009209
Liu A, Kovacik P, Peard N, et al. Monolithic flexible supercapacitors integrated into single sheets of paper and membrane via vapor printing. Adv Mater, 2017, 29: 1606091
Li X, Liu L, Wang X, et al. Flexible and self-healing aqueous supercapacitors for low temperature applications: Polyampholyte gel electrolytes with biochar electrodes. Sci Rep, 2017, 7: 1685
Tao F, Qin L, Wang Z, et al. Self-healable and cold-resistant supercapacitor based on a multifunctional hydrogel electrolyte. ACS Appl Mater Interfaces, 2017, 9: 15541–15548
Jian Y, Handschuh-Wang S, Zhang J, et al. Biomimetic anti-freezing polymeric hydrogels: Keeping soft-wet materials active in cold environments. Mater Horiz, 2020, 8: 351–369
Liu X, Taiwo OO, Yin C, et al. Aligned ionogel electrolytes for high-temperature supercapacitors. Adv Sci, 2019, 6: 1801337
Evanko B, Boettcher SW, Yoo SJ, et al. Redox-enhanced electrochemical capacitors: Status, opportunity, and best practices for performance evaluation. ACS Energy Lett, 2017, 2: 2581–2590
Guo Y, Bae J, Fang Z, et al. Hydrogels and hydrogel-derived materials for energy and water sustainability. Chem Rev, 2020, 120: 7642–7707
Vieira MGA, da Silva MA, dos Santos LO, et al. Natural-based plasticizers and biopolymer films: A review. Eur Polym J, 2011, 47: 254–263
Zhang Z, Xiao F, Xiao J, et al. Functionalized carbonaceous fibers for high performance flexible all-solid-state asymmetric supercapacitors. J Mater Chem A, 2015, 3: 11817–11823
Liu L, Niu Z, Chen J. Unconventional supercapacitors from nanocarbon-based electrode materials to device configurations. Chem Soc Rev, 2016, 45: 4340–4363
Liu W, Yan X, Chen J, et al. Novel and high-performance asymmetric micro-supercapacitors based on graphene quantum dots and polyaniline nanofibers. Nanoscale, 2013, 5: 6053–6062
Li X, Lou D, Wang H, et al. Flexible supercapacitor based on organohydrogel electrolyte with long-term anti-freezing and anti-drying property. Adv Funct Mater, 2020, 30: 2007291
Huang L, Li C, Shi G. High-performance and flexible electrochemical capacitors based on graphene/polymer composite films. J Mater Chem A, 2014, 2: 968–974
Zhou Q, Li Y, Huang L, et al. Three-dimensional porous graphene/polyaniline composites for high-rate electrochemical capacitors. J Mater Chem A, 2014, 2: 17489–17494
Kurra N, Jiang Q, Nayak P, et al. Laser-derived graphene: A three-dimensional printed graphene electrode and its emerging applications. Nano Today, 2019, 24: 81–102
Wang X, Lu Q, Chen C, et al. A consecutive spray printing strategy to construct and integrate diverse supercapacitors on various substrates. ACS Appl Mater Interfaces, 2017, 9: 28612–28619
Liu C, Yu Z, Neff D, et al. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett, 2010, 10: 4863–4868
Zhong J, Meng J, Yang Z, et al. Shape memory fiber supercapacitors. Nano Energy, 2015, 17: 330–338
Hu X, Fan L, Qin G, et al. Flexible and low temperature resistant double network alkaline gel polymer electrolyte with dual-role KOH for supercapacitor. J Power Sources, 2019, 414: 201–209
Fan E, Li L, Wang Z, et al. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects. Chem Rev, 2020, 120: 7020–7063
Wang Y, Song Y, Xia Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem Soc Rev, 2016, 45: 5925–5950
Shao Y, El-Kady MF, Sun J, et al. Design and mechanisms of asymmetric supercapacitors. Chem Rev, 2018, 118: 9233–9280
Acknowledgements
This work was supported by the Natural Science Foundation of Tianjin (18JCJQJC46300 and 19JCZDJC31900), the National Natural Science Foundation of China (51822205 and 21875121), the Ministry of Science and Technology of China (2019YFA0705600 and 2017YFA0206701), the Ministry of Education of China (B12015), and the “Frontiers Science Center for New Organic Matter”, Nankai University (63181206). The authors thank Professor Zhou Z (Nankai University) for supporting Materials Studio calculations.
Author information
Authors and Affiliations
Contributions
Author contributions Wang R performed the experiments and wrote the original manuscript; Yao M contributed to the electrochemical measurements and revised the manuscript; Huang S and Tian J contributed to the synthesis of electrolytes; Niu Z proposed the concept, supervised the experiments and revised the manuscript. All authors contributed to the general discussion.
Corresponding author
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Supporting data are available in the online version of the paper.
Rui Wang received his BS degree in chemistry from Nankai University in 2018. He then joined the Key Laboratory of Advanced Energy Materials Chemistry at Nankai University under the supervision of Prof. Zhiqiang Niu. His research focuses on the design of smart electrolytes for energy storage devices.
Zhiqiang Niu is a professor at the College of Chemistry, Nankai University. He received his PhD degree from the Institute of Physics, Chinese Academy of Sciences in 2010. After his postdoctoral research at the School of Materials Science and Engineering, Nanyang Technological University (Singapore), he started his independent research career at Nankai University in 2014. His research interests focus on the unconventional energy storage devices from electrode materials to device configurations.
Rights and permissions
About this article
Cite this article
Wang, R., Yao, M., Huang, S. et al. An anti-freezing and anti-drying multifunctional gel electrolyte for flexible aqueous zinc-ion batteries. Sci. China Mater. 65, 2189–2196 (2022). https://doi.org/10.1007/s40843-021-1924-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-021-1924-2