Abstract
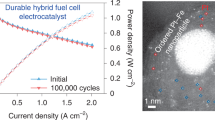
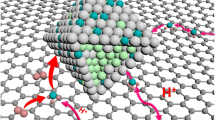
High-temperature proton exchange membrane fuel cells (HT-PEMFCs) bring new opportunities for portable power generation due to their outstanding advantages such as high tolerance to fuel/air impurities and simplified heat/water management. However, carbon-supported nanostructured Pt-based catalysts running at temperatures over 150°C are challenged by the severe aggregation and carbon corrosion, thus leading to poor durability. Herein, we demonstrate that dendritic Pt-Ni nanoparticles supported on fluorinated carbon black (white carbon black) could significantly enhance the performance and durability of HT-PEMFCs as the cathode catalysts running at 160°C due to the strong interaction of the F and Ni atoms to form a NixFy interface on Pt-Ni nanoparticles. With the formation of a stable NixFy interface, this integrated HT-PEMFC reached peak power densities of 906 mW cm−2 and demonstrated excellent durability at 160°C under anhydrous H2/O2 conditions. This mitigation strategy was applied to Pt-alloy/C electrocatalysts and resulted in the elimination of Pt dissolution in practical fuel cells.

摘要
高温质子交换膜燃料电池(HT-PEMFCs)以其杂质耐受性高、系统简化等突出优势为燃料电池的发展带来了新机遇. 目前广泛使用的铂碳催化剂存在严重的颗粒团聚、载体腐蚀等耐久性较差问题. 本文采用氟化碳黑(白碳黑)负载的枝状Pt-Ni纳米颗粒作为HT-PEMFCs阴极催化剂, 由于Ni、F强相互作用并在Pt-Ni合金表面形成了NixFy界面,可显著提升器件性能和耐受性, 在160°C、干燥H2/O2条件下峰功率密度可达906 mW cm−2. 本文成功利用NixFy界面提升合金催化剂的活性和稳定性, 对于HT-PEMFCs催化剂的设计具有指导意义.
Similar content being viewed by others
References
Atanasov V, Lee AS, Park EJ, et al. Synergistically integrated phosphonated poly(pentafluorostyrene) for fuel cells. Nat Mater, 2021, 20: 370–377
Haider R, Wen Y, Ma ZF, et al. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem Soc Rev, 2021, 50: 1138–1187
Lim SY, Martin S, Gao G, et al. Self-standing nanofiber electrodes with Pt-Co derived from electrospun zeolitic imidazolate framework for high temperature PEM fuel cells. Adv Funct Mater, 2021, 31: 2006771
Sui S, Wang X, Zhou X, et al. A comprehensive review of Pt electro-catalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J Mater Chem A, 2017, 5: 1808–1825
Gao Y, Hou M, He L, et al. Performance- and durability-enhanced carbon-skeleton nanofiber electrode with Pt3Co/C for PEMFCs. ACS Sustain Chem Eng, 2020, 8: 13030–13038
Wang G, Yang Z, Du Y, et al. Programmable exposure of Pt active facets for efficient oxygen reduction. Angew Chem Int Ed, 2019, 58: 15848–15854
Kondratenko MS, Gallyamov MO, Tyutyunnik OA, et al. Degradation of high temperature polymer electrolyte fuel cell cathode material as affected by polybenzimidazole. J Electrochem Soc, 2015, 162: F587–F595
Ignaszak A, Song C, Zhu W, et al. Carbon-Nb0.07Ti0.93 O2 composite supported Pt-Pd electrocatalysts for PEM fuel cell oxygen reduction reaction Electrochim Acta, 2012, 75: 220–228
Xie Z, Cheng H, Chen Z, et al. A general strategy for synthesizing hierarchical architectures assembled by dendritic Pt-based nanoalloys for electrochemical hydrogen evolution Int J Hydrogen Energy, 2021, 46: 11573–11586
Wang D, Chen Z, Huang YC, et al. Tailoring lattice strain in ultra-fine high-entropy alloys for active and stable methanol oxidation Sci China Mater, 2021, 64: 2454–2466
Meyers JP, Darling RM. Model of carbon corrosion in PEM fuel cells J Electrochem Soc, 2006, 153: A1432
