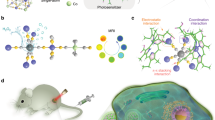
Highlights
-
Gene-engineering tumor cell membrane with CD47 over-expression is achieved to improve the macrophage-mediated phagocytosis of tumor cells by blocking CD47 immune checkpoint.
-
A cascade-enzyme nanoreactor combining tumor enzyme dynamic therapy, phototherapy, and deoxygenation-activated chemotherapy is proposed.
-
Glucose oxidase and chloroperoxidase can generate sufficient HClO to kill normoxic tumor cells, and tirapazamine can be subsequently activated to kill hypoxic tumor cells.
Abstract
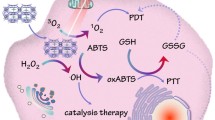
The enzyme-mediated elevation of reactive oxygen species (ROS) at the tumor sites has become an emerging strategy for regulating intracellular redox status for anticancer treatment. Herein, we proposed a camouflaged bionic cascaded-enzyme nanoreactor based on Ti3C2 nanosheets for combined tumor enzyme dynamic therapy (EDT), phototherapy and deoxygenation-activated chemotherapy. Briefly, glucose oxidase (GOX) and chloroperoxidase (CPO) were chemically conjugated onto Ti3C2 nanosheets, where the deoxygenation-activated drug tirapazamine (TPZ) was also loaded, and the Ti3C2-GOX-CPO/TPZ (TGCT) was embedded into nanosized cancer cell-derived membrane vesicles with high-expressed CD47 (meTGCT). Due to biomimetic membrane camouflage and CD47 overexpression, meTGCT exhibited superior immune escape and homologous targeting capacities, which could effectively enhance the tumor preferential targeting and internalization. Once internalized into tumor cells, the cascade reaction of GOX and CPO could generate HClO for efficient EDT. Simultaneously, additional laser irradiation could accelerate the enzymic-catalytic reaction rate and increase the generation of singlet oxygen (1O2). Furthermore, local hypoxia environment with the oxygen depletion by EDT would activate deoxygenation-sensitive prodrug for additional chemotherapy. Consequently, meTGCT exhibits amplified synergistic therapeutic effects of tumor phototherapy, EDT and chemotherapy for efficient tumor inhibition. This intelligent cascaded-enzyme nanoreactor provides a promising approach to achieve concurrent and significant antitumor therapy.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Malignant tumors are still major diseases that threaten human health, and the number of cancer death has increased by nearly 20% in the past 10 years [1]. Among kinds of conventional clinical cancer therapies, chemotherapy is still widely used in clinic as the main treatment mean except for surgery, but the toxic side effects caused by drug resistance, high invasiveness and lack of specific targeting significantly limit its therapeutic effect [2,3,4]. Therefore, it is urgent to study advanced treatments of cancer. In most recent study, a new kind of strategy so called enzyme dynamic therapy (EDT) provided an innovative approach for tumor treatment by fully taking advantage of the enzyme-catalyzed reactions in tumor microenvironment (TME) to generate different kinds of cytotoxic reactive oxygen species (ROS) [5, 6]. Proverbially, ROS is responsible for regulating intracellular signaling pathways and redox environments [7,8,9]. The redox environment of tumor cells has better selectivity than normal cells, and rapid accumulation of ROS in a short time can break the vulnerable redox balance and increase the oxidative stress levels in the cells, eventually leading to cancer cell damage and death [10,11,12,13]. Therefore, the ROS elevation is considered as an effective way to improve the effect of tumor treatment .
