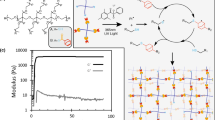
Abstract
Mechanical cues present in the stem cell niche resulting from intracellular processes or external force sources significantly affect the basic functions of stem cells such as self-renewal and differentiation. Creation of artificial cellular matrices exhibiting intrinsic mechanical cues generated by mechanical movements remains scarce. Herein, we reported on mechanically dynamic hydrogel matrices undergoing photo-induced directional domain sliding movement and their role in regulating embryonic stem cell (ESC) differentiation. The mechanically dynamic hydrogels were prepared via the self-assembly of an alternating hydrophilic and hydrophobic peptide with a photocaged cysteine residue. Upon light irradiation, the assemblies of the caged peptide were converted to non-equilibrated non-caged peptide bilayers that underwent the directional domain sliding motion induced by the thermodynamically favorable hydrophobic collapse transition. Culturing murine ESCs on the mechanically dynamic hydrogels resulted in biased differentiation toward the ectodermal lineage. We further showed that the mechanically dynamic hydrogels stimulated the translocation of a mechanotransduction protein Yes-associated protein (YAP) into the nucleus, implicating a potential mechanotransduction mechanism for the biased differentiation of ESCs. The finding of the biased ectodermal differentiation of ESCs induced by the mechanically dynamic hydrogels implies the great potency of the mechanically dynamic hydrogels as biomaterials for disease therapy and tissue regeneration in the future.
摘要
由细胞内行为或外源力引起的干细胞龛中存在的机械信号对干细胞的自我恢复和分化等基本功能具有重要影响. 然而, 关于具有分子机械运动产生的内在机械信号的人工细胞外基质鲜有报 道. 在此, 我们报道了含光诱导片段定向滑动的机械动态水凝胶的合成及其作为人工细胞外基质在调节胚胎干细胞(ESC)分化中的功能. 通过引入光笼蔽的半胱氨酸残基调控亲疏水交替多肽的自 组装制备机械动态水凝胶. 光笼蔽多肽组装体在光照射下转化为热力学非平衡的非笼蔽多肽双分子层, 其进一步发生热力学有利的疏水性塌陷转变诱导的片段定向滑动. 在机械动态水凝胶上培养鼠胚胎干细胞, 该片段定向滑动诱导干细胞向外胚层谱系定向分化. 进一步揭示了机械动态水凝胶促进机械转导蛋白YAP进入细胞核, 表明其用于ESCs定向分化的潜在机械转导机制. 细胞定向分化结果表明了机械动态水凝胶作为潜在的生物材料, 有望用于疾病治疗和组织再生.
Similar content being viewed by others
References
Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell, 2006, 126: 677–689
Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol, 2007, 8: 633–644
Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls α5β1 function. Science, 2009, 323: 642–644
Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol, 2017, 18: 728–742
Bao M, Xie J, Huck WTS. Recent advances in engineering the stem cell microniche in 3D. Adv Sci, 2018, 5: 1800448
Dalby MJ, Gadegaard N, Tare R, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater, 2007, 6: 997–1003
McMurray RJ, Gadegaard N, Tsimbouri PM, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater, 2011, 10: 637–644
Ondeck MG, Kumar A, Placone JK, et al. Dynamically stiffened matrix promotes malignant transformation of mammary epithelial cells via collective mechanical signaling. Proc Natl Acad Sci USA, 2019, 116: 3502–3507
Huang G, Li F, Zhao X, et al. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem Rev, 2017, 117: 12764–12850
Brown TE, Anseth KS. Spatiotemporal hydrogel biomaterials for regenerative medicine. Chem Soc Rev, 2017, 46: 6532–6552
Guvendiren M, Burdick JA. Stiffening hydrogels to probe shortand long-term cellular responses to dynamic mechanics. Nat Commun, 2012, 3: 792
Hadden WJ, Young JL, Holle AW, et al. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc Natl Acad Sci USA, 2017, 114: 5647–5652
Zhang J, Cheng C, Cuellar-Camacho JL, et al. Thermally responsive microfibers mediated stem cell fate via reversibly dynamic mechanical stimulation. Adv Funct Mater, 2018, 28: 1804773
Yang C, Tibbitt MW, Basta L, et al. Mechanical memory and dosing influence stem cell fate. Nat Mater, 2014, 13: 645–652