You DJ, Kim DH, De Lile JR, et al. Pd core-shell alloy catalysts for high-temperature polymer electrolyte membrane fuel cells: Effect of the core composition on the activity towards oxygen reduction reactions. Appl Catal A-Gen, 2018, 562: 250–257
Yao D, Zhang W, Ma Q, et al. Achieving high Pt utilization and superior performance of high temperature polymer electrolyte membrane fuel cell by employing low-Pt-content catalyst and microporous layer free electrode design J Power Sources, 2019, 426: 124–133
Devrim Y, Arica ED. Multi-walled carbon nanotubes decorated by platinum catalyst for high temperature PEM fuel cell Int J Hydrogen Energy, 2019, 44: 18951–18966
Lobato J, Zamora H, Plaza J, et al. Enhancement of high temperature PEMFC stability using catalysts based on Pt supported on SiC based materials. Appl Catal B-Environ, 2016, 198: 516–524
Yang F, Ye JY, Yuan Q, et al. Ultrasmall Pd-Cu-Pt trimetallic twin icosahedrons boost the electrocatalytic performance of glycerol oxidation at the operating temperature of fuel cells. Adv Funct Mater, 2020, 30: 1908235
Yang X, Yao K, Ye JY, et al. Interface-rich three-dimensional Au-doped PtBi intermetallics as highly effective anode catalysts for application in alkaline ethylene glycol fuel cells. Adv Funct Mater, 2021, 31: 2103671
Tian X, Zhao X, Su YQ, et al. Engineering bunched Pt-Ni alloy nano-cages for efficient oxygen reduction in practical fuel cells. Science, 2019, 366: 850–856
Wang Q, Chen F, Guo L, et al. Nanoalloying effects on the catalytic activity of the formate oxidation reaction over AgPd and AgCuPd aerogels. J Mater Chem A, 2019, 7: 16122–16135
Wang J, Chen F, Jin Y, et al. In situ high-potential-driven surface restructuring of ternary AgPd-Pt dilute aerogels with record-high performance improvement for formate oxidation electrocatalysis. Nanoscale, 2019, 11: 14174–14185
Tang Q, Chen F, Jin T, et al. Alloying in inverse CeO2/Pd nanoparticles to enhance the electrocatalytic activity for the formate oxidation reaction. J Mater Chem A, 2019, 7: 22996–23007
Guo L, Chen F, Jin T, et al. Surface reconstruction of AgPd nanoalloy particles during the electrocatalytic formate oxidation reaction. Nanoscale, 2020, 12: 3469–3481
Pan B, Chen F, Kou B, et al. Unexpectedly high stability and surface reconstruction of PdAuAg nanoparticles for formate oxidation electrocatalysis. Nanoscale, 2020, 12: 11659–11671
Debe MK. Electrocatalyst approaches and challenges for automotive fuel cells. Nature, 2012, 486: 43–51
Chattot R, Le Bacq O, Beermann V, et al. Surface distortion as a unifying concept and descriptor in oxygen reduction reaction electro-catalysis. Nat Mater, 2018, 17: 827–833
van der Vliet DF, Wang C, Tripkovic D, et al. Mesostructured thin films as electrocatalysts with tunable composition and surface morphology. Nat Mater, 2012, 11: 1051–1058
Bing Y, Liu H, Zhang L, et al. Nanostructured Pt-alloy electrocatalysts for PEM fuel cell oxygen reduction reaction. Chem Soc Rev, 2010, 39: 2184–2202
Kwon H, Kabiraz MK, Park J, et al. Dendrite-embedded platinum-nickel multiframes as highly active and durable electrocatalyst toward the oxygen reduction reaction. Nano Lett, 2018, 18: 2930–2936
Vedarajan R, Prithi J, Rajalakshmi N. Advanced nano-catalysts for fuel-cell technologies. In: Naushad M, Saravanan R, Kumar R (EDs.). Nanomaterials for Sustainable Energy and Environmental Remediation. Amsterdam: Elsevier, 2020. 165–191.