Among the ROS family, highly reactive hypochlorous acid (HClO) is the strongest factor, which can be produced by chloroperoxidase (CPO) catalysis to increase intracellular oxidative stress level and irreversibly destroy proteins, nucleic acids, lipids and carbohydrates [14]. As a highly active peroxidase of calcium-rich yeast with strong antioxidant inactivation ability, CPO enzyme can catalyze the reaction of chlorine ion (Cl−) and hydrogen peroxide (H2O2) to generate HClO and the subsequent decomposition of HClO can produce singlet oxygen (1O2), forming the CPO-H2O2-Halogen system for antitumor effect and killing microorganisms [15,16,17]. However, CPO-mediated catalytic reaction alone is inefficient for HClO generation owing to the low level of H2O2 in the tumor region [18]. Therefore, it is necessary to elevate the level of H2O2 in the tumor region for the improvement of the HClO-mediated antitumor effect. So far, various kinds of strategies have been designed to elevate the endogenous H2O2, such as glucose oxidase (GOX) biocatalysis, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase biocatalysis and superoxide dismutase (SOD) biocatalysis [5, 17, 19]. There into, GOX has been applied repeatedly as an H2O2 generator for its particular enzyme activity of catalyzing glucose into abundant H2O2 and gluconic acid, and this process can also consume tumor cell energy to achieve tumor starvation and death [20,21,22,23].
In this work, GOX and CPO were designed as the cascaded-enzyme reactor to cut off the intratumoral glucose supply and generate sustained HClO for effective tumor starvation treatment and EDT without external energy. However, the O2 consumption in the tumor tissue during starvation treatment exacerbates the degree of hypoxia in the TME, which in turn limits the effectiveness of tumor treatment and induces tumor recurrence and metastasis [24, 25]. To kill the hypoxic tumor cell, tirapazamine (TPZ), as an deoxygenation-sensitive prodrug, can be converted to cytotoxic instantaneous intermediates (Benzotriazine, BTZ) that can induce double-stranded DNA rupture and the hypoxic cells death by topoisomerase II-dependence process [26, 27]. Therefore, the combination of the cascaded-enzyme and TPZ may achieve amplified antitumor therapeutic effects. Besides, to protect enzymes from protein structure degradation and deactivation and deliver multiple components with high drug loading for effectively EDT and chemotherapy simultaneously, two-dimensional transition metal carbides/nitrides (2D MXenes) with good biocompatibility and high specific surface area [28, 29] were applied to co-load the cascaded-enzyme and drug to improve the accumulation in the tumor sites. In the meantime, 2D MXenes can transfer external energy for the generation of exogenous ROS to effectively cause tumor cell apoptosis under laser irradiation [30,31,32]. Therefore, a strategy of combining physical and biological enzymatic reactions for the enhanced antitumor effect is constructed, for which the laser irradiation can provide short-term exogenous ROS and the enzyme can provide sustainable endogenous ROS.
Herein, we designed and proposed a camouflaged nano-cascaded catalytic system by combining tumor enzyme dynamic therapy, phototherapy and hypoxic-activated chemotherapy for efficient antitumor effects (Scheme 1). In brief, the nano-cascaded enzymes Ti3C2-GOX-CPO/TPZ (TGCT) were prepared by conjugating GOX and CPO onto the Ti3C2 2D nanosheets with TPZ drug loading, then CD47-overexpressed cancer cell membrane biomimetic modification was utilized to form a bionic cascaded-enzyme nanoreactor (denoted as meTGCT). As CD47 can combine with the signal regulatory protein α (SIRPα) to form an effective “don’t eat me” signal, the meTGCT can avoid being eliminated by the innate immune system and block the phagocytosis by the first responder cells (such as macrophages) to realize long blood circulation in vivo [33, 34]. At the same time, because of superior immune escape and homologous targeting capacities, meTGCT would preferentially accumulate in the tumor sites and target tumor cells, which could reduce side effects. Moreover, when meTGCT arrived at the tumor site, high-expressed CD47 could also block the SIRPɑ receptor of the macrophage, thus enhancing the phagocytosis of tumor cells. On the other hand, once meTGCT is internalized by tumor cells, the cascaded-enzyme consumes the glucose and O2 in tumor regions to generate the cytotoxic HClO. Simultaneously, Ti3C2 can produce heat and ROS under NIR laser irradiation, and heat can accelerate the enzyme-catalyzed reaction rate and ROS production, further aggravating the hypoxic status in TME. Then, TPZ as a hypoxia-activated prodrug can be activated by reductase and further cause double-stranded DNA rupture and cell apoptosis to enhance the effects of EDT and phototherapy. Consequently, this camouflaged cascaded-enzyme nanoreactor can realize amplified synergistic effects of the phototherapy/enzyme dynamic therapy and hypoxia-activated chemotherapy to inhibit tumor growth efficiently.
