Abstract
Hydroformylation is one of the most important homogeneously catalyzed reactions on an industrial scale. The manufacture of bulk chemicals clearly dominates. Large cobalt- and rhodium-based processes are mature technologies that have been developed over the past 80 years. Meanwhile, the potential of hydroformylation for the production of fine chemicals (perfumes, pharmaceuticals) has also been recognized. This review gives insight into the state-of-the-art of the reaction and its development. It commences with some remarks on the accidental discovery by the German chemist Otto Roelen within the historical and personal framework of the Fischer–Tropsch process, followed by the mechanistic basics of the catalytic cycle, metals used for the catalyst as well as their organic ligands. In addition, the stability of ligands and catalysts is addressed. The huge potential of this transformation is demonstrated using a variety of substrates. Finally, the use of some surrogates for syngas is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydroformylation is the addition of synthesis gas (“syngas”), a mixture of CO and H2, to olefins in the presence of a catalyst to form aldehydes. Hydrogen (“hydro”) and a formyl group (H–C=O) are added to the double bond (Fig. 1). The reaction proceeds in an atom-economical manner, i.e., no other products are theoretically formed. The reaction only proceeds in the presence of a catalyst. In general, elevated temperatures and syngas pressures are applied.
The reaction leads, unless ethylene or cycloalkenes are used as a substrate, to a mixture of isomeric products, n-aldehydes (linear) and iso-aldehydes (branched). Since double bond isomerization of longer chain olefins may occur prior to the hydroformylation, different branched aldehydes can be formed even when a single terminal olefin is subjected to the reaction. In addition to the rate of the reaction, the ratio of the isomers (regioselectivity) is therefore an important parameter of each hydroformylation. Independent of the catalyst used, hydroformylation proceeds in a cis-manner, i.e., H and CHO are added from the same side to the C=C–bond. If R is not H or CH3 (Fig. 1), the iso-aldehydes become chiral, thus the possibility for a stereoselective transformation arises. The same happens when α,α-disubstituted olefins with different substituents are employed as substrates.
History
Hydroformylation was discovered accidently by Otto Roelen (1897–1993), a fascinating person and scientist (Fig. 2) [1, 2].
After returning as a soldier from the First World War he started to work as a chemist at the Kaiser-Wilhelm-Institut for coal research in Mülheim (Germany). The discovery of the hydroformylation happened in the scientific and personal framework of the Fischer–Tropsch process. In 1925, this process, where a mixture of carbon monoxide, hydrogen or water gas is converted into liquid hydrocarbons (Fig. 3) was developed by the German chemists Franz Fischer and Hans Tropsch. Not only saturated hydrocarbons are formed in the Fischer–Tropsch process but also short and long chain olefins. Several metallic catalysts can be used. Among them cobalt is of special importance.
After the First World War the unrestricted access to synthetic lubrication oil and synthetic fuel became of strategic importance for Germany. As a result of not owning overseas colonies, only limited resources of natural oil and gas were available. Therefore, the German Reich endorsed a secret program of military self-sufficiency of fuels in the 1920s. This problem was addressed and finally solved by the Fischer–Tropsch protocol.
One of the main sources of carbon monoxide for the Fischer–Tropsch process is derived from the reaction of water with carbon at high temperature. The resulting mixture is called synthesis gas (“syngas”) (Fig. 4).
Owing to the large excess of coking gas (a mixture of hydrogen, methane, nitrogen, and carbon monoxide) which was a consequence of the modernization of coking plants in this historical period new applications were sought. This situation also led to an increase of academic interest in the utilization of syngas.
In this historical setting Otto Roelen started his investigations as an associate in the department of Dr. Hans Tropsch. At that time Professor Franz Fischer was the director of the institute. In the beginning, most of Roelen’s work was purely academic, but he hoped for some practical relevance. His preliminary interest was in the decomposition of formaldehyde at elevated temperatures, which resulted in 1924 in the successful defense of his dissertation. The decomposition reaction of formaldehyde leads to CO and H2 and received only recently (70 years later!) again some importance as a surrogate for syngas in hydroformylation [3]. Strangely enough the thesis of this extraordinary scientist was graded by the academic supervisor only with the mark “good”.
In 1934, Roelen moved from Kaiser-Wilhelm-Institut to Kohlenchemie AG, a German company collectively founded by a consortium of mining companies, which was renamed Ruhrchemie in 1928. During an attempt to lead ethylene produced by the Fischer–Tropsch synthesis back into the reaction, Roelen surprisingly found in the presence of ammonia the corresponding imine of propionaldehyde as a white solid product when a catalyst containing a mixture of cobalt, thorium, and magnesium oxide commonly used at this time was applied [2]. Soon he also discovered that other cobalt salts could mediate the reaction. Roelen concluded that the formation of the aldehyde could not merely be a side reaction of the Fischer–Tropsch process, and he started to investigate the scope and limitations of this new reaction in detail. Immediately the huge chemical and economic potential of his discovery was recognized. Roelen added a patent application for the so-called oxo synthesis at the end of 1938 [4]. The names “oxo synthesis” or “oxo process” were later replaced by “hydroformylation”, but they are still used in industry [5]. The first pilot unit began to operate only a few years later at IG Farben Leuna/Merseburg. Later, Roelen also included Fischer–Tropsch olefins with chain lengths of 11–17 carbon atoms in his investigations. In 1940, Ruhrchemie started the construction of a plant with an output of 10,000 tons of fatty acids per year. However, as a result of the Second World War, the plant could not go onstream.
Otto Roelen never intended to start an academic career, although some of his advisors and colleagues recognized his great intellectual potential. In fact, he mostly neglected to publish his exciting results. He was always more interested in the solution of specific technical challenges. Directly after the war his hesitancy to publish became a personal advantage for Roelen, because the Soviet occupier did not realize his scientific importance and let him leave the zone they occupied. In this way one of the greatest discoveries in homogeneous catalysis was not really appreciated by the scientific community, although Roelen’s findings were at the same level compared to those of other outstanding chemists of this period who were awarded with the Nobel Prize.
Current industrial importance of hydroformylation
Today, the transformation represents one of the largest homogeneously catalyzed reactions in industry (Fig. 5). Nearly 10 million metric tons of oxo chemicals are produced every year. Almost the entire volume is manufactured worldwide in plants with an output of several hundred thousand metric tons each.
The aldehydes formed are valuable final products and intermediates in the synthesis of bulk chemicals like alcohols, esters, and amines (Fig. 6). Moreover, a reduction-elimination sequence gives access to isomerically pure olefins prolonged by an additional C1-unit.
Hydroformylation is also of value in academic laboratories and for the manufacture of fine chemicals. However, this great potential only recently came into focus. This odd situation is probably attributed to having to work with extremely toxic CO under pressure. Moreover, development costs are too high for specific, small applications. But there is no doubt that hydroformylation will see an increasing importance in the future, especially in the production of perfumes and scents [6]. This is because aldehydes belong to odor-conferring functional groups. Famous perfumes, like Chanel N°5, get their olfactorial impression mainly from the high concentration of several aldehydes. Asymmetric variants of hydroformylation have become useful only very recently and general-purpose ligands, which have seen application in other enantioselective reactions, are still missing. Another reason for the rare application of hydroformylation in fine chemical production may be that synthetic chemists working at the interface between academic and industrial research often have only limited knowledge about the potential of modern hydroformylation methodologies. A detailed analysis showed that the industrial research is carried out by only a few companies. Meanwhile some books and reviews have been published in the field of hydroformylation that may change this situation in the future [7,8,9].
Mechanism of the hydroformylation reaction and types of catalysts
There have been many investigations concerning the evaluation of different metals in hydroformylation and the effect of reaction conditions (choice of ligands, temperature, syngas pressure, ratio of H2/CO, solvent, etc.) [10]. To date, homogeneous cobalt and rhodium catalysts are used exclusively in industry. In some cases, heterogeneous versions were also tested, but they did not achieve industrial relevance. One can differentiate between processes with unmodified catalysts and those based on ligand-modified catalysts. In contrast to “naked” catalysts, the use of organic ligands as cocatalysts allows one to tune the activity of the catalyst, as well as the rate of the reaction and selectivity in the product.
Mechanism
Some general conclusions can be made about a successful setup of the reaction independent of the type of the metal used: only hydrido metal carbonyl complexes mediate the reaction independent of the type of metal used (Fig. 7). Usually, these complexes are added to the reaction as the more stable precatalyst, which is converted under hydroformylation conditions (H2/CO) into the active catalyst. In accordance with the broadly accepted Heck and Breslow mechanism [11], crucial steps follow in the presence of olefin, such as the formation of a metal–alkyl complex by hydride migration (a), subsequent insertion of CO into the M–alkyl bond by migration of a ligated CO ligand (b), and the final hydrogenolysis of the M–acyl bond to liberate the desired aldehyde and to reconstruct the catalyst (c). The type of transient M–alkyl complex is responsible for the formation of isomeric aldehydes, here distinguished as Cycle I and II [12]. Besides the reaction conditions, the choice of metal and its coordinated ligands are pivotal for a successful passage of these catalytic events.
Adequate hydroformylation activity of hydrido carbonyl complexes is attributed to the polarity of the M–H bond. It is assumed that high acidity facilitates the addition to an olefin and the hydrogenolysis of the transient metal–acyl complex in a later stage of the catalytic cycle. In this respect, HCo(CO)4 is a much stronger acid than, e.g., H2Ru(CO)4, H2Fe(CO)4, H2Os(CO)4, or HMn(CO)5 [13]. The stability of the catalysts depends on the syngas pressure and the H2/CO ratio. Thus, HCo(CO)4 undergoes fast reaction into Co2(CO)8 owing to the fast intermolecular elimination of H2 at reduced CO partial pressure.