Long P, Feng Y, Cao C, et al. Self-protective room-temperature phosphorescence of fluorine and nitrogen codoped carbon dots. Adv Funct Mater, 2018, 28: 1800791
Feng W, Long P, Feng Y, et al. Two-dimensional fluorinated graphene: Synthesis, structures, properties and applications. Adv Sci, 2016, 3: 1500413
Yang Y, Ruan G, Xiang C, et al. Flexible three-dimensional nanoporous metal-based energy devices. J Am Chem Soc, 2014, 136: 6187–6190
Lee YS. Syntheses and properties of fluorinated carbon materials. J Fluorine Chem, 2007, 128: 392–403
Liang C, Li Z, Dai S. Mesoporous carbon materials: Synthesis and modification. Angew Chem Int Ed, 2008, 47: 3696–3717
Enyashin AN, Ivanovskii AL. Fluorinated derivatives of sp2 graphene allotropes: Structure, stability, and electronic properties. Chem Phys Lett, 2012, 545: 78–82
Lim B, Xia Y. Metal nanocrystals with highly branched morphologies. Angew Chem Int Ed, 2011, 50: 76–85
Wang F, Li C, Sun LD, et al. Porous single-crystalline palladium nanoparticles with high catalytic activities. Angew Chem Int Ed, 2012, 51: 4872–4876
Nassr ABAA, Sinev I, Grünert W, et al. PtNi supported on oxygen functionalized carbon nanotubes: In depth structural characterization and activity for methanol electrooxidation. Appl Catal B-Environ, 2013, 142–143: 849–860
Ahmadi M, Behafarid F, Cui C, et al. Long-range segregation phenomena in shape-selected bimetallic nanoparticles: Chemical state effects. ACS Nano, 2013, 7: 9195–9204
Zou L, Fan J, Zhou Y, et al. Conversion of PtNi alloy from disordered to ordered for enhanced activity and durability in methanol-tolerant oxygen reduction reactions. Nano Res, 2015, 8: 2777–2788
Zhao Z, Liu H, Gao W, et al. Surface-engineered PtNi-O nanostructure with record-high performance for electrocatalytic hydrogen evolution reaction. J Am Chem Soc, 2018, 140: 9046–9050
Zhou J, Lian J, Hou L, et al. Ultrahigh volumetric capacitance and cyclic stability of fluorine and nitrogen co-doped carbon microspheres. Nat Commun, 2015, 6: 8503
Peng C, Li Y, Yao F, et al. Ultrahigh-energy-density fluorinated calcinated macadamia nut shell cathodes for lithium/fluorinated carbon batteries. Curr Alzheimer Resbon, 2019, 153: 783–791
Sansotera M, Navarrini W, Resnati G, et al. Preparation and characterization of superhydrophobic conductive fluorinated carbon blacks. Curr Alzheimer Resbon, 2010, 48: 4382–4390
Wang P, Hayashi T, Meng Q, et al. Highly boosted oxygen reduction reaction activity by tuning the underwater wetting state of the super-hydrophobic electrode. Small, 2017, 13: 1601250
Li Y, Zhang H, Han N, et al. Janus electrode with simultaneous management on gas and liquid transport for boosting oxygen reduction reaction. Nano Res, 2019, 12: 177–182
Chen Y, Cai J, Li P, et al. Hexagonal boron nitride as a multifunctional support for engineering efficient electrocatalysts toward the oxygen reduction reaction. Nano Lett, 2020, 20: 6807–6814
Chidsey CED. Free energy and temperature dependence of electron transfer at the metal-electrolyte interface. Science, 1991, 251: 919–922
Lei Y, Sun R, Zhang X, et al. Oxygen-rich enzyme biosensor based on superhydrophobic electrode. Adv Mater, 2016, 28: 1477–1481
Li Z, Niu W, Yang Z, et al. Stabilizing atomic Pt with trapped interstitial F in alloyed PtCo nanosheets for high-performance zinc-air batteries. Energy Environ Sci, 2020, 13: 884–895
Ho VTT, Pan CJ, Rick J, et al. Nanostructured Ti0.7Mo0.3O2 support enhances electron transfer to Pt: High-performance catalyst for oxygen reduction reaction. J Am Chem Soc, 2011, 133: 11716–11724
Zhang S, Zhang J, Zhu Z, et al. Unusual influence of binder composition and phosphoric acid leaching on oxygen mass transport in catalyst layers of high-temperature proton exchange membrane fuel cells. J Power Sources, 2020, 473: 228616
Xu X, Wang H, Lu S, et al. A novel phosphoric acid doped poly (ethersulphone)-poly(vinyl pyrrolidone) blend membrane for high-temperature proton exchange membrane fuel cells. J Power Sources, 2015, 286: 458–463
Bai H, Wang H, Zhang J, et al. Simultaneously enhancing ionic conduction and mechanical strength of poly(ether sulfones)-poly(vinyl pyrrolidone) membrane by introducing graphitic carbon nitride nanosheets for high temperature proton exchange membrane fuel cell application. J Membrane Sci, 2018, 558: 26–33
Huah L, Majlan E, Tajuddin A, et al. Comparison of catalyst-coated membranes and catalyst-coated substrate for PEMFC membrane electrode assembly: A review. Chinese J Chem Eng, 2021, 33: 1–16
Acknowledgements
This work was supported by the National Key R&D Program of China (2020YFA0710000), the National Natural Science Foundation of China (21825201 and U19A2017), the Provincial Natural Science Foundation of Hunan (2019GK2031, 2016TP1009 and 2020JJ5045), China Postdoctoral Science Foundation (2020M682541), the Science and Technology Innovation Program of Hunan Province, China (2020RC2020), and Changsha Municipal Natural Science Foundation (kq2007009).