2 Experimental Methods
2.1 Materials and Instruments
2.1.1 Materials
The transfection CD47-expression vector was obtained from Vector Builder Inc. (Guangzhou). Ti3C2 crystals were bought from Hangzhou Nano Technology Co. Ltd. Carbodiimide hydrochloride (EDC·HCl), N-Hydroxysuccinimide (NHS) and lipopolysaccharide (LPS) were from Sigma-Aldrich. Thiazolyl Blue Tetrazolium Bromide (MTT), Cyanine5.5 amine (Cy5.5-NH2) and diphenylisobenzofuran (DPBF) were from Aladdin Reagents. Calcein-AM/PI and Annexin V-FITC/PI Detection Kit were bought from Key GEN Bio TECH (Shanghai, China). Phenylmethanesulfonyl fluoride (PMSF), SDS polyacrylamide gel, chemiluminescent kit, bovine serum albumin (BSA), BCA Protein Assay Kit were bought from the Beytotime Institute of Biotechnology (Guangzhou). Polyvinylidene fluoride (PVDF) membrane was from Millipore Ltd (Shanghai). The anti-CD47, anti-GAPDH and the second antibody were bought from Solarbio life Science (Guangzhou). 2′, 7′-dichlorofluorescein diacetate (DCFH-DA) and 4′, 6-diamidino-2-phenylindole (DAPI) were obtained from Biosharp. Fetal bovine serum and DMEM medium were acquired from Gibco Life Technologies. Anti-CD47/PE antibody was purchased from Bio Legend, Inc. (San Diego, USA). Glucose oxidase (GOX, 1 g), Chloroperoxidase (CPO, 12.5 KU mL−1) and Coomassie blue were purchased from Sigma-Aldrich. The Amplite® Colorimetric Hypochlorite (Hypochlorous Acid) Assay Kit was from AAT Bioquest (USA). Hypoxyprobe™-1 plus kit was from Beijing Biolead Biology SCI & TECH Co. Ltd (Beijing), methylene blue (MB), coumarin-6 (C6) and Tirapazamine (TPZ) were from Aladdin Reagents. Mouse mammary tumor cells (4T1) and macrophages (RAW264.7) were from the cell bank of Chinese Academy of Sciences (Shanghai).
2.1.2 Instruments
The particle size and zeta potential were measured by Nano zetasizer (Malven). The morphologies were observed using transmission electron microscope (TEM, H7650, Japan) and field emission scanning electron microscope (CFSEM, ZEISS GeminiSEM500, Germany). The crystal structure was measured by X-ray diffraction (XRD, Bruker Quantax Flat Quad 5060, Germany). The Avanti mini extruder was obtained from Avanti Polar Lipids. The UV spectra were obtained by a spectrophotometer (DU-730, USA). Real-time detection of O2 concentration was measured by a dissolved O2 meter (JPBJ-608, Rex). The cell fluorescence images were performed using a Confocal laser scanning microscope (CLSM, FV3000, Olympus). The cell fluorescence quantitative assay was carried out using flow cytometry (FACSCalibur, BD). The cell viability was assessed with a microplate reader (Bio-Rad, model 550, USA). The temperature change was measured by an infrared thermal imager (Ti27, Fluke). The fluorescence imaging experiments in vivo were performed via IVIS Lumina Vivo Imaging System (PerkinElmer). The tissue sections were detected by Motic AE31 optical microscope (Xiamen).