The number of CO moieties ligated to the same metal may affect the catalytic properties (Fig. 8) [14]. With cobalt (but also with rhodium), both the tetra- and tricarbonyl complexes are considered as catalysts. It is thought that the coordinatively unsaturated complex HCo(CO)3 is more active than HCo(CO)4. As a result of different steric congestion around the metal center, it is assumed that the two complexes have different regiodiscriminating propensities for the formation of transient alkyl complexes and, consequently, for the formation of isomeric aldehydes. Moreover, at a low CO partial pressure, the beta-hydride elimination of the metal–alkyl complex is favored, leading to both terminal and isomeric olefins. Density functional theory (DFT) studies revealed that terminal olefins form M–alkyl complexes preferred both thermodynamically and kinetically over those with branched alkenes [15]. Therefore, different CO partial pressures usually result in various regioselectivities. If the CO partial pressure is extremely low, the formation of HCo3(CO)9 in a solution containing HCo(CO)4 and its precursor Co2(CO)8 under hydrogen should be taken into account. HCo3(CO)9 reacts with hydrogen to form HCo(CO)3. The latter is more active in isomerization and, consequently, forms more isomeric aldehydes as final products.
In comparison to HCo(CO)4, the rhodium congener has a greater tendency to liberate one CO ligand. In other words, the equilibrium in Fig. 9 is less markedly displaced to the left-hand side in comparison to the cobalt-based system.
Bearing in mind the greater atomic radius of Rh, it is thus apparent why an unmodified rhodium catalyst generates a greater amount of branched aldehydes in comparison to the cobalt congener.
Organic ligands used in hydroformylation
Some basic considerations about structure and effects
The properties of the catalytically active metal can be modified by organic ligands. Independent of the metal used, only trivalent phosphorus compounds are employed as ancillary ligands in industrial applications. Other potentially ligating compounds based on elements of the 5th row of the periodic table have not played a role to date, although trivalent compounds of As, Sb, and Bi are occasionally claimed in patents [16].
Phosphine ligands (also called phosphanes) are usually characterized by three carbon atoms surrounding the central phosphorus atom (Fig. 10). Replacement of one C-substituent in phosphines by an oxy group produces esters of phosphinous acid (phosphinites). Further transesterification with alcohol gives esters of phosphonous acid (phosphonites), and esters of phosphorus acid (phosphites). Another possibility of modification is the stepwise incorporation of N-substituents, which produces aminophosphines, diaminophosphines, or triaminophosphines. Further variations can be produced by combining different heteroatoms. A frequently used ligand motif used in asymmetric hydroformylation is the structure of phosphoramidite. The organic backbone can be acyclic, but the incorporation of the phosphorus in aromatic or non-aromatic heterocycles of different ring sizes is also possible [17].
To optimize known processes and develop new catalytic systems, knowledge of some crucial ligand parameters can be of value. There are numerous methods to test the steric and electronic properties of phosphorus ligands. These experimental data are nowadays supported by computational methods. It should be noted, however, that simple correlations between a set of limited analytical data and catalytic results or stabilities rarely exist.
31P NMR spectroscopy is the method of choice for characterization of phosphorus ligands and corresponding metal complexes. Numerous data for the characterization of P-ligands have been accumulated after incorporation into the corresponding metal carbonyl complexes. A frequently used model compound is the rapidly formed complex of the type [Ni(CO)3(phosphine)]. The Tolman cone angle, θ, characterizes the size of the organic ligand in this complex (Fig. 11a) [18]. σ-Donor and π-acceptor properties of ligands can be assessed by recording the IR spectra of corresponding metal–CO complexes, since they show very specific and intensive CO stretching bands usually in the range from 1900 to 2100 cm−1. The Tolman electronic parameter (TOP) can be extracted using this procedure. In the meantime, Tolman ligand maps have become a guiding principle for the evaluation of ligand effects. In addition, the concept of the natural bite angle, α, of bidentate ligands has been developed for hydroformylation in order to get some insights into the relationship between activity and regioselectivity of homogeneous rhodium catalysts (Fig. 11b) [19]. The parameter, which is derived from molecular mechanics calculations, has been designed for bidentate ligands and describes the preferred angle created by two phosphorus atoms and a “dummy” metal atom. In general, one can differentiate between a steric and an electronic bite angle effect.
Ligands designed for industrial applications
The price and the long-term stability of the phosphorus ligand are pivotal issues for calculating the overall costs of an industrial process. In academic labs, literally thousands of ligands have been prepared mainly to explain structure–activity/selectivity relationships. Only very few of them have any chance of finding broader application. Short and high yield synthetic routes based on cheap starting materials are ideal. The modular preparation of related structures can be a further advantage for final optimization in terms of activity, selectivity, and stability. As is usual in such systems, small changes in the ligand structure may cause large effects.
Historically, in the first ligand-modified hydroformylation process with Co, and subsequently with Rh, PPh3 was usually used as an accessible, inexpensive, and quite air-stable ligand. The use of rather volatile trialkyl phosphines (PEt3, PBu3) has also been reported in the literature [20]. Owing to their higher σ-donor properties, trialkylphosphines coordinate stronger to the metal in comparison to triarylphosphines, a property which is particularly useful for the stability of relevant cobalt complexes under hydroformylation conditions [21]. Stanley reported only recently on the use of monoaryl phosphines [22]; however, the nature of the active catalysts and the benefit for hydroformylation have yet to be verified. Rhodium alkylphosphine catalysts frequently display a high hydrogenation activity which may increase the concentration of alkanes or alcohols in the product.
Shell was the first company to utilize trialkylphosphines for optimization of Co catalysts. Regioisomeric phosphabicyclononanes (“phobanes”) are applied as monophosphines (Fig. 12) [23]. Sasol claimed similar bicyclic monophosphines (LIM-ligands) [24].
Surprisingly, phosphine oxides can also dramatically improve the preactivation of classic cobalt precatalysts, as reported recently by Gusevskaya and Beller [25].
Organophosphites are less sensitive towards oxidation than phosphines are. As a result of the P–O bonds, organophosphites are weak σ-donors, but strong π-acceptors. This property facilitates the dissociation of CO from the metal center. The replacement of phosphines by phosphites therefore contributes to a substantial increase in the reaction rate in Rh-catalyzed hydroformylation. The n-regiodirecting properties of the catalyst can be enhanced by incorporation of sterically demanding substituents in the organic backbone. Bulky alkyl groups near the P–O bond fulfill this requirement and also contribute to the hydrolysis stability of the ligand. These properties were combined for the first time by van Leeuwen in the structure of phosphite Alkanox bearing a tert-butyl group in the ortho-position of the three aromatic rings (Fig. 13) [26].
Typically, such phosphites are produced by condensation of PCl3 with substituted phenols in the presence of a base. Some of these phenols and phosphites are available in a wide variety and on a large scale as they are used as antioxidants for the stabilization of polymers. The first series of phosphites as ligands for hydroformylation were claimed by Shell [27] and Union Carbide Chemicals & Plastics (since 2001 Dow Chemical) [28] and are still used industrially and in academia.
Sterically hindered, chelating P ligands predominantly induce the formation of n-aldehydes. Owing to their short and easy synthesis, diphosphites have been the first choice when the aim is n-regioselective hydroformylation of large quantities of olefins. Out of several chelating and non-chelating polyphosphites, diphenylphosphites of the Biphephos type became the prototype of all subsequently developed phosphite ligands [29]. Hybrid phosphite acylphosphite ligands were developed by Börner’s group in cooperation with Evonik in order to create a nonsymmetric electronic and steric environment for the reaction [30]. In general, these phosphorous acid triesters can be prepared in one or two steps starting from substituted biphenols.
The BASF group of Röper and Paciello correlated product linearity in the hydroformylation of 1-octene with structural data of closely related diphosphite ligands (Fig. 14) [31]. A key finding was that ligands which stabilize a P–Rh–P angle of 120° lead to superior n-regioselectivity as realized perfectly with ligand a. Linearity is predominantly determined by steric interactions in such closely related catalysts.
Replacement of phenol substituents by pyrrole units produces corresponding phosphoramidites or triaminophosphines. Trzeciak and Ziółkowski showed that phosphoramidites are stronger π-acceptor ligands in comparison to phosphites [32]. The order of π-acceptor and σ-donor properties is shown in Fig. 15.
In general, phosphoramidites/triaminophosphines can be easily prepared by a simple reaction of PCl3 in the presence of amine bases. In contrast the formation of P–C bonds is frequently based on multistep sequences and requires the use of metal organic reagents as well as the application of low temperature. Therefore, safety issues and high costs may hamper the production of these ligands on a large scale. Exceptions can be justified either by extremely high activities of the corresponding catalysts or their use for the manufacture of expensive aldehydes.
Xantphos is the generic name of a class of diphosphine ligands consisting of numerous individuals developed by van Leeuwen and Kamer (Fig. 16) [33]. These ligands are of particular value for the exploration of structure–activity/selectivity relationships and for the manufacture of expensive aldehydes. Several of them are commercially available even on a larger scale. By varying the heterocycle, different P–Rh–P bite angles can be adjusted in the final Rh catalyst.