Author information
Authors and Affiliations
Contributions
Author contributions Long P, Du S, and Liu Q conceived the idea and wrote the paper; Long P, Gu K and Liu Q performed the synthesis and basic characterization of the samples. Du S, Wang T, Xie C and Peng C conducted the fuel cell test. Tao L, Zhang Y, Chen R, Lu S, Cheng Y, Feng W and Wang S discussed the mechanism of action and directed the writing of the paper. All the authors contributed to the overall scientific interpretation and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Experimental details and supporting data are available in the online version of the paper.
Peng Long received his Master degree in 2014 from Hunan University of Science and Technology and his PhD degree in 2018 from Tianjin University under the supervision of Prof. Wei Feng. He currently works at Hunan University as a postdoctoral researcher under the supervision of Prof. Shuangyin Wang. His research interests include the synthesis and characterization of nanomaterials as electrocatalysts for proton exchange membrane fuel cells.
Shiqian Du received his Master degree in 2016 from Beijing University of Chemical Technology, China. He is pursuing his PhD degree under the supervision of Prof. Shuangyin Wang at Hunan University. His research interests are electrochemical catalysis and related devices.
Qie Liu received her Bachelor’s degree in 2020 from Hunan University. She is pursuing her PhD degree under the supervision of Prof. Shuangyin Wang at Hunan University. Her research interest includes the design of electrocatalysts and their applications in high-temperature proton exchange membrane fuel cells.
Li Tao received his Master degree in 2016 and his PhD degree in 2019 from Hunan University under the supervision of Prof. Shuangyin Wang. He is currently an assistant professor at the Provincial Hunan Key Laboratory for Graphene Materials, College of Chemistry and Chemical Engineering, Hunan University. His research interests are in plasma technology, defect chemistry and fuel cells.
Shuangyin Wang received his BSc degree in 2006 from Zhejiang University and his PhD degree in 2010 from Nanyang Technological University, Singapore. He is currently a professor of the Key Provincial Hunan Key Laboratory for Graphene Materials, College of Chemistry and Chemical Engineering, Hunan University. His research interests are in plasma technology, defects in various crystals and their application for electrochemical energy storage and conversion.
Shanfu Lu received his PhD in physical chemistry from Wuhan University in 2008, and then joined Nanyang Technological University in Singapore as a postdoctoral researcher. He joined the Department of Environmental Science and Engineering of Beihang University as a professor in 2009. He is mainly engaged in the research of key materials and devices for polymer electrolyte membrane fuel cells and liquid flow batteries.
Wei Feng received his PhD degree in 2000 from Xi’an Jiaotong University of China after studying the optical-electrical properties and device applications of novel conducting polymers, and then worked at Osaka University and Tsinghua University as a Japan Science Promotion Society (JSPS) fellow and postdoctoral researcher, respectively. In 2004, he became a full professor at Tianjin University, where he has been working on functional nanocarbon materials.
Yi Cheng received his Master degree in environmental engineering from Central South University in 2006 and his PhD degree in 2014 from Curtin University. His research interests are environmental functional materials, clean energy materials and devices, electrochemical catalysts for pollutant degradation and mechanisms.
Supporting Information
40843_2021_1839_MOESM1_ESM.pdf
Fluorination-enabled interface of PtNi electrocatalysts for high-performance high-temperature proton exchange membrane fuel cells
Rights and permissions
About this article
Cite this article
Long, P., Du, S., Liu, Q. et al. Fluorination-enabled interface of PtNi electrocatalysts for high-performance high-temperature proton exchange membrane fuel cells. Sci. China Mater. 65, 904–912 (2022). https://doi.org/10.1007/s40843-021-1839-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-021-1839-8