2.2 Synthesis of meTGCT (CD47-Engineering Membrane@Ti3C2-GOX-CPO/TPZ)
2.2.1 Extraction and Characterization of 4T1 Cell Membrane
4T1 cells with high expression of CD47 were prepared according to our previous study by transfection using lentiviruses plasmid [35]. Subsequently, 4T1 cells were plated in a 12-well plate with incubation for 12 h and then were cultured with lentiviruses (multiplicity of infection (MOI) was 10) and polybrene (10 μg mL−1) for 24 h. Then, the transfected 4T1 (4T1CD47) cells were screened out with dihydropenicillin and were detected by CLSM and flow cytometry. Besides, the proteins of 4T1 and 4T1CD47 cells were obtained from RIPA buffer and loaded with 10% SDS polyacrylamide gel, which were then transferred to PVDF membrane and sealed with BSA for 2 h. Finally, the membranes were treated with anti-CD47 and anti-GAPDH and then were imaged by a chemiluminescence analyzer. To obtain 4T1 cell membrane with high CD47 expression, 4T1CD47 cells were resuspended with precooled 0.25 × PBS (phosphate buffer saline) containing PMSF, then the above cell solution was repeated freezing and thawing for three times and centrifuged at a speed of 3000 rpm min−1 (10 min) at 4 °C, the supernatant was further centrifuged (13,500 rpm min−1, 30 min) at 4 °C. Finally, 4T1CD47 cell membrane fragments were extruded 12 times by an Avanti mini extruder.
2.2.2 Synthesis of Ti 3 C 2 Nanosheets
The Ti3C2 nanosheets were obtained by a sonication exfoliation. In brief, bulk Ti3C2 were added into deionized water at the concentration of 2 mg mL−1, and then, the dispersion was treated with an ultrasonic probe (1200 W) for 15 h, followed by ultrasonic cleaning (300 W) for 12 h. Then, the final dispersion was centrifuged at 7000 rpm min−1 for 10 min to remove large Ti3C2, and the suspension was further centrifuged (13,000 rpm min−1) for 30 min to obtain Ti3C2 nanosheets.
2.2.3 Synthesis of TGCT (Ti 3 C 2 -GOX-CPO/TPZ)
To obtain TGC, GOX (2 mg), CPO (50 U) and Ti3C2 nanosheets (2 mg) were dispersed into PBS (2 mL) with magnetic stirring for 24 h. During the time, EDC (20 mg) and NHS (30 mg) were joined to active Ti3C2 nanosheets. At last, the obtained mixture was centrifuged (13,000 rpm min−1, 10 min) and rinsed with PBS to obtain TGC. TG and TC were obtained by the same above method. To obtain TGCT, TPZ (0.5, 1, 2, 4 or 8 mg) was dissolved into 2 mL of deionized water containing 2 mg of TGC (Ti3C2-GOX-CPO) under vigorous stirring. Then, TGCT was obtained by centrifugation at the speed of 13,000 rpm min−1, the drug loading efficiency and entrapment efficiency were calculated by UV–Vis at 470 nm.
To obtain meTGCT, the TGCT was mixed with equal amounts of the above obtained cell membrane, and the mixture was extruded for 12 passes by Avanti mini extruder (200 nm membrane). Finally, obtained meTGCT was characterized by SDS-PAGE protein analysis. Specifically, meTGCT was dissolved in RIPA lysis buffer with an ice bath for 30 min and prepared in SDS loading buffer. Then, we heated the samples loading buffer to 99 °C. Next, the samples were loaded each well of 10% SDS polyacrylamide gel, and then, the samples were run at the voltage of 80 V (0.5 h) and 120 V (1.5 h), the resulting polyacrylamide gel was stained by Coomassie blue and washed overnight.
The release of TPZ from meTGCT solution (1 mg mL−1) was detected in dialysis bag (MWCO = 3000 Da) and placed into 20 mL of PBS with shaking at 37 °C. Then, 0.5 mL of sample solution was got from dialysis bag and the same volume of PBS was added, the release content of TPZ was detected by UV–Vis at 470 nm. Besides, the TPZ release was detected under 808 nm laser irradiation with or without the addition of glucose (4 mg mL−1) and Cl− (25 mM) when the sample was exposed to 808 nm laser (1.5 W cm−2, 3 min) at 2, 8, and 24 h, respectively.
