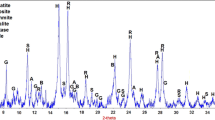
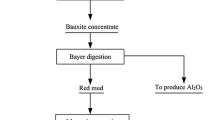
Abstract
The Bayer process for alumina production generates more than 160 million tons of bauxite residue annually. The current global stockpiles of bauxite residue have reached more than 4 billion tons with less than 2% annual recycling rate. Critical elements such as Sc and Y present an opportunity to explore bauxite residue as a secondary resource; however, low concentration affects the process economics. The following research focuses on the recovery of Ti, Al, and rare earth elements (Sc, Y, La, Ce) from upgraded bauxite residue obtained after Fe, Na, Ca, and Si separation. Recovery of major elements resulted in upgradation of Ti and RE values up to fourfold. Thermodynamic and kinetic aspects of the proposed recovery process are critically evaluated, and optimized conditions are reported to obtain high recovery of Ti, Al, and RE values. The major elements are recovered as high-purity (> 99.5%) TiO2 and Al2(SO4)2.14H2O, whereas Sc and Y are concentrated into a liquid solution for downstream recovery with solvent extraction.
Graphical Abstract












Similar content being viewed by others
References
Habashi F (2016) A hundred years of the bayer process for alumina production. In: Donaldson D, Raahauge BE (eds.) Essential readings in light metals. Springer, Cham, pp. 85–93. https://doi.org/10.1007/978-3-319-48176-0_12
Healy S (2022) International Aluminum Institute, Sustainable Bauxite Residue Management Guidance 2022. https://international-aluminium.org/resource/sustainable-bauxite-mining-guidelines-second-edition-2022-2/
Statistical annual report of USGS. U.S. Geological Survey, Mineral Commodity Summaries, “Aluminum” 2021. https://www.usgs.gov/centers/national-minerals-information-center/aluminum-statistics-and-information
Evans K (2016) The history, challenges, and new developments in the management and use of bauxite residue. J Sustain Metall 2(4):316–331
Di Carlo E, Boullemant A, Courtney R (2020) Ecotoxicological risk assessment of revegetated bauxite residue: Implications for future rehabilitation programmes. Sci Total Environ 698:134344
Khairul MA, Zanganeh J, Moghtaderi B (2019) The composition, recycling and utilisation of Bayer red mud. Resour Conserv Recycl 141:483–498
Ujaczki E et al (2018) Re-using bauxite residues: benefits beyond (critical raw) material recovery. J Chem Technol Biotechnol 93(9):2498–2510
Borra CR et al (2016) Recovery of rare earths and other valuable metals from bauxite residue (red mud): a review. J Sustain Metall 2(4):365–386
Mishra B, Gostu S (2017) Materials sustainability for environment: red-mud treatment. Front Chem Sci Eng 11(3):483–496
Swain B, Akcil A, Lee J-C (2020) Red mud valorization an industrial waste circular economy challenge; review over processes and their chemistry. Critical Rev Environ Sci Technol 52:1–51
Hammond K et al (2013) CR3 communication: red mud—a resource or a waste? Jom 65(3):340–341
Abhilash, et al (2014) Extraction of lanthanum and cerium from Indian red mud. Int J Miner Process 127:70–73
Agatzini-Leonardou S et al (2008) Titanium leaching from red mud by diluted sulfuric acid at atmospheric pressure. J Hazard Mater 157(2–3):579–586
Agrawal S, Dhawan N (2021) Microwave acid baking of red mud for extraction of titanium and scandium values. Hydrometallurgy 204:105704
Alkan G et al (2018) Novel approach for enhanced scandium and titanium leaching efficiency from bauxite residue with suppressed silica gel formation. Sci Rep 8(1):5676
Borra CR et al (2016) Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner Eng 92:151–159
Narayanan RP, Kazantzis NK, Emmert MH (2017) Selective process steps for the recovery of scandium from jamaican bauxite residue (red mud). ACS Sustain Chem Eng 6(1):1478–1488
Borra CR et al (2015) Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J Sustain Metall 2(1):28–37
Cardenia C, Balomenos E, Panias D (2018) Iron recovery from bauxite residue through reductive roasting and wet magnetic separation. J Sustain Metall 5(1):9–19
Liu X et al (2020) Clean utilization of high-iron red mud by suspension magnetization roasting. Minerals Eng 157:106553
Kaußen F, Friedrich B (2015) Reductive smelting of red mud for iron recovery. Chem Ing Tec 87(11):1535–1542
Mishra B, Staley A, Kirkpatrick D (2002) Recovery of value-added products from red mud. Mining Metall Explor 19:87–94
Azof FI et al (2020) The leachability of a ternary CaO-Al2O3-SiO2 slag produced from smelting-reduction of low-grade bauxite for alumina recovery. Hydrometallurgy 191:105184
Erçağ E, Apak R (1997) Furnace smelting and extractive metallurgy of red mud: Recovery of TiO2, Al2O3 and pig iron. J Chem Technol Biotechnol 70(3):241–246
Anawati J, Azimi G (2020) Recovery of strategic materials from canadian bauxite residue by smelting followed by acid baking-water leaching. Springer International Publishing, Cham
Archambo MS, Kawatra SK (2020) Utilization of bauxite residue: recovering iron values using the iron nugget process. Miner Process Extr Metall Rev 42(4):222–230
Anawati J, Azimi G (2022) Integrated carbothermic smelting – acid baking – water leaching process for extraction of scandium, aluminum, and iron from bauxite residue. J Cleaner Prod 330:129905
Tanvar H, Mishra B (2021) Hydrometallurgical recycling of red mud to produce materials for industrial applications: alkali separation, iron leaching and extraction. Metall and Mater Trans B 52:3543–3557
US Patent US1504672A. Preparation of titanium hydroxide. Inventor: Joseph Blumenfeld, https://patents.google.com/patent/US1504672A/en
Grzmil BU, Grela D, Kic B (2008) Hydrolysis of titanium sulphate compounds. Chem Pap 62(1):18–25
Schwartz D et al (2000) A kinetic study of the decomposition of spent sulfuric acids at high temperature. Ind Eng Chem Res 39(7):2183–2189
Han KN, Rubcumintara T, Fuerstenau MC (1987) Leaching behavior of ilmenite with sulfuric acid. Metall Trans B 18(2):325–330
Dubenko AV et al (2020) Mechanism, thermodynamics and kinetics of rutile leaching process by sulfuric acid reactions. Processes 8(6):640
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201(4914):68–69
Tanvar H, Mishra B (2022) Comprehensive utilization of bauxite residue for simultaneous recovery of base metals and critical elements. Sustain Mater Technol 33:e00466
Acknowledgements
The authors are thankful to Mr. Glenn Yee for the fellowship he instituted at the Worcester Polytechnic Institute. Thanks are due to the NSF Center for Resource Recovery & Recycling for their technical support through Global Minerals Recovery, LLC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Atsushi Shibayama.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanvar, H., Mishra, B. Extraction of Titanium, Aluminum, and Rare Earth Values from Upgraded Bauxite Residue. J. Sustain. Metall. 9, 665–677 (2023). https://doi.org/10.1007/s40831-023-00678-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00678-1