A crucial point in the workup of a hydroformylation reaction is the separation of product and catalyst. In best case scenarios, the latter can be recycled and used for subsequent runs. A breakthrough was the use of the sulfonated phosphine ligand TPPTS [trisodium salt of 3,3′,3″-phosphinidynetris(benzenesulfonic acid)] [34] (Fig. 17) in the Ruhrchemie/Rhône-Poulenc (now OXEA) process for the hydroformylation of propene, which is the only aqueous two-phase hydroformylation used in industry to date. TPPTS exhibits excellent solubility in water (about 1.1 kg/l) and is in general insoluble in most organic solvents used for two-phase catalytic reactions. Bidentate sulfonated diphosphine ligand like BISBIS and BINAS were developed by Herrmann together with the former Hoechst company, but their industrial use is still not clear [35].
Several chiral ligands were also prepared and investigated for use in the asymmetric version of the hydroformylation (Fig. 18). However, elaborate multistep syntheses frequently prevent application on industrial scale. One of the first ligands, (R,S)-BINAPHOS, with some potential for the manufacture of chiral fine chemicals was developed by Takaya and Nozaki almost 30 years ago [36].
The stereopreference of the asymmetric hydroformylation with BINAPHOS is governed mainly by the absolute configuration of the binaphthyl moiety bearing the diphenylphosphine group [37]. Moreover, the relative configuration of the phosphite unit plays a crucial role in the degree of enantioselectivity. Usually (R,R)-BINAPHOS induces inferior ee values. (S,S,S)-Bisdiazophos, developed by researchers of the Dow Chemical Company, is one of the most frequently tested chiral ligands in asymmetric hydroformylation [38]. Kelliphite with a biphenol backbone was used to produce some fine chemicals on a multi-ton scale [39]. A ligand called (Sax,S,S)-bobphos (“the best of both phosphorus ligands”) gives particularly promising results in the asymmetric hydroformylation of alkyl alkenes in favor of the formation of branched aldehydes (e.g., 1-hexene: b:l = 3:1, 93% ee) [40].
Classification of mature industrial processes
Cornils differentiated industrial hydroformylation processes by the technology that was used [41]. The first-generation processes followed the original procedure of Roelen or used similar conditions with cobalt-based catalysts. They operated under quite smooth conditions, but production did not exceed 10 kt/year.
The second generation benefitted from an unmodified Co catalyst under much more severe conditions. Differences originated from varying methods of cobalt recovery from the product (“decobalting”) [42], which were implemented either by redox processes or by transformation of [HCo(CO)4] into water-soluble salts, followed by extraction. The feedstock basis changed from higher olefins in the first plants to mainly propene. On the basis of these processes, large units manufactured aldehydes up to 300 kt/year. This allowed world-wide production of dialkyl phthalates, the most important outlet for aldehydes, to increase to several hundreds of kt/year. A crucial problem of unmodified Co catalysts is the relatively low l/b-butyraldehyde ratio, which led to a fast change to rhodium catalysts for propylene hydroformylation when they became available. This disadvantage could be overcome in some cases through modification with monodentate phosphine ligands [43]. However, as a result of the coordination of organic phosphorus ligands, a lower level of reactivity and the formation of alcohols and alkanes became a critical issue, which could be accepted when alcohols specifically are needed. The new processes could be conducted under much less syngas pressure and are still used today. The reactions are usually carried out in a temperature range of 120–190 °C and a syngas pressure of 40–300 bar in large industrial companies, such as BASF, Exxon, Sasol, and Shell. The production of high boiling aldehydes or alcohols from long-chain and branched olefins still remains a domain of cobalt catalysis.
The third generation started in the 1970s and involves processes operating with P-ligand-modified Rh catalysts at low syngas pressure (18–60 bar) and medium temperatures (85–130 °C). These “low-pressure oxo processes” (LPO) are still state of the art and are carried out at, e.g., Dow Chemical, BASF, and Mitsubishi. Preferentially, short unfunctionalized olefins are used as substrates. About 70% of the total hydroformylation capacity, i.e., the transformation of ethylene, propene, and butenes, is based on LPOs. In general, a high excess of P ligand is required to stabilize the Rh complex and to prevent formation of the less-desired branched aldehydes. In some recent approaches, monodentate phosphines have been replaced by bidentate diphosphites.
One of the main differences between cobalt- and rhodium-catalyzed hydroformylations is the technology used to separate the product and the catalyst with the aim of metal reuse. Because of cobalt’s relatively low price compared to rhodium, less efficient recycling methods can be used. However, deposition products of Co clusters and metallic cobalt cause fouling on reactor surfaces and serious blockage of valves that can result in a shutdown of the plant. Moreover, the formation of Co carbides (CoxC, x = 2, 3), which are responsible for the formation of methane from syngas in dry spots of the reactor, must be prevented.
Wiese estimated an annual financial loss of several million euros when just 1 ppm Rh/kg product is lost in a 400-kt plant [44]. Efficient catalyst recycling is, therefore, indispensable. It may be achieved by stripping off the low-boiling product with an excess of syngas (“gas recycling”). The technology is limited to the hydroformylation of alkenes up to pentene. An alternative separation process is based on the distillative removal of the products (“liquid recycling”). The catalyst remains in the residue, consisting of high boiling condensation products, and is used for the next run. This technology can also be used in the workup procedure in the hydroformylation of alkenes with chain lengths greater than C6. The lifetime of a catalyst charge may exceed 1 year if sufficient purity of the feed and careful process control are guaranteed.
An example of aqueous two-phase hydroformylation went onstream at Ruhrchemie AG in 1984 (fourth generation) with an annual capacity of 100 kt/year. The current capacity is 500 kt/year. The Rh catalyst is immobilized in the aqueous phase. A sulfonated phosphine ligand (TPPTS) induces the high catalyst solubility in water. The catalyst is removed into the aqueous phase before distillation of the product, which avoids thermal stress. The rhodium losses are in the range of parts per billion.
The catalytic reaction
Preparation of precatalysts
Catalysts for hydroformylation may contain ancillary organic ligands or not. In all cases, a corresponding more stable precatalyst is added to the reaction, which is converted under syngas into the catalytically active species. Modification of the metal with organic ligands can be accomplished in a separate vessel before the hydroformylation or in situ. Unmodified Co catalysts can be generated from Co2(CO)8, an air-sensitive solid. The compound is a strong irritant and harmful to eyes, skin, and mucous membranes. Alternatively, cobalt octanoate is used as well. As precursors for rhodium catalysts RhCl3·(H2O)x, Rh(OAc)3, Rh(II) carboxylates, Rh(acac)(CO)2 (acac = acetylacetonate), Rh(acac)(COD) (COD = 1,5-cyclooctadiene), and Rh4(CO)12 are used.
Modification of Co catalysts with phosphorus ligands can be realized by mixing Co2(CO)8 with an excess of the ligand to produce a salt, which is rapidly converted at higher temperatures into the dimeric species Co2(CO)6P2 (Fig. 19). The corresponding precatalyst is formed in the presence of H2 or syngas.
In addition, the commercially available hydrido complex HRh(CO)(PPh3)3 can be used directly as a precatalyst; however, addition of a bidentate ligand is occasionally needed for stabilization. Today, Rh(acac)(CO)2, which is reacted with the P ligand under syngas, is mainly used for synthetic purposes (Fig. 20). The precatalysts formed, such as [HRh(CO)3P] (P = bulky monophosphite), usually cannot be isolated easily and are only observable by spectroscopic methods, as exemplarily shown by Kubis and Selent [45]. The progress of the formation of the P-ligand-modified precatalyst can be investigated by means of UV–vis spectroscopy or in the case of chiral ligands with UV–vis CD spectroscopy [46]. Different high pressure NMR techniques coupled with IR provide valuable structural information about the precatalyst formed.
It is highly recommended that hydroformylation batches should be set up under an inert atmosphere. Otherwise, precursors may introduce oxygen and moisture in an undesired way. In the literature, a startup routine can occasionally be found where the precatalyst, ligand, and even olefin are mixed under air. The mixture is transferred into the autoclave and then flushed with syngas. This straightforward approach will lead to contamination with olefin hydroperoxides. Furthermore, the transformation of the precursor to the catalyst takes place in parallel to hydroformylation, and thus few reliable results on catalyst activity and selectivity are obtained.
General reaction conditions
The conversion of olefin, the chemoselectivity, and the regioselectivity towards the formation of the desired aldehyde are strongly dependent on the reaction conditions. In the literature numerous and varying setups have been described. Only some typical tendencies are, therefore, discussed here. In general, the n-selectivity decreases with increasing pressure. A high syngas pressure favors the hydroformylation over the isomerization of the starting olefin. The ratio of the partial pressures of CO/H2 also affects the result of hydroformylation. Hydroformylation occurs with equal partial pressures of CO and H2. A slightly higher H2 pressure assists in the formation of the active precursors RhH(CO)2P2, thereby improving the rate of the reaction. Best results were obtained with a CO/H2 ratio of 1:2. It should be noted, however, that an excess of hydrogen may lead to the hydrogenation of olefin and product aldehyde as undesired side reactions.
As shown by Breit, hydroformylation on a laboratory scale can be even performed in a Schlenk tube equipped with a cross-type magnetic stirring bar at room temperature and 1 bar syngas pressure (RTAP = room temperature/ambient pressure), provided a particularly active catalyst is used (Fig. 21) [47].
Hydroformylation can be accelerated by the effect of microwaves [48]. At low syngas pressure (ca. 3.7 bar) and a temperature of 110 °C, the reaction with a Rh-Xantphos catalyst was finished within a few minutes. Several terminal olefins were successfully converted under these conditions.