2.3 Loading Amount and Cascade Catalytic Activity of GOX and CPO
The loading amount of GOX and CPO was detected by BCA protein assay Kit. In brief, GOX (2 mg), CPO (50 U) and Ti3C2 nanosheets (2 mg) were dispersed into 2 mL of PBS, and then EDC (20 mg) and NHS (30 mg) were added and stirred overnight. TGC was acquired by centrifugal separation, and the supernatant was analyzed to calculate the amount of residual enzyme. The calculation of GOX and CPO loading efficiency (LE) is confirmed as follows:
where mi and mr stand for the initial enzyme mass and the residual enzyme mass, respectively.
To evaluate the cascade catalytic activity of GOX and CPO, glucose (4 mg mL−1) was added into the meTGCT (50 μg mL−1) solution, and then, the changes of dissolved O2 level and the pH were detected.
HClO detection was performed by HClO detection Kit (Amplite fluorescence method). Briefly, different samples were dispersed in PBS solution with glucose (4 mg mL−1) and Cl− (25 mM), and laser irradiation groups were treated with 808 nm (1.5 W cm−2, 3 min) and 635 nm (0.5 W cm−2, 5 min) lasers, and then, the supernatants were collected and treated with HClO detection Kit, the HClO content was monitored according to the OD value of 550 nm. Besides, meTGCT of different concentrations were dispersed in PBS solution containing glucose (4 mg mL−1) and Cl− (25 mM) and were treated with HClO detection Kit, the HClO content was monitored according to the OD value of 550 nm.
2.4 Detection of Singlet Oxygen In vitro
The production efficiency of 1O2 in meTGCT was detected by the absorbance change of the DPBF. Typically, Ti3C2, meT (CD47-engineering membrane@Ti3C2) and meTGCT were added into DPBF ethanol solution containing 4 mg mL−1 of glucose and 20 mM of NaCl, respectively. Then, the mixture was placed in darkness and exposed to 635 nm (0.5 W cm−2) laser. The DPBF absorption at 420 nm was detected at different points of time. Besides, 1O2 quantum yields (ΦΔ) were also detected via DPBF indicator, and the O2-saturated solution of Ti3C2, meT, meTGCT and MB with DPBF (20 μg mL−1) was exposed to 635 nm laser (0.5 W cm−2) for 3 min; the absorbance change of DPBF at 420 nm was recorded every 30 s by the UV–Vis system. Finally, the ΦΔ values of all samples were calculated by the standard of MB (ΦΔ = 0.52) [36].
2.5 Detection of the Photothermal Effect In vitro
808 nm laser was used as the NIR light source to evaluate the photothermal effects of meTGCT. Various concentrations of meTGCT (1.5 mL) were added into transparent quartz plates under 808 nm laser (1.5 W cm−2) exposure, and the temperature was recorded every 30 s. The photothermal conversion efficiency (η) was measured by detecting the temperature change of meTGCT solution under 808 nm laser (1.5 W cm−2).
Besides, the photothermal stability of meTGCT was examined by the 808 nm laser, meTGCT solution was exposed (5 min) and then cooled to inceptive temperature naturally with repetition (4 times). In order to investigate the impact of the physiological environment on the stability of meTGCT, which was dispersed in PBS and kept at 37 °C, the particle size and PDI were recorded at the various time by dynamic light scattering (DLS).
2.6 Detection of Cellular Uptake
The cell internalization and subcellular distribution of meTGC were assessed with C6 fluorescent dye via flow cytometry and CLSM, respectively. At a word, 4T1 cells were cultured for 12 h and subsequently cultured with TGC/C6 (50 μg mL−1) and meTGC/C6 (50 μg mL−1), and then, 4T1 cells were collected for flow cytometry detection and stained by DAPI (10 μg mL−1) for CLSM.
2.7 meTGC Promoted M1 Macrophages Phagocytosis of Tumor Cells
To detect the impact of meTGC on the M1 macrophage phagocytosis of 4T1 cells, 1000 ng mL−1 of LPS was used to polarize RAW264.7 cells into M1 macrophages, which were cultured with mwTGC (wild-type cell membrane@Ti3C2-GOX-CPO/TPZ) and meTGC for 2 h, respectively. After that, mCherry-labeled 4T1 cells were collected and added into per well at the quantity ratio of 1:5 (macrophages/cancer cells). After 4 h incubation, M1 macrophages were analyzed via CLSM and flow cytometry.
