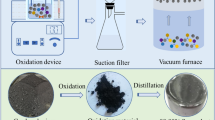
Abstract
90% of selenium (Se) originates from the anode slime byproduct of copper electrorefining. A sulfurizing-vacuum distillation process was proposed to remove Hg, a typical impurity element exhibiting high toxicity in metallurgical-grade Se, from high-value waste selenium material (HWSM). 97.7% Hg was removed from the Se sample. It was obtained after the raw material of Se with high amounts of Hg was sulfurized at 473 K for 30 min. It was subsequently sealed in the atmosphere with 5 wt% sulfur addition and then underwent the first-stage vacuum distillation at 523 K for 60 min under 1–10 Pa. Moreover, 99.84% Hg was removed from the HWSM after the secondary distillation at 473 K for 60 min under 1–10 Pa. In the mentioned process, no waste gases and materials were produced. Compared with other metallurgical methods of removing Hg from the HWSM, this study demonstrated a clean, short, efficient and low energy consumption route.
Graphical Abstract











Similar content being viewed by others
References
Nasim J, Ali W, Enrique Domínguez-lvarez et al (2017) Reactive selenium species: redox modulation, antioxidant, antimicrobial and anticancer activities. In: Jain VK, Priyadarsini KI (eds) Organoselenium compounds in biology and medicine: synthesis, biological and therapeutic treatments. Royal Society of Chemistry, pp 277–302
Lei H, Jiao S, Jiguo Tu, Song W-L, Zhang X, Wang M, Li S, Chen H, Fang D (2020) Modified separators for rechargeable high-capacity selenium-aluminium batteries. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123452
Guozheng Z, Bin Y, Huan L, Daxin H, Jiang Wenlong Xu, Baoqiang LD (2021) Innovative green approach for the selective extraction of high-purity selenium from waste selenium sludge. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2021.118536
Lee WR, Eom Y, Tai GL (2016) Mercury recovery from mercury-containing wastes using a vacuum thermal desorption system. Waste Manage 60:546–551. https://doi.org/10.1016/j.wasman.2016.12.017
Yau VM, Green PG, Alaimo CP, Yoshida CK, Lutsky M, Windham GC, Delorenze G, Kharrazi M, Grether JK, Croen LA (2014) Prenatal and neonatal peripheral blood mercury levels and autism spectrum disorders. Environ Res 133:294–303
Yu J-G, Yue B-Y et al (2016) Removal of mercury by adsorption: a review. Environ Sci Pollut Res 23(6):5056–5076. https://doi.org/10.1007/s11356-015-5880-x
Anyuan W (2020) Experimental study on wet recovery of mercury and selenium from acid mud. China Nonferrous Metall 49(05):42–47
Shuang Z, Zhong Yu, Renjun X et al (2020) Study on extraction and separation technology of selenium and mercury from acid mud. J Xiangtan Univ 042(001):86–94
Xiaowu W, Xingxiang F, Yongxiang Li (2013) Experimental study on extraction of selenium from selenium-containing mud. Wet Metall 5:316–318
Yi L et al (2012) Alkylthiol-enabled Se powder dissolution in oleylamine at room temperature for the phosphine-free synthesis of copper-based quaternary selenide nanocrystals. J Am Chem Soc 134(17):7207–7210. https://doi.org/10.1021/ja300064t
Qiongqiong W, Qifeng D, Chengsheng L, Xiaoyu W, Li Boyuan Xu, Qing LH, Xuzhou Ji, Bonian Z, Dongsu C (2021) Novel amidinothiourea-modified chitosan microparticles for selective removal of Hg (II) in solution. Carbohyd Polym. https://doi.org/10.1016/j.carbpol.2021.118273
Singh N, Srivastava I, Dwivedi J et al (2021) Ultrafast removal of ppb levels of Hg (II) and volatile Hg (0) using post modified metal organic framework. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.129490
Shi T, He J, Zhu R et al (2021) Arsenic removal from arsenic–containing copper dust by vacuum carbothermal reduction–sulfurizing roasting. Vacuum 2:110213. https://doi.org/10.1016/j.vacuum.2021.110213
Wang F et al (2020) Preparation of vanadium powders by calcium vapor reduction of V2O3 under vacuum. Vacuum 173:109133–109133. https://doi.org/10.1016/j.vacuum.2019.109133
Ji W, Xie K, Yan S, Huang H, Chen H (2020) A new method of recycling gallium from yellow phosphorus flue dust by vacuum thermal reduction process. J Waste Mater. https://doi.org/10.1016/j.jhazmat.2020.123234
Liu T, Qiu K (2018) Removing antimony from waste lead storage batteries alloy by vacuum displacement reaction technology. J Waste Mater 347:334–340. https://doi.org/10.1016/j.jhazmat.2018.01.017
Yong-nian D, Bin Y (2000) Vacuum metallurgy of nonferrous metal materials. Metallurgical Industry Press, Beijing
Liang Y, Yinchang C (1994) Inorganic substances thermodynamic data handbook. Northeastern University Press, Shenyang
Dean JA (2005) Lange’s hand book of chemistry, 16th edn. McGraw-Hill, New York
Zhai X, Zhou Y (2009) Dilute metals: scattered metals. China University of Science and Technology Press, Hefei
Zha G, Wang Y, Cheng M et al (2020) Purification of crude selenium by vacuum distillation and analysis. J Market Res. https://doi.org/10.1016/j.jmrt.2020.01.04
Acknowledgements
This study was supported by the National Science Foundation of China (U1902221); Plan of Yunling Scholars Project of Yunnan Province, the Scientist Studio of Yunnan Province, the Leading Talents of Industrial Technology in Ten Thousand Talents Plan of Yunnan Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Sharif Jahanshahi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, H., Zha, G., Liu, L. et al. Sustainable and Clean Approach to Remove High Toxicity Element from High-Value Waste Selenium Material. J. Sustain. Metall. 8, 112–121 (2022). https://doi.org/10.1007/s40831-021-00474-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00474-9