Decomposition of ligands and catalysts and measures to combat this
Catalysts may lose their catalytic properties because of degradation over the reaction time. This applies to batch reactions and is even more pronounced in continuous processes. As a result, a group of more or less active and selective catalytic species is responsible for the overall result. Moreover, the properties of the product change over time. Only in recent years has this feature, which is a crucial point in technical hydroformylation, been recognized to some extent in academia; before this, it was exclusively a domain of industrial research [49].
Degradation of ligands
Information about the degree of degradation of ligand and catalyst is essential for maintaining the quality and quantity of the product aldehydes over the whole reaction time [50]. This holds especially for continuous processes. Degradation products containing phosphorus can be analyzed by means of 31P NMR spectroscopy. Unfortunately, their concentrations in technical hydroformylation solutions are very small and may be below the detection limit. Moreover, NMR measurements are rather time-consuming and may be affected by problems of mass transfer of reactive gases from the head space of the NMR tube into the solution, although a technical arrangement which avoids these problems is now commercially available [51]. The method of choice is in situ FTIR spectroscopy with different techniques (Fig. 22) [52].
This spectroscopic technique can be directly linked to the reactor and immediately provides continuous information about nearly all components of the reaction mixture, which may be present in very low concentrations. The actual status of the catalytically active metal can be investigated on the basis of characteristic CO bands. For an accurate analysis of single IR bands of a single component in mixtures of compounds, programs for spectra deconvolution are required. High quality algorithms for this purpose were developed by the groups of Garland (BTEM, band-target entropy minimization) [53] and Neymeyr (PCD, pure component spectral recovery) [54].
Basically, every ligand must compete with CO for coordination sites on the metal center. As a result, metal species are formed which differ in the number of coordinated CO and P ligands. The shift of the equilibria depends on CO pressure, temperature, excess and coordination properties of the P ligand. The last of these are determined by electronic and steric properties. The situation is shown in Fig. 23 with the example of a typical Rh catalyst. It should be borne in mind that only [RhH(CO)3P] (I, P = monodentate ligand) and [RhH(CO)2P2] (II, P2 = bidentate ligand) represent the desired precatalysts, whereas increasing displacement of the organic ligand by CO produces unmodified rhodium with its typically poor hydroformylation performance (low activity and regioselectivity). Moreover, displacing the P ligand and lowering the CO pressure leads to the formation of catalytically less active Rh clusters (“dead ends”). With an increasing ratio of Rh/CO, the metal might eventually “plate out”. If bidentate P,P ligands are used, an increasing CO pressure and higher temperatures favor the monodentate coordination mode, which reduces the regioselectivity.
An optimal P/Rh ratio results in relation to these features for the catalytic properties of each catalytic system. In general, an excess of P ligand in relation to the metal (usually 2:1–200:1) is used. With an increase in the P/Rh ratio the rate of the reaction is affected because of the formation of RhHP4 complexes, which also causes a “dead end” in the reaction. In addition, the P/Rh ratio may influence the regioselectivity [55].
It is clear that the subtle equilibria described above are dramatically affected by the degradation of P ligand. In general, the main enemies of the catalyst are oxygen, carbon dioxide, water, enones, alkynes, and butadienes in the feed. Chemical and technology-based measures have been developed to remove these poisons prior to or during the reaction. In addition, product aldehydes, alcohols, and acidic degradation products of phosphorus ligands may interfere with the original ligand.
The situation becomes even more complicated since the catalytic metal itself is able to stabilize the ligand or to accelerate its deterioration. The mechanism and products of the degradation are dependent on the structure of the trivalent phosphorus compound and can therefore vary even within one class of ligands. Degradation products of ligands do not necessarily lose their catalytic properties. However, the catalytic performance is altered in comparison to the original system.
The most prominent degradation reaction of phosphines is their reaction with oxygen or alkylhydroperoxides. The major products are the corresponding phosphine oxides. Phosphinate esters, phosphonates, and phosphates are formed in lesser amounts. Phosphine oxides are not entirely inactive as ligands. For instance, triphenylphosphine oxide (TPPO) has a slightly coordinating property and may therefore also contribute to the rhodium-catalyzed hydroformylation [56].
Phosphorus ligands with P–O bonds are less prone to oxidation but react easily with water to produce the corresponding pentavalent species. Water is continuously formed in the continuous reactor by aldol reaction of product aldehydes under hydroformylation conditions. After hydrolysis of the P–O bond and formation of the pentavalent phosphorus compound, the ligating properties are not completely lost. Secondary phosphine oxides (SPOs) or heteroatom-substituted secondary phosphine oxides (HASPOs) form an equilibrium consisting of a pentavalent and a trivalent species (Fig. 24). The latter is capable of binding metals. As a result of hydrolysis and subsequent equilibrium, new ligands are formed in the catalytic system. SPOs and HASPOs can therefore be considered as “preligands” [57] and show typical properties in rhodium-catalyzed hydroformylation [58]. Consequently, the results expected with the original ligand are adulterated.
Degradation of rhodium complexes
Apart from the degradation of the non-coordinated ligand, its ligation to the metal may lead to additional deterioration. For example, in the continuous hydroformylation of propene with PPh3, in addition to benzene and benzaldehyde, a considerable amount of n-propyldiphenylphosphine has been detected as a ligand, which arises from a Rh-mediated replacement of one P-aryl by an alkyl group [59]. The conversion is dependent on the partial pressure of propene. A similar decomposition process was observed with TPPTS [60]. Since the alkyldiarylphosphine formed has a higher basicity, it binds more strongly to the metal and, therefore, the catalytic property of the original catalyst is modified (hydrogenation!).
Measures against degradation
Different measures to counteract deterioration of ligands are suggested, mainly in the patent literature. Observations made with phosphites in the stabilization of polymeric materials, which are widely used as antioxidants, provide additional information. Two general methodologies of increasing the long-term stability of ligands and catalysts can be differentiated:
-
1.
Appropriate steric and electronic design
-
2.
External stabilization in the presence of additives
Phosphines with an alkyl group are more prone to oxidation than their aryl counterparts, which should be taken into consideration in the ligand design. The rate of hydrolysis of tri-n-alkyl phosphites is dependent on the length and branching of the alkyl chain. In polymer chemistry, where phosphites are used as flame retardants and antioxidants, a typical “rule of thumb” is that the cost of high-performance phosphites is directly proportional to their hydrolytic stability. In other words, efforts for their synthesis can be correlated with their resistance towards water and, ultimately, a compromise has to be found for commercial uses.
Several patents claim enhancement of the hydrolytic stability of phosphites by inorganic bases or amines. It should be noted that many amines also catalyze the aldol condensation of product aldehydes, which leads to the formation of high-boiling by-products. Therefore, organic phosphonites and phosphites containing sterically hindered amines (hindered amine light stabilizers, HALS) were developed [61]. In the reaction with water, the amine unit neutralizes the formed acid to form a betaine (Fig. 25). This structure is extremely stable against hydrolysis. Neither H3PO3 nor water at 70 °C for 90 h causes hydrolysis, which was ascribed to a decrease in the enhanced electron density at the phosphorus atom.
Substrates and reactions
General remarks
Non-functionalized olefins of different chain lengths are mainly employed in bulk chemical processes, but functionalized substrates are also interesting targets. Principally terminal C=C bonds react faster than internal olefins. The rate of the hydroformylation falls with increasing steric hindrance of the substrate [62]. The reactivity order in Fig. 26 was found independent of the metal (Rh, Co) used by the Botteghi group [63].
The reaction of branched olefins requires more severe reaction conditions or alternatively a more active catalyst. Principally, the hydroformylation of trifold substituted sp2-configured C atoms is unfavorable (Keulemans’ rule) [64]. Meanwhile, there are exceptions to this rule, but they are mostly restricted to olefins with neighboring activating groups, e.g., hydroxyl or ester groups which allow the chelation of the substrate to the metal center (“substrate-directed hydroformylation”) [65]. Moreover, functional groups may alter the “normal” regioselectivity of the hydroformylation of the parent olefin. A typical example is styrene, where the branched aldehyde is usually formed.
Prior isomerization of the double bond may change the structure of the original substrate. In the presence of a small amount of a Rh diphosphite catalyst under syngas 1-octene is already immediately converted into cis- and trans-2-octene before hydroformylation begins [66]. The isomerization is a reversible reaction and dependent on the temperature.
In most industrial bulk processes, single olefins are not available at an economically attractive price. Therefore, mixtures of isomers are used as feedstock. In mixtures of acyclic olefins, internal and branched compounds are also present in addition to terminal compounds. Since the production of terminal aldehydes is desired in many cases, hydroformylation with prior isomerization is targeted. In the optimal case, this tandem reaction is achieved by a single Rh catalyst based on sterically demanding bidentate ligands. High branched selectivity is predominantly achieved using an internal olefin together with a catalyst of low isomerization activity.
Important unfunctionalized acyclic alkenes used in industry are, in particular, ethylene (C2) and propene (C3), isomeric butenes (C4), octenes (C8), and olefins up to a chain length of C18. In general, a distinction is made between short-chain (C3–C4), medium-chain (C5–C12), and long-chain (C13–C19) oxo products. Some linear α-olefins (LAOs), such as 1-butene, 1-hexene, 1-octene, or 1-decene, can be extracted selectively from Fischer–Tropsch processes. As exemplarily conducted in Sasol’s SYNTHOL® process, a range of olefins with a broad distribution of odd and even carbon numbers can be obtained. Using low-cost ethylene, dimerization may lead to 1-butene. Trimerization or tetramerization affords 1-hexene or and 1-octene. Further oligomerization yields higher even numbered α-olefins.