2.8 Cellular ROS and Thermal Imaging Evaluation
The ROS generation of meTGCT was confirmed using DCFH-DA. In short, 4T1 cells were plated for 24 h and subsequently cultured with meTGCT for 4 h, and then DCFH-DA was added into the different samples containing Ti3C2, meT (me@Ti3C2), meTG, meTC, meTGC and meTGCT (50 μg mL−1) for ROS detection. With or without 635 nm laser (0.5 W cm−2, 5 min) treatment, 4T1 cells were analyzed via flow cytometry and CLSM.
2.9 Intracellular Hypoxia Detection In vitro
The intracellular hypoxia status was evaluated by Hypoxyprobe™-1 plus kit. The composition of hypoxyprobe-1 is a pimonidazole hydrochloride, which could be reduced by the intracellular nitroreductase to produce the stable adducts with thiol (sulphydryl) groups in the tissue, and then FITC-marked antibody (FITC-Mab1) could bind to these adducts to reflect the cellular O2 level by immunofluorescence method. In brief, 4T1 cells were plated for 12 h of incubation, and then, the medium was replaced with meT, meTG, meTC and meTGC (50 μg mL−1). After 4 h incubation, laser groups were treated with 635 nm laser (0.5 W cm−2, 5 min), and then, pimonidazole HCl was used to pre-treat the cells for 1 h and stained using FITC-Mab1 for another 30 min. Finally, the cell hypoxia status was analyzed via CLSM and flow cytometry.
2.10 2.10. Cytotoxicity Evaluation in vitro
The cytotoxicity was measured via MTT method. 4T1 cells were cultured for 12 h, and then, meT, meTG, meTC, meTGC, TGCT, and meTGCT with concentration of 50 μg mL−1 were used to treat with 4T1 cells for 4 h, which were irradiated with 808 nm (1.5 W cm−2, 3 min) and 635 nm (0.5 W cm−2, 5 min) lasers and continued to be cultured to 24 h. MTT (5 mg mL−1) was added for a further 4 h incubation, the absorption value was recorded at 570 nm after the addition of DMSO.
Besides, the efficacy of every formulation was evaluated by a live/dead cell experiment. Briefly, 4T1 cells were processed with different formulations (50 μg mL−1 of meT, meTG, meTC, meTGC, and meTGCT) for 24 h, and the laser irradiation conditions were the same as the MTT assay. Finally, the cells were stained by Calcein-AM/PI and recorded by CLSM.
Finally, the anti-proliferation capacities of meTGCT were further measured by cell apoptosis experiments. Firstly, 4T1 cells were cultured with meT, meTG, meTC, meTGC, and meTGCT at the same concentration (50 μg mL−1) for 24 h, and laser irradiation condition was the same as the MTT assay, and then, the cells were treated with Annexin V-FITC/PI and detected by flow cytometry subsequently.
2.11 2.11. Tumor Targeting Capacity of meTGC
All animal experiments were approved in agreement with the Animal Ethics Committee and Use of Sun Yat-sen University (2,021,000,656). BALB/c female mice (3–5 weeks old) were given 4T1 cells (1 × 106) by subcutaneous injection to create the animal model, and then, the mice were intravenously injected with Ti3C2/Cy5.5, mwTGC/Cy5.5 or meTGC/Cy5.5 (2 mg kg−1) when the tumor volume reached to 100 mm3. The fluorescence images were monitored (excitation: 670 nm, emission: 710 nm) at different time. Lastly, the tumors and major organs were dissected and monitored after 24 and 120 h administration.