Wen JH, Vincent LG, Fuhrmann A, et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater, 2014, 13: 979–987
Chaudhuri O, Gu L, Klumpers D, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater, 2015, 15: 326–334
Ma Y, Lin M, Huang G, et al. 3D spatiotemporal mechanical microenvironment: a hydrogel-based platform for guiding stem cell fate. Adv Mater, 2018, 30: 1705911
Hörner M, Raute K, Hummel B, et al. Phytochrome-based extracellular matrix with reversibly tunable mechanical properties. Adv Mater, 2019, 31: 1806727
Zhang S, Gelain F, Zhao X. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin Cancer Biol, 2005, 15: 413–420
Wang H, Yang Z, Adams DJ. Controlling peptidebased hydrogelation. Mater Today, 2012, 15: 500–507
Du X, Zhou J, Shi J, et al. Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Chem Rev, 2015, 115: 13165–13307
Silva GA, Czeisler C, Niece KL, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science, 2004, 303: 1352–1355
Gelain F, Silva D, Caprini A, et al. BMHP1-derived self-assembling peptides: hierarchically assembled structures with self-healing propensity and potential for tissue engineering applications. ACS Nano, 2011, 5: 1845–1859
Ye K, Wang X, Cao L, et al. Matrix stiffness and nanoscale spatial organization of cell-adhesive ligands direct stem cell fate. Nano Lett, 2015, 15: 4720–4729
Ferris DP, Zhao YL, Khashab NM, et al. Light-operated mechanized nanoparticles. J Am Chem Soc, 2009, 131: 1686–1688
SzymaŃski W, Yilmaz D, Koçer AĞ, et al. Bright ion channels and lipid bilayers. Acc Chem Res, 2013, 46: 2910–2923
Peng H, Li XF, Zhang H, et al. A microRNA-initiated DNAzyme motor operating in living cells. Nat Commun, 2017, 8: 14378
García-López V, Chen F, Nilewski LG, et al. Molecular machines open cell membranes. Nature, 2017, 548: 567–572
Liu D, García-López V, Gunasekera RS, et al. Near-infrared light activates molecular nanomachines to drill into and kill cells. ACS Nano, 2019, 13: 6813–6823
Lussis P, Svaldo-Lanero T, Bertocco A, et al. A single synthetic small molecule that generates force against a load. Nat Nanotech, 2011, 6: 553–557
Juluri BK, Kumar AS, Liu Y, et al. A mechanical actuator driven electrochemically by artificial molecular muscles. ACS Nano, 2009, 3: 291–300
Berná J, Leigh DA, Lubomska M, et al. Macroscopic transport by synthetic molecular machines. Nat Mater, 2005, 4: 704–710
Lou S, Wang X, Yu Z, et al. Peptide tectonics: encoded structural complementarity dictates programmable self-assembly. Adv Sci, 2019, 335: 1802043
Bowerman CJ, Liyanage W, Federation AJ, et al. Tuning β-sheet peptide self-assembly and hydrogelation behavior by modification of sequence hydrophobicity and aromaticity. Biomacromolecules, 2011, 12: 2735–2745
Haines LA, Rajagopal K, Ozbas B, et al. Light-activated hydrogel formation via the triggered folding and self-assembly of a designed peptide. J Am Chem Soc, 2005, 127: 17025–17029
Geisler IM, Schneider JP. Evolution-based design of an injectable hydrogel. Adv Funct Mater, 2012, 22: 529–537
Muraoka T, Cui H, Stupp SI. Quadruple helix formation of a photoresponsive peptide amphiphile and its light-triggered dissociation into single fibers. J Am Chem Soc, 2008, 130: 2946–2947
He M, Li J, Tan S, et al. Photodegradable supramolecular hydrogels with fluorescence turn-on reporter for photomodulation of cellular microenvironments. J Am Chem Soc, 2013, 135: 18718–18721
Smith DJ, Brat GA, Medina SH, et al. A multiphase transitioning peptide hydrogel for suturing ultrasmall vessels. Nat Nanotech, 2016, 11: 95–102
Fasman GD. Circular Dichroism and the Conformational Analysis of Biomolecules. New York: Plenum Publishers, 1996
Yu Z, Tantakitti F, Palmer LC, et al. Asymmetric peptide nanoribbons. Nano Lett, 2016 16: 6967–6974
Haris PI, Chapman D. The conformational analysis of peptides using fourier transform IR spectroscopy. Biopolymers, 1995, 37: 251–263
Yu Z, Tantakitti F, Yu T, et al. Simultaneous covalent and noncovalent hybrid polymerizations. Science, 2016, 351: 497–502
Suter DM, Tirefort D, Julien S, et al. A Sox1 to Pax6 switch drives neuroectoderm to radial glia progression during differentiation of mouse embryonic stem cells. Stem Cells, 2009, 27: 49–58
Bernal A, Arranz L. Nestin-expressing progenitor cells: function, identity and therapeutic implications. Cell Mol Life Sci, 2018, 75: 2177–2195
Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell, 2012, 150: 780–791
Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature, 2011, 474: 179–183
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFC1313003), the Fundamental Research Funds for the Central Universities, the National Natural Science Foundation of China (21774065 and 31622038), and the Natural Science Foundation of Tianjin (18JCQNJC14100 and 18JCJQJC48400).
Author information
Authors and Affiliations
Contributions
Author contributions Cheng Z and Yu Z conceived and designed the study. Cheng Z and Song S carried out the preparation and characterization of the hydrogels. Nai S and Chen L designed and performed all the cellular studies. Cheng Z prepared the manuscript with assistance from Nai S. Chen L and Yu Z revised the manuscript. All the authors discussed the results and have the approval of the submission of the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Zhifei Cheng received his MSc degree from Hefei University of Technology in 2017. Then he continued his study as a PhD candidate under the guidance of Prof. Zhilin Yu at Nankai University. His current research interest focuses on the development of peptide-based dynamic materials for biomedical applications.
Shanshan Nai received her MSc degree from the College of Life Sciences, Capital Normal University in 2018. She is now a PhD student in Prof. Lingyi Chen’s laboratory at the College of Life Sciences, Nankai University. Her current interest focuses on novel Mek1 downstream targets in maintaining the pluripotency of embryonic stem cells.
Lingyi Chen is a professor and vice dean of the College of Life Sciences, Nankai University, and an awardee of the National Science Fund for Excellent Young Scholars. He obtained his PhD degree in biochemistry from Northwestern University in 2004, and then conducted his postdoctoral research in Harvard University. He established his laboratory at Nankai University in 2008, and his research interests include molecular mechanism of pluripotency maintenance in embryonic stem cells and molecular regulation of early mouse embryonic development.
Zhilin Yu was awarded his PhD degree under the supervision of Prof. Stefan Hecht at the Humboldt- Universität zu Berlin in 2013. He conducted his postdoctoral training with Prof. Samuel I. Stupp at Northwestern University focusing on self-assembly of peptide-based amphiphilic molecules. In 2017, he started his independent career at the Institute of Polymer Science of Nankai University. His current research interests focus on the self-assembly of peptides into dynamic nanostructures and their broad applications as biomaterials including disease diagnosis and therapy.
Supporting Information
40843_2019_1184_MOESM1_ESM.pdf
Photoinduced Directional Domain Sliding Motion in Peptide Hydrogels Promotes Ectodermal Differentiation of Embryonic Stem Cells
Rights and permissions
About this article
Cite this article
Cheng, Z., Nai, S., Song, S. et al. Photoinduced directional domain sliding motion in peptide hydrogels promotes ectodermal differentiation of embryonic stem cells. Sci. China Mater. 63, 467–478 (2020). https://doi.org/10.1007/s40843-019-1184-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-019-1184-y