From a quantitative point of view, the most important oxo chemical represents n-butyraldehyde with a worldwide annual consumption of more than 50% of all aldehydes by weight, based on the total weight of all oxo aldehydes consumed. In comparison, the world production of iso-butyraldehyde is just 15%. Main producers are, for example, BASF, Dow Chemical, Celanese, and Eastman.
Hydroformylation of selected olefins
n-Butyraldehyde is used for the manufacture of n-butanol and n-butyric acid (Fig. 27). The main part is subjected to aldol condensation to produce the corresponding unsaturated C8-aldehyde. The latter is hydrogenated on a heterogeneous catalyst to produce 2-ethylhexanol (2-EH), which is used to manufacture bis(2-ethylhexyl)phthalate (DEHP), the standard plasticizer used in today’s polyvinyl chloride (PVC) industry. Alternatively, esterification with adipic acid produces bis(2-ethylhexyl)adipate (DEHA), another plasticizer ester, which has also been used as a hydraulic fluid and as a component of aircraft lubricants.
Butenes are usually derived from Crack-C4 from naphtha steam cracking [67]. After the removal of butadiene and isobutene from the crude stream, the so-called Raffinate II contains 1-butene, cis/trans-2-butene, and the isomeric butanes. The non-selective hydroformylation of linear butenes produces n- and iso-valeraldehyde; the former is predominantly required for further transformations (Fig. 28).
Oxeno (now Evonik Industries) patented a process for the n-regioselective hydroformylation of Raffinate II, which can be operated continuously [68]. Figure 29 shows the general process. The products are isolated by condensation and distillation. The reactor is linked to a filter where insoluble decomposition products of the phosphorus ligand are removed at a lower pressure and temperature.
The main application for n-valeraldehyde is the transformation into 2-propylheptanol (2-PH) by aldol condensation and subsequent hydrogenation of the product. Like 2-EH, 2-PH is also an important plasticizer alcohol.
1-Heptene is used as a substrate in the first step of Sasol’s process (using likely Dow/UCC technology) for its homologation to 1-octene [69]. The hydroformylation is presumably carried out either with a rhodium catalyst based on PPh3 or with a diphosphite as a ligand, which would eventually produce n-regioselectivities of 85–92%. 1-Octene is formed by hydrogenation of the aldehyde followed by thermally induced elimination of water.
The hydroformylation of mixtures of C8-olefins is a task with large economic importance. A typical example is “di-n-butene”, consisting of isomeric n-octenes, methylheptenes, and dimethylhexenes. This mixture is produced from Raffinate II, where isomeric butenes are dimerized (e.g., by the IFP Dimersol® or Octol® process). Hydroformylation of di-n-butene produces linear and alkyl-branched C9-aldehydes, which are hydrogenated and converted to diisononyl phthalate (DINP), another additive for flexible PVC with industrial relevance. For this application, the use of less branched aldehydes is preferred.
Hydroformylation of 1-decene for the production of 1-undecanal is an important process in perfume manufacturing (Fig. 30).
1-Undecanal can be converted by aldol condensation with formaldehyde and subsequent hydrogenation into 2-methylundecanal (methylnonyl acetaldehyde, MNA) [70]. MNA is a natural product found in kumquat peel oil. It is a sought-after principal ingredient in perfumery because of its strong fragrance of oranges and incense. Its current market to produce flavors and fragrances is estimated to be 500–600 t/year. Important producers or/and suppliers are Symrise, Givaudan, Firmenich, and Kao Corporation. Alternatively, after hydrogenation and elimination, 1-undecene can be derived from 1-decene via hydroformylation (homologation).
Because of serious decomposition problems with Rh catalysts during the separation of high-boiling products, most commercial plants for long chain aldehydes (> C10) operate with Co catalysts. These approaches are based on unmodified catalysts under rather severe conditions (300 bar, 200 °C). Besides alcohols, alkanes are also formed because of the high hydrogenation activity of Co complexes. Through modification of the Co catalyst with phosphines the pressure can be lowered (< 100 bar) and selectivity towards the formation of the linear alcohols is enhanced as a result. A suitable feedstock of higher olefins (up to C20) can be derived from Fischer–Tropsch feed (Sasol) or is produced by SHOP (Shell Higher Olefin Process). Products are commonly used for the production of surfactant alcohols.
Hydroformylation of conjugated dienes is much slower compared to that of mono-olefins. There are only a few reports relating to the conversion of butadiene to adipaldehyde [71], although this compound is a high-value intermediate for producing ε-caprolactam and adipic acid/hexamethylene-1,6-diamine (HMDA) (Fig. 31). Both are key monomers in nylon-6.6 and nylon-6 manufacture. Alternatively, hexane-1,6-diol can be derived by hydrogenation, which is a valuable monomer in the synthesis of polyesters.
The reaction with conjugated dienes does not necessarily produce the desired dialdehydes. Thus, the rhodium-mediated reaction with 1,3-butadiene may produce mainly n-valeraldehyde with high regioselectivity (Fig. 32). The reason for this preference is the intermediate formation of the corresponding α,β-unsaturated aldehyde [72].
Hydroformylation of an endo/exo-mixture of dicyclopentadiene, which is an important component of the C5-fraction of the steam cracker, produces more than 90% tricyclodecanedialdehyde (TCD-dialdehyde) within a few hours with unmodified Rh at high pressure [73]. The product is used as a starting material for the manufacture of TCD-diol and TCD-diamine (Fig. 33). The former is widely used as a diol component for the production of glass fiber reinforced plastic and solvent-free fast-drying lacquers [74].
Meanwhile considerable progress has also been documented in the hydroformylation of alkynes [75] and allenes [76].
Hydroformylation of functionalized olefins provides access to aldehydes with one or more additional functional groups. Such functionalized aldehydes can be sold as final products or used as intermediates in the synthesis of fine chemicals, pharmaceuticals, and fragrances.
The transformation may be significantly influenced by the functional group/heteroatom (“substrate-directed hydroformylation”) [77] and show distinct differences compared to the reaction with unfunctionalized alkenes. These differences are ascribed to the intermediate formation of stable metallacycles affecting the rate and the regioselectivity of the reaction. In order to cleave the metallacycle, the use of high pressure or a reaction in aqueous biphasic systems is frequently required. Under these reaction conditions vinyl groups conjugated with a carbonyl moiety can be hydrogenated. Meanwhile also remote supramolecular control was advocated as a tool for achieving high regioselecitivity [78].
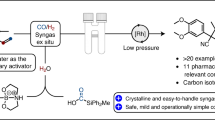
A group at Chirotech Technology showed that when crotonaldehyde is protected prior to the hydroformylation as cyclic acetal, it can be converted with a Rh-Biphephos catalyst into glutaraldehyde monoethylene acetal with l/b-selectivity of approx. 15:1 (Fig. 34) [79]. The reaction was used for the large-scale synthesis of allysine ethylene acetal, which is a key intermediate in the manufacture of angiotensin-I-converting enzyme (ACE) and neutral endopeptic (NEP) inhibitors, such as ilepatril and omapatrilat.
Besides 1-octene, styrene is the most frequently used standard substrate for testing new ligands, catalysts, and additives and is widely used for mechanistic studies. Usually, vinyl aromatics and vinyl heteroaromatics direct the hydroformylation towards the formation of the branched aldehydes. This result has been rationalized by the formation of a stabilizing η3-allyl intermediate [80]. p-Substituents on the phenyl ring can alter the α-selectivity, which increases in the following order [81]:
Amgen claimed an approach for the total synthesis of cinacalcet (Sensipar®, Mimpara®) prescribed as a calcimimetic (Fig. 35) [82]. The synthesis commences with the hydroformylation of m-trifluormethyl styrene. The formed aldehyde is converted by reductive amination into the pharmaceutically active compound. Hydroformylation and reductive amination can even be carried out advantageously in a single step without isolation of intermediate aldehyde and imine.
The hydroformylation of isomeric butenyl-diacetates with unmodified Rh catalysts is a main step in the manufacture of vitamin A acetate, originally developed by BASF (Fig. 36) [83]. Hoffmann-La Roche suggested a similar procedure but based on the hydroformylation of the isomeric 1,4-diacetoxybut-2-ene [84]. Several thousand tons of vitamin A are consumed annually [85].
Functionalized allyl amides were hydroformylated with a Rh complex containing monophosphite Alkanox or the diphosphine Xantphos as a ligand (Fig. 37) [86].
The reaction proceeds with excellent n-regioselectivity and provides intermediates for the manufacture of β-lactams frequently prescribed as antibiotics. Subsequent incorporation of an enamido moiety produces compounds with potential anticancer activity.
The highly iso-regioselective hydroformylation of allyl arenes is of considerable interest [87], since the reactions with the monoterpenes eugenol, safrole, estragol, and their double bond isomers produce aldehydes such as Helional® with many applications in the flavor, perfume, and pharmaceutical industries (Fig. 38).
The use of unsaturated fatty compounds is an interesting alternative for a non-petroleum-based feedstock. Their hydroformylation was first investigated in the late 1960s and early 1970s. Thereafter, both university and industrial research became more interested in olefins derived from petrochemical sources. However, a real renaissance of the hydroformylation of these substrates has been observed for the last decade mainly driven by the group of Behr [88]. The global market for fats and oils from renewable resources amounts to approx. 130 million tons, with soybean, palm, rapeseed, peanut, linseed, and sunflower oil being the most important [89]. These unsaturated compounds provide an interesting and environmentally friendly alternative to the use of alkenes derived from mineral oil. However, while the petrochemical alkenes are mainly short and sometimes branched hydrocarbons without substituents, unsaturated fatty acid compounds are linear and predominantly have a chain length of C18 or longer. They contain a carboxylic or ester group (esters of glycerol or methanol) and often more than one double bond (e.g., ω3- and ω6-fatty acids). Ricinolic acid also contains a hydroxyl function. These functional groups may interact with transition metal catalysts, causing their deactivation. Moreover, multiple double bonds are frequently hydrogenated and/or isomerization takes place prior to the reaction with syngas. Therefore, the experimental knowledge accumulated in the hydroformylation of unfunctionalized petrochemicals cannot simply be transferred to the transformation of fatty compounds.
The aldehydes produced can be incorporated, for instance, after hydrogenation of ester and/or formyl groups as an alcohol component in plasticizers for PVC or in novel polyurethanes. The properties of the polyurethanes are dependent on the metal used for the hydroformylation [90]. At high conversion rates with a rhodium catalyst a rigid polyurethane is formed, whereas under the conditions of Co catalysis and low conversion a hard rubber with lower mechanical strength is produced.
In the past, methyl oleate has frequently been used as a model substrate, but in some cases linoleates and linolenates have also been added to the reaction. Technical feedstock usually contains a mixture of unsaturated and saturated fatty acids as shown in Fig. 39.
The use of a homogeneous rhodium catalyst with a bulky monophosphite allows the conversion of methyl oleate in a yield of 85–90% at a syngas pressure of 20–80 bar and 80–100 °C [91]. Mainly 9- and 10-formyl octadecanoic acid methyl esters were produced. The use of a technical feedstock decreased conversion and yield. Detailed investigations showed that cis-double bonds isomerize rapidly to trans-configurated olefins; the latter undergo hydroformylation only slowly.
A particular challenge with rhodium is the isomerizing hydroformylation of fatty acid esters to form ω-formyl esters. Behr reacted methyl oleate with syngas in the presence of a Rh-Biphephos catalyst at relatively low syngas pressure (Fig. 40) [92]. The desired 18-formyl stearic acid methyl ester was produced with a yield of 26%. Some higher yields were observed with the methyl ester of linoleic acid.
Stereoselective hydroformylations
General remarks
iso-Regioselective hydroformylation of olefins, with the exception of ethylene and propene, produces chiral aldehydes. When the reaction is carried out with stereoface control, products with considerable potential for the production of chiral fine chemicals are formed. Stereoface differentiation can be achieved by stereodirecting chiral groups in the substrate or by using a chiral catalyst. In order to enhance the stereoselectivity, catalysts with chiral ligands are sometimes applied to chiral substrates in order to achieve a matched pair effect [93].
Syngas pressure as well as the ratio of the individual partial gas pressures may not only influence the rate and regioselectivity but also the stereoselectivity. The effects are strongly dependent on the particular catalytic system and general conclusions are hard to draw. An appropriate chiral ligand has to provide not only for high iso-selectivity but also for high enantioselectivity. In early studies chiral trivalent phosphorus ligands, which have been successfully applied in other asymmetric transformation, were tested. But in most cases, they did not meet the high expectations. Therefore, new structures were designed, frequently mimicking motifs of well-established ligands for non-asymmetric hydroformylation. Their preparation is always based on multistep syntheses, which explains their high prices on the market.
Usually, asymmetric hydroformylation (AHF) is associated with the iso-regioselective reaction of olefins. With the exception of ethylene and propene, chiral aldehydes are produced in this reaction (Fig. 41).
One would expect that this type of regioselectivity is supported by sterically less hindered ligands. However, an effective steric interaction between the substrate and catalyst is necessary to achieve high enantioselectivity, which also accounts for bulky ligands. It seems that high iso-regioselectivity may preclude high enantioselectivity and vice versa. This probably explains the minor progress in enantioselective hydroformylation as compared to other metal-catalyzed asymmetric reactions, such as hydrogenation. Only a small number of highly selective catalytic systems are known, and the range of substrates has been extended only over the last few years. The problem of high iso-regioselectivity is less pronounced in functionalized prochiral substrates, like vinyl arenes or vinyl ethers, which direct the reaction in favor of the branched product.
Currently, most efforts have focused on the asymmetric hydroformylation of vinyl arenes to get access to enantiomerically pure 2-aryl propionic acids (Fig. 42). Some of these compounds constitute a class of non-steroidal inflammatory drugs, such as (S)-naproxen, (S)-ibuprofen, (R)-flurbiprofen, and (S)-ketoprofen [94].
For this reason, asymmetric hydroformylation of styrene and related vinyl arenes derivatives has been addressed frequently in the literature. Styrene usually induces high iso-selectivity and is therefore particularly convenient for testing the stereoface-discriminating abilities of new chiral ligands. In spite of these more academic efforts, enantioselective synthesis of pharmaceutical aryl propionic acids by hydroformylation remains a challenge on an industrial scale. The high manufacturing costs of the vinyl substrates and the hitherto achieved low catalytic activities in the hydroformylation have to compete with other methods of preparation (mainly resolution of racemates).
Researchers from Dowpharma were able to convert allyl cyanide with (R,R)-Kelliphite with a ratio of b/l of 20:1 and 80% ee (Fig. 43) [95]. After optimization of the reaction conditions the hydroformylation was carried out on a 0.93-mmol scale of substrate in a 300-ml vessel.
Hydrogenation of the functional groups has been carried out in two steps by using two different heterogeneous catalysts. The chiral methyl-substituted 1,4-aminoalcohol is useful for the synthesis of Merck’s nonpeptide gonadotropin-releasing hormone antagonist or a novel tachykinin NK1 receptor antagonist developed by Ono Pharmaceuticals.
Alternatives to syngas
Syngas can be manufactured by partial oxidation technology or by steam reforming. In general, it can be derived from almost every carbon source as a side product of the water gas shift reaction [96]. In addition to low-boiling hydrocarbons, heavier oils, and by-products from various processes, including hydroformylation, have also been employed. Sometimes, other solid materials like biomass and waste plastics are used in addition to coal. Most large companies engaging in hydroformylation run their own facilities for the production of syngas. For small-scale hydroformylation, in-house production is not efficient. The price of CO or syngas can vary considerably on the market. Special safety conditions for the transportation of the highly toxic gases may also contribute to the current high price and can be decisive as regards the economic efficiency of a process.
For several years there has been increased interest in easier-to-handle alternatives for syngas [3]. Back in 1994, Alper successfully used formic acid as a hydrogen source together with CO gas for the hydroformylation of 1-decene in the presence of a heterogeneous Rh catalyst [97]. The use of an aqueous solution of formaldehyde (formalin) or paraformaldehyde seems even more promising. Principally the reaction could be run in an open vessel. However, additional hydrogen gas can be advantageous in increasing the reaction rate [98].
Morimoto showed that the use of two different Rh catalysts (Rh-BINAP, Rh-Xantphos) can be beneficial, one for the decomposition of formaldehyde and the second for the hydroformylation of olefins (Fig. 44) [99]. With this system, aldehydes were produced with a yield of 95% and excellent n-regioselectivity without using CO under pressure.
Alternatively, CO can be derived in situ from the reversed water–gas shift reaction (RWGS) (Fig. 45) [100]. For the generation of CO preferentially ruthenium catalysts are appropriate. As a result of the high hydrogenation activity, intermediate aldehydes are finally converted to alcohols [101].
Quite recently, Ding and Zhou advocated the use of formic acid as an alternative for syngas for the hydroformylation of alkynes [102].
There is no doubt that these and other syngas-free systems will be of particular value for the manufacture of fine chemicals, provided their efficiency can be improved in the near future. An intrinsic problem of the CO generation in situ which must be overcome is the low partial concentration of CO. As a consequence, this leads to low reaction rates of hydroformylation.
Conclusions and outlook
Hydroformylation is one of the most important homogeneously catalyzed reactions on an industrial scale. A clear dominance in the manufacture of bulk chemicals can be observed. Large cobalt- and rhodium-based processes are mature technologies that have been developed over the past 60 years. Currently, the search for more stable ligands and more active and regioselective catalytic systems is the focus of research. Interestingly, the potential of hydroformylation for the production of fine chemicals, especially for the manufacture of optically pure compounds, has not been fully explored to date. The reasons for this disparate behavior are the enormous price of the chiral ligands and the average activities and selectivities of corresponding catalysts. Moreover, safety issues in working with the toxic CO, relatively high investment costs, and the current high price of carbon monoxide hamper the broad use of this innovative technology for the production of fine chemicals. There is no doubt that mainly economic reasons will reopen the discussion about alternative syngas sources and the best metals in industrial hydroformylation in the near future.
References
Rasch M (2013) Otto Roelen: life and research. 75 years of oxo synthesis. Klartext Verlag, Essen, pp 95–158
Cornils B, Herrmann WA, Rasch M (1994) Otto Roelen als Wegbereiter der industriellen homogenen Katalyse. Angew Chem 106:2219–2238
Wu L, Jackstell R, Beller M (2014) Carbonylation of alkenes with CO surrogates. Angew Chem Int Ed 53:6310–6320
Roelen O (1938/1952) Verfahren zur Herstellung von sauerstoffhaltigen Verbindungen. DE 849548
Adkins H, Krsek GJ (1949) Hydroformylation of unsaturated compounds with a cobalt carbonyl catalyst. Am Chem Soc 71:3051–3055
Gusevskaya EV, Jiménez-Pinto J, Börner A (2014) Hydroformylation in the realm of scents. ChemCatChem 6:382–411
van Leeuwen PWNM, Claver C (2000) Rhodium catalyzed hydroformylation. Kluwer Academic, Dordrecht
Börner A, Franke R (2016) Hydroformylation. Fundamentals, processes and applications in organic synthesis. Wiley-VCH, Weinheim
Franke R, Selent D, Börner A (2012) Applied hydroformylation. Chem Rev 112:5675–5732
Pospech J, Fleischer I, Franke R, Buchholz S, Beller M (2013) Alternative metals for homogeneous catalyzed hydroformylation reactions. Angew Chem Int Ed 52:2852–2872
Heck RF, Breslow DS (1961) The reaction of cobalt hydrotetracarbonyl with olefins. J Am Chem Soc 83:4023–4027
Evans JA, Osborn JA, Wilkinson G (1968) Hydroformylation of alkenes by use of rhodium complex catalysts. J Chem Soc A. https://doi.org/10.1039/j19680003133
Moore EJ, Sullivan JM, Norton JR (1986) Kinetic and thermodynamic acidity of hydrido transition-metal complexes. 3. Thermodynamic acidity of common mononuclear carbonyl hydrides. J Am Chem Soc 108:2257–2263
Cornils B (1980) Hydroformylation. Oxo synthesis, Roelen reaction. In: Falbe J (ed) New syntheses with carbon monoxide, reactivity and structure, concepts in organic chemistry 11. Springer-Verlag, Berlin, pp 38–45
Kégl T (2015) Computational aspects of hydroformylation. RSC Adv 5:4304–4327
Richter, W, Schwirten, K, Stops P (1984) Verfahren zur kontinuierlichen Hydroformylierung olefinisch ungesättigter Verbindungen. EP 0114611
Matthey F (2001) Phosphorus-carbon heterocyclic chemistry: the rise of a new domain. Pergamon, Amsterdam
Tolman CA (1977) Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis. Chem Rev 77:313–348
Birkholz M-N, Freixa Z, van Leeuwen PWNM (2009) Bite angle effects of diphosphines in C-C and C–X bond forming cross coupling reactions. Chem Soc Rev 38:1099–1118
Slaugh LH, Mullineaux RD (1968) Novel hydroformylation catalysts. J Organomet Chem 13:469–477
Hebrard F, Kalck P (2009) Cobalt-catalyzed hydroformylation of alkenes: generation and recycling of the carbonyl species, and catalytic cycle. Chem Rev 109:4272–4282
Hood DM, Johnson RA, Carpenter AE, Younker JM, Vinyard DJ, Stanley GG (2020) Highly active cationic cobalt(II) hydroformylation catalysts. Science 367:542–548
Mason RF, van Winkle, JL (1968) Bicyclic heterocyclic sec- and tert-phosphines. US 3400163
Steynberg JP, Govender K, Steynberg PJ (2002) Bicyclic phosphine comprising hydroformylation catalyst and use thereof in production of oxygenated products. WO 2002014248
Delolo FG, Yang J, Neumann H, dos Santos EN, Gusevskaya EV, Beller M (2021) Cobalt-catalyzed hydroformylation under mild conditions in the presence of phosphine oxides. ACS Sustain Chem Eng 14:5148–5154
van Leeuwen PWNM, Roobeek CF (1983) Hydroformylation of less reactive olefins with modified rhodium catalysts. J Organomet Chem 258:343–350
van Leeuwen PWNM, Roobeek C (1982) Verfahren zur Hydroformylierung von Olefinen. EP 0054986
Abatjoglou AG, Bryant DR, Maher JM (1995) Stabilization of phosphite ligands in hydroformylation process. EP 0697391
Billig E, Abatjoglou AG, Bryant DR (1987) Bisphosphite compounds. EP 213639
Selent D, Hess D, Wiese KD, Röttger D, Kunze C, Börner A (2001) Rhodiumkatalysierte isomerisierende hydroformylierung interner olefine mit einer klasse neuartiger phosphorliganden. Angew Chem 113:1739–1741
Paciello R, Siggel L, Kneuper HJ, Walker N, Röper M (1999) Structure activity relationship for chelating phosphite ligands used in rhodium-catalyzed hydroformylations. J Mol Catal A Chem 143:85–97
Trzeciak AM, Glowiak T, Grzybek R, Ziółkowski JJ (1997) Novel rhodium complexes with N-pyrrolylphosphines: attractive precursors of hydroformylation catalysts. J Chem Soc Dalton Trans. https://doi.org/10.1039/a607961j
Kamer PCJ, van Leeuwen PWNM, Reek JNH (2001) Wide bite angle diphosphines: xantphos ligands in transition metal complexes and catalysis. Acc Chem Res 34:895–904
Gärtner L, Cornils B, Lappe P (1983) Process for the preparation of sulfonated aryl phosphines. EP 0107006
Herrmann WA, Kohlpaintner R, Manetsberger B, Bahrmann H, Kottmann H (1995) Water-soluble metal coplexes and catalysts. Part 7. New efficient water-soluble catalysts for two phase hydroformylation: BINAS-Na, a superlative in propene hydroformylation. J Mol Catal A Chem 97:65–72
Sakai N, Mano S, Nozaki K, Takaya H (1993) Highly enantioselective hydroformylation of olefins catalyzed by new phosphinephosphite-Rh(I) complexes. J Am Chem Soc 115:7033–7034
Nozaki K, Sakai N, Nanno T, Higashijima T, Mano S, Horiuchi T, Takaya H (1997) Highly enantioselective hydroformylation of olefins catalyzed by rhodium(I) complexes of new chiral phosphine-phosphite ligands. J Am Chem Soc 119:4413–4423
Klosin J, Landis CR (2007) Ligands for practical rhodium-catalzed asymmetric hydroformylation. Acc Chem Res 40:1251–1259
Clark TP, Landis CR, Freed SL, Klosin J, Abboud KA (2005) Highly active, regioselective, and enantioselective hydroformylation with Rh catalysts ligated by bis-3,4-diazophospholanes. J Am Chem Soc 127:5040–5042
Noonan GM, Fuentes JA, Cobley CJ, Clarke ML (2012) An asymmetric hydroformylation catalyst that deliver branched aldehydes from alkyl alkenes. Angew Chem Int Ed 51:2477–2480
Cornils B (1980) Hydroformylation. Oxo synthesis, Roelen reaction. In: Falbe J (ed) New syntheses with carbon monoxides. Springer, Berlin, pp 1–181
Blankertz HJ, Grenacher AV, Sauer F, Schwahn H, Schönmann W (1998) Verfahren zur Hydroformylierung. WO 98/12235.
Canell LG, Slaugh LH, Mullineaux RD (1965) Verfahren zur Herstellung von Aldehyden und/oder Alkoholen durch die Oxo-Synthese. DE 1186455
Wiese KD, Obst D (2008) Hydroformylation. In catalytic carbonylation reactions. In: Beller M (ed) Topics in organometallic chemistry. Springer, Heidelberg, pp 1–33
Kubis C, Ludwig R, Sawall M, Neymeyr K, Börner A, Wiese KD, Hess D, Franke R, Selent D (2010) A comparative in situ HP-FTIR spectroscopic study of bi- and monodentate phosphite modified hydroformylation. ChemCatChem 2:287–295
Cheng S, Gao F, Krummel KI, Garland M (2008) The application of BTEM to UV-vis and EUV-vis CD spectroscopies. The reaction of Rh4(CO)12 with chiral and achiral ligands. Talanta 74:1132–1140
Seiche W, Schuschkowski A, Breit B (2005) Bidentate ligands by self-assembly through hydrogen bonding: a general room temperature/ambient pressure regioselective hydroformylation of terminal alkenes. Adv Synth Catal 347:1488–1494
Petricci E, Mann A, Schoenfelder A, Rota A, Taddei M (2006) Microwaves make hydroformylation a rapid and easy process. Org Lett 8:3725–3727
van Leeuwen PWNM, Chadwick JC (2011) Homogeneous catalysts, activity-stability-deactivation. Wiley, Weinheim, pp 213–274
Zhang B, Jiao H, Michalik D, Kloß S, Deter L, Selent D, Spannenberg A, Franke R, Börner A (2016) Hydrolysis stability of bidentate phosphites utilized as modifying ligands in the Rh-catalyzed n-regioselective hydroformylation olefins. ACS Catal 6:7554–7565
Selent D, Baumann W, Börner A (2003) Gaseinleitungs- und zirkulationsvorrichtung zur Verfolgung von Reaktionen in flüssiger Phase unter Beteiligung gasförmiger Reaktanden unter Normal- und Hochdruck mittels Kernresonanzspektroskopie (Druck-NMR-Spektroskopie) unter stationären Bedingungen. DE 10333143
Allmendinger M, Zintl M, Eberhardt R, Luinstra GA, Molnar F, Rieger B (2004) Online ATR-IR investigations and mechanistic understanding of the carbonylation of epoxides—the selective synthesis of lactones or polyesters from epoxides and CO. J Organomet Chem 689:971–979
Garland M, Li C (2009) A review of BTEM analysis for catalytic studies and a recent homogenous catalytic example. Top Catal 52:1334–1341
Neymeyr K, Sawall M, Hess D (2010) Pure component spectral recovery and constrained matrix factorization. J Chemom 24:67–74
Bernas A, Mäki-Arvela P, Lehtonen J, Salmi T, Murzin DY (2008) Kinetic modeling of propene hydroformxlation with Rh/TPP and Rh/CHDPP catalysts. Ind Eng Chem Res 47:4317–4324
He D, Liu J, Liu Y, Wang T, Pang D, Chen Y, Liang Y, Zhu Q (2001) Effect of ammonium salts as additives in Rh-Ph3PO catalytic system on hydroformylation of mixed octenes. Chem Lett 73:221–224
Dubrovina NV, Börner A (2004) Enantioselective catalysis with chiral phosphine oxide preligands. Angew Chem Int Ed 43:5883–5886
Christiansen A, Li C, Garland M, Selent D, Ludwig R, Franke R, Börner A (2010) Secondary phosphane oxides as preligands in rhodium-catalyzed hydroformylation. ChemCatChem 2:1278–1285
Abatjoglou AG, Billig E, Bryant DR (1984) Mechanism of rhodium-promoted triphenylphosphine reaction in hydroformylation processes. Organometallics 3:923–926
Herrmann W, Kohlpaintner CW (1993) Water-soluble ligands, metal complexes, and catalysts: synergism of homogeneous and heterogeneous catalysis. Angew Chem Int Ed Engl 32:1524–1544
Bauer I, Körner S, Pawelke B, Al-Malaika S, Habicher WD (1998) Hydroperoxide dcomposing ability and hydrolytic stability of organic phosphites containing hindered amine moieties (HALS-phosphites). Polym Degrad Stab 62:175–186
van Rooy A, De Bruijn JNH, Roobeek KF, Kamer PCJ, van Leeuwen PWNM (1996) Rhodium-catalyzed hydroformylation of branched 1-alkenes; bulky phosphite vs. triphenylphosphine as modifying ligand. J Organomet Chem 507:69–73
Botteghi C, Ganzerla R, Lenarda M, Moretti G (1987) Advances in the hydroformylation of olefins containing functional groups. J Mol Catal 40:129–134
Keulemans AIM, Kwantes A, van Bavel T (1948) The structure of the formylation (OXO) products obtained from olefines and watergas. Recl Trav Chim Pays-Bas 67:298–3008
Krauss IJ, Wang CC-Y, Leighton JL (2001) Highly regioselective and diastereoselective directed hydroformylation of allyl ethers: a new approach to propionate aldol synthesis. J Am Chem Soc 123:11514–11515
Moasser B, Gladfelter WL, Roe DC (1995) Mechanistic aspects of a highly regioselective catalytic alkene hydroformylation using a rhodium chelating bis(phosphite) complex. Organometallics 14:3832–3838
Weissermel K, Arpe HJ (1988) Industrielle organische chemie. VCH, Weinheim, p 69
Wiese KD, Protzmann G, Koch J, Büschken W (2001) Verfahren zur katalytischen Durchführung von Aldolkondensationen mittels Mehrphasenreaktion. DE 19957522
van Leeuwen PWNM, Clément ND, Tschan MJ-L (2011) New processes for the selective production of 1-octene. Coord Chem Rev 255:1499–1517
Surburg H, Panten J (2006) Common fragrance and flavor materials: preparation, properties and uses. Wiley, Weinheim, p 14
Smith SE, Rosendahl T, Hofmann P (2011) Towards the rhodium-catalyzed bis-hydroformylation of 1,3-butadiene to adipic aldehyde. Organometallics 30:3643–3651
van Leeuwen PWNM, Roobeek CFJ (1985) The synthesis, co-ordination chemistry and catalytic application of phosphine ligands containg long-chain perfluoro-alkyl groups. Mol Catal 31:345–353
Papp R, Paciello R, Benisch C (2005) Verfahren zur Herstellung von Tricyclodecandialdehyd. WO 2005058786
Cornils B, Payer B (1974) Derivate des dicyclopentadiens—aktuelle schlüsselverbindungen. Chemiker-Ztg 98:70–76
Agabekov V, Seiche W, Breit B (2013) Rhodium-catalyzed hydroformylation of alkynes employing a self-assembling ligand system. Chem Sci 4:2418–2422
Eshon J, Landis CR, Schomaker JM (2017) Regioselective Rh-catalyzed hydroformylation of 1,1,3-trisubstituted allens using BisDiazaPhos ligand. J Org Chem 82:9270–9278
Reinius HK, Krause AOI (2000) Hydroformylation of functional alkenes with heterodonor phosphine rhodium catalysts: substrate or ligand directed regioselectivity. Catal Lett 70:149–154
Linnebank PR, Ferreira SF, Kluwer AM, Reek JNH (2020) Regioselective hydroformylation of internal and terminal alkenes via remote supramolecular control. Chem Eur J 26:8214–8219
Cobley CJ, Hanson CH, Loyd MC, Simmonds S (2011) The combination of hydroformylation and biocatalysis for the large-scale synthesis of (S)-allysine ethylene acetal. Org Proc Res Dev 15:284–290
del Rio I, Pàmies O, van Leeuwen PWNM, Claver C (2000) Mechanistic study of the hydroformylation of styrene catalyzed by the rhodium/BDPP system. J Organomet Chem 608:115–121
Panda AG, Bhor MD, Ekbote SS, Bhanage BM (2009) Regioselectivity in hydroformylation of aryl olefins using novel rhodium polyether phosphinite catalysts. Catal Lett 131:649–655
Thiel O, Bernard C, Larsen R, Martinelli MJ, Raza MT (2008) Methods of synthesizing cinacalcet and salts thereof. WO 2009002427
Himmele W, Aquila W (1969) Bismonocarbonsäureester des 3-Formylbutandiol-(1,2) sowie ein Verfahren zu deren Herstellung. DE 1945479
Fitton PP, Moffet HF (1976) Verfahren zur Hersteellung einer Formylverbindung. DE 2621224
Schäfer B (2007) Naturstoffe der chemischen industrie. Elsevier, München, p 413
Dekeukeleire S, D’hooghe M, Müller C, De Kimpe N (2010) Rhodium-catalyzed hydroformylation of N-(2-propenyl)-β-lactams as a key step in the synthesis of functionalized N-[4-(2-oxoazedidin-1-yl)but-1-enyl]acetamides. New J Chem 34:1079–1083
Carrilho RMB, Neves ACB, Lourenço MAO, Abreu AR, Rosado MTS, Abreu PE, Eusébio MES, Kollár L, Bayón JC, Pereira MM (2012) Rhodium/tris-binaphthyl chiral monophosphite complexes: efficient catalysts for the hydroformylation of distubstituted aryl olefins. Organomet Chem 698:28–34
Behr A, Westfechel A, Pérez Gomes J (2008) Catalytic process for the technical use of natural fats and oils. Chem Eng Technol 31:700–714
Behr A, Seidensticker T (2018) Chemistry of renewables. Springer, Berlin, pp 17–36
Guo A, Demydov D, Zhang W, Petrovic ZS (2002) Polyols and polyurethanes from hydroformylation of soybean oil. J Polym Environ 10:49–52
Muilwijk KF, Kamer PCJ, van Leeuwen PWNM (1997) A bulky phosphite-modified rhodium catalyst for the hydroformylation unsaturated fatty acid esters. J Oil Chem Soc 74:223–228
Behr A, Obst D, Westfechtel A (2005) Isomerizing hydroformylation of fatty acid esters: formation of ω-aldehydes. Eur J Lipid Sci Technol 107:213–219
Masamune S, Choy W, Petersen JS, Sita LR (1985) Double asymmetric synthesis and a new strategy for stereochmical control in organic synthesis. Angew Chem Int Ed Eng 24:1–76
Chakrabortty S, Almasalma AA, de Vries JG (2021) Recent developments in asymmetric hydroformylation. Catal Sci Technol 11:5388–5411
Cobley CC, Gardner K, Klosin J, Praquin C, Hill C, Whiteker GT, Zanotti-Gerosa A (2004) Synthesis and application of a new bisphosphosphite ligand collection for asymmetric hydroformylation of allyl cyanide. J Org Chem 69:4031–4040
Newsome DS (1980) The water-gas reaction. Catal Rev 21:275–318
Somasunderam A, Alper H (1994) Use of rhodium on carbon and 1,3-bis(diphenylphosphino)propane to catalyze the regioselective hydroformylation of alkenes with formic acid as the hydrogen source. J Mol Catal 92:35–40
Uhlemann M, Doerfelt S, Börner A (2013) Rhodium catalyzed hydroformylation with formaldehyde and an external H2-source. Tetrahedron Lett 54:2209–2211
Makado G, Morimoto T, Sugimoto Y, Tsutsumi K, Kagawa N, Kakiuchi K (2010) Highly linear-selective hydroformylation of 1-alkenes using formaldehyde as a syngas substitute. Adv Synth Catal 352:299–304
Stepić R, Wick CR, Strobel V, Berger D, Vučemilović-Alagić N, Haumann M, Wasserscheid P, Smith AS (2019) Mechanism of the water-gas shift reaction catalyzed by efficient ruthenium-based catalysts: a computational and experimental study. Angew Chem Int Ed 58:741–745
Wu L, Fleischer I, Jackstell R, Profir I, Franke R, Beller M (2013) Ruthenium-catalyzed hydroformylation/reduction of olefins to alcohols: extending the scope to internal alkenes. J Am Chem Soc 135:14306–14312
Fan C, Hou J, Chen Y-J, Ding K-L, Zhou QL (2021) Rhodium-catalyzed regioselective hydroformylation of alkynes to α,β-unsaturated aldehydes using formic acid. Org Lett 23:2074–2077
Funding
Open Access funding enabled and organized by Projekt DEAL. Naval Medical Research Unit-3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, B., Peña Fuentes, D. & Börner, A. Hydroformylation. ChemTexts 8, 2 (2022). https://doi.org/10.1007/s40828-021-00154-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40828-021-00154-x