2.12 2.12 Antitumor Evaluation in vivo
BALB/c female mice were given 4T1 cells by subcutaneous injection to create the animal model. After fed for 7 days, the mice (n = 5 per group) were intravenously injected with different samples at a dosage 2 mg kg−1 as follows: PBS, meT (CD47-engineering membrane@Ti3C2), meTG (CD47-engineering membrane@Ti3C2-GOx), TGC (Ti3C2-GOX-CPO), meTGC (CD47-engineering membrane@Ti3C2-GOX-CPO), TGCT (Ti3C2-GOX-CPO/TPZ), meTGCT (CD47-engineering membrane@Ti3C2-GOX-CPO/TPZ), TGCT+laser, meTCGT+laser, at the first day and fourth day, respectively. Then, tumors of the laser groups were irradiated by 808 nm (1.5 W cm−2, 2 min) and 635 nm (0.5 W cm−2, 5 min) lasers at 12 h post-injection. And the tumor volume and body weight were measured regularly during a month. Afterward, the major organs were peeled for hematoxylin and eosin (H&E) and terminal transferase dUTP (TUNEL) detection, and the tumor tissues were weighed and photographed. The tumor volume was calculated via the formula: V = length × (width)2/2, and V/V0 in each group was used to normalize tumor volume, V0 is the inceptive tumor volume. Furthermore, obtained serum was used for evaluating the alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine (CR), γ-glutamyltransferase (γ-GT), total protein (TP) and glucose (GLU) values. In addition, the tumors were stained FITC-Mab1 by immunofluorescence staining to assess the deoxy-generation ability of meTGCT in vivo.
2.13 Statistical Analysis
All data were used in the manuscript as mean ± standard deviation. Statistical analysis was constructed by the two-tailed Student’s t test.
3 Results and Discussion
3.1 Characterization of 4T1 Cell Membrane with High-expressed CD47
To obtain 4T1 cell membrane with high-expressed CD47, we firstly transfected 4T1 cells with lentiviruses vectors to prepare gene-engineering 4T1 cells with overexpression of CD47, and the transfected 4T1 cells (4T1CD47) were detected using anti-CD47/PE staining by CLSM and flow analysis. As illustrated in Fig. 1a, the non-transfected 4T1 cell group showed slightly red fluorescence signal, while 4T1CD47 cells displayed the strongest fluorescence signal intensity, verifying the successful transfection of 4T1 cells with high CD47 expression. Quantitatively, the percentage of CD47/PE positive cells in 4T1CD47 cells group reached 98.4%, which was dramatically as high as that in 4T1 cells group (Fig. 1b), and the fluorescence intensity of 4T1CD47 cells was 12.8 times as vigorous as that of 4T1 cells by quantitative analysis (Fig. 1c). Besides, it was also identified the high expression of CD47 on 4T1CD47 cells by western blot analysis (Fig. S1). These results all suggested the successful preparation of 4T1 cells with high-CD47 expression.
3.2 Characterization of meTGCT (CD47-engineering Membrane @Ti3C2-GOX-CPO/TPZ)
The cascaded-enzyme nanoreactor (meTGCT) was synthesized by conjugating GOX and CPO onto the surface of Ti3C2 nanosheets loading with TPZ and further encapsulated with 4T1 cell membrane with high-CD47 expression (Fig. 2a). Ti3C2 nanosheets were prepared from the bulk layer-structured Ti3C2 by ultrasonication. Scanning electron microscopy (SEM) images showed the accordion-like layer structures of origin Ti3C2, and the element mapping of the bulk Ti3C2 sample confirmed the absence of the Al element and the existence of O and F elements (Fig. 2b). The morphology of obtained Ti3C2 nanosheets was characterized by TEM (Fig. 2c) and SEM (Fig. S2a) images, revealing the ultrathin and transparent flake with the size of 100 nm and exhibiting the typical 2D topology. The atomic force microscopy (AFM) image suggested that the thickness of the as-synthesized Ti3C2 nanosheets was ∼6 nm (Fig. 2d). The exfoliated Ti3C2 nanosheets also performed a size of 115 nm (Fig. 2e) with a charge of -28.9 mV (Fig. 2f). The infrared spectroscopy (Fig. S2b) and Raman spectroscopy (Fig. S2c) confirmed that the surface of as-exfoliated Ti3C2 nanosheets was rich in –COOH and –OH groups. Subsequently, GOX and CPO were conjugated onto Ti3C2 nanosheets with amