Abstract
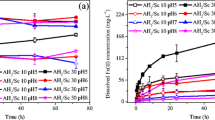
The migratory characteristic of iron and its effects on the oxidative dissolution of low-grade polymetallic Ni–Cu–Fe-bearing sulfide ores in sulfuric acid solutions were investigated. This study involved three parts. First, the influence of Fe on the Ni and Cu leaching efficiencies was measured using leaching tests. It was revealed that the dissolution of Fe-bearing sulfide limits the dissolution of Ni–Cu sulfide to a certain extent, the leached Fe3+ ion significantly affects Ni and Cu leaching, and the Fe3+ ion has a synergistic impact with hydrogen and alkali-metal ions in the solutions. Second, this work presents the thermodynamic solution status and mineralogical phase transformation of the Fe species. Fe sulfide tends to oxidize into Fe3+ ions, precipitating into Fe oxide/sulfate sediment. Third, the electrochemical test results illustrate that a lower reduction potential leads to the preferential dissolution of Fe sulfide relative to Ni–Cu sulfides; the dissolved Fe3+ ion results in a better exchange efficiency of Fe3+/Fe2+ and a higher potential and conductivity of the Fe3+-containing solution, increasing the Ni and Cu dissolution rates by a factor of 3.65 and 2.92 times at pH 0.8, relative to that of Fe3+-free solutions, respectively. Furthermore, the coupling of Fe3+with Na+/Mg2+ions slows down the Ni–Cu leaching rate by more than 10% compared to that of Fe3+ ions in acidic solutions. Based on these results, the influence of Fe migration on the Ni–Cu oxidative leaching was significantly affected by the acidity and ionic state of the lixivium. This study elucidates the effect of ionic migration, which is related to the metallurgical return water, on the Ni–Cu oxidative extraction for the low-grade Ni-Cu-Fe sulfide ore.
Graphical Abstract
Fe migration of the low-grade Ni–Cu–Fe-bearing sulfide ore oxidative leaching












Similar content being viewed by others
References
Zhai M, Hu R, Wang Y et al (2021) Mineral resource science in China: review and perspective. Geogr Sustain 2:107–114. https://doi.org/10.1016/j.geosus.2021.05.002
Henckens M, Worrell E (2020) Reviewing the availability of copper and nickel for future generations. The balance between production growth, sustainability and recycling rates. J Clean Prod 264:121460. https://doi.org/10.1016/j.jclepro.2020.121460
Cui F, Mu W, Zhai Y et al (2020) The selective chlorination of nickel and copper from low-grade nickel-copper sulfide-oxide ore: mechanism and kinetics. Sep Purif Technol 239:116577. https://doi.org/10.1016/j.seppur.2020.116577
Xie Y, Xu Y, Yan L et al (2005) Recovery of nickel, copper and cobalt from low-grade Ni–Cu sulfide tailings. Hyrometallurgy 80:54–58. https://doi.org/10.1016/j.hydromet.2005.07.005
Kang J, Wang Y, Yu C et al (2022) Selective extraction performance of Ni, Cu Co, and Mn adopted by a coupled leaching of low-grade Ni-sulfide ore and polymetallic Mn-oxide ore in sulfuric acid solutions. J Phys Chem Solids 168:110814. https://doi.org/10.1016/j.jpcs.2022.110814
Eksteen J, Oraby E, Nguyen V (2020) Leaching and ion exchange based recovery of nickel and cobalt from a low grade, serpentine-rich sulfide ore using an alkaline glycine lixiviant system. Miner Eng 145:106073. https://doi.org/10.1016/j.mineng.2019.106073
Mu W, Cui F, Huang Z et al (2017) Synchronous extraction of nickel and copper from a mixed oxide-sulfide nickel ore in a low-temperature roasting system. J Clean Prod 177:371–377. https://doi.org/10.1016/j.jclepro.2017.12.260
Watling H (2008) The bioleaching of nickel-copper sulfides. Hydrometallurgy 91:70–88. https://doi.org/10.1016/j.hydromet.2007.11.012
Zhong P, Mu W, Li L et al (2022) Extraction of metals from nickel concentrate via FeCl3·6H2O chlorination followed by water leaching and the preparation of (Ni, Cu)Fe2O4 catalyst. Miner Eng 188:107826. https://doi.org/10.1016/j.mineng.2022.107826
Muravyov M, Panyushkina A, Fomchenko N (2022) Bulk flotation followed by selective leaching with biogenic ferric iron is a promising solution for eco-friendly processing of complex sulfidic ores. J Environ Manage 318:115587. https://doi.org/10.1016/j.jenvman.2022.115587
Mu W, Xiao T, Shi S et al (2022) Co-extraction of valuable metals and kinetics analysis in chlorination process of low-grade nickel-copper sulfide ore. T Nonferr Metal Soc 32:2033–2045. https://doi.org/10.1016/S1003-6326(22)65928-4
Kang J, Chen L, Yu S et al (2022) Chromite geochemistry of the Jinchuan Ni-Cu sulfide-bearing ultramafic intrusion (NW China) and its petrogenetic implications. Ore Geol Rev 141:104644. https://doi.org/10.1016/j.oregeorev.2021.104644
Wang Y, Zhou M, Zhao D (2005) Mineral chemistry of chromite from the Permian Jinbaoshan Pt–Pd–sulfide-bearing ultramafic intrusion in SW China with petrogenetic implications. Lithos 83:47–66. https://doi.org/10.1016/j.lithos.2005.01.003
Zhao K, Yan W, Wang X et al (2020) Effect of a novel phosphate on the flotation of serpentine-containing copper-nickel sulfide ore. Miner Eng 150:106276. https://doi.org/10.1016/j.mineng.2020.106276
Muravyov M, Panyushkina A, Fomchenko N (2022) Effect of copper/nickel ratio on the efficiency of biobeneficiation of bulk copper-nickel sulfide concentrates. Miner Eng 182:107586. https://doi.org/10.1016/j.mineng.2022.107586
Kelebek S, Tukel C (2018) Separation of nickeliferous hexagonal pyrrhotite from pentlandite in Ni-Cu sulfide ores: recovery by size performance. Miner Eng 125:223–230. https://doi.org/10.1016/j.mineng.2018.06.015
Huang K, Li Q, Chen J (2007) Recovery of copper, nickel and cobalt from acidic pressure leaching solutions of low-grade sulfide flotation concentrates. Miner Eng 20:722–728. https://doi.org/10.1016/j.mineng.2007.01.011
Maley M, Bronswijk W, Waltling H (2009) Leaching of a low-grade, copper–nickel sulfide ore: impact of aeration and pH on Cu recovery during abiotic leaching. Hydrometallurgy 98:66–72. https://doi.org/10.1016/j.hydromet.2009.03.016
Watling H (2013) Chalcopyrite hydrometallurgy at atmospheric pressure: review of acidic sulfate, sulfate–chloride and sulfate–nitrate process options. Hydrometallurgy 140:163–180. https://doi.org/10.1016/j.hydromet.2005.07.005
Mu W, Cui F, Xin H et al (2020) A novel process for simultaneously extracting Ni and Cu from mixed oxide-sulfide copper-nickel ore with highly alkaline gangue via FeCl3∙6H2O chlorination and water leaching. Hydrometallurgy 191:105187. https://doi.org/10.1016/j.hydromet.2019.105187
Wang S, Fang Z (2006) Mechanism of influence of ferric ion on electrogenerative leaching of sulfide minerals with FeCl3. T Nonferr Metal Soc 16:473–476. https://doi.org/10.1016/S1003-6326(06)60081-2
Bochmann G, Vielstich W (1988) On the reaction rate of the Fe2+/Fe3+ redox couple in sulfate solution. Electrochimi Acta 33:805–809. https://doi.org/10.1016/S0013-4686(98)80011-X
Karimi S, Ghahreman A, Rashchi F (2018) Kinetics of Fe(III)-Fe(II) redox half-reactions on sphalerite surface. Electrochimi Acta 281:624–637. https://doi.org/10.1016/j.electacta.2018.05.132
Olvera O, Quiroz L, Dison D et al (2014) Electrochemical dissolution of fresh and passivated chalcopyrite electrodes. Effect of pyrite on the reduction of Fe3+ ions and transport processes within the passive film. Electrochimi Acta 127:7–19. https://doi.org/10.1016/j.electacta.2014.01.165
Warner T, Rice N, Toylor N (1996) Thermodynamic stability of pentlandite and violarite and new Eh-pH diagrams for the iron-nickel sulphur aqueous system. Hydrometallurgy 41:107–118. https://doi.org/10.1016/0304-386X(95)00081-Q
Li H, Li C, Zhang Z (2012) Decomposition mechanism of pentlandite during electrochemical bio-oxidation process. T Nonferr Metal Soc 22:731–739. https://doi.org/10.1016/S1003-6326(11)61238-7
Ahmadi A, Schaffie M, Petersen J et al (2011) Conventional and electrochemical bioleaching of chalcopyrite concentrates by moderately thermophilic bacteria at high pulp density. Hydrometallurgy 106:84–92. https://doi.org/10.1016/j.hydromet.2010.12.007
Thornber M (1983) Mineralogical and electrochemical stability of the nickel-iron sulfides-pentlandite and violarite. J Appl Electroch 13:253–267. https://doi.org/10.1007/BF00612487
Acknowledgements
This study was supported by China Ocean Biological Resources Development Program (DY135-B2-15).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
The contributing editor for this article was Atsushi Shibayama.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, Jx., Yu, C., Liu, Zg. et al. Fe Migration and its Influence on the Oxidative Leaching of Low-Grade Ni–Cu–Fe-Bearing Sulfide Ores in Sulfuric Acid Solutions. J. Sustain. Metall. 9, 723–737 (2023). https://doi.org/10.1007/s40831-023-00679-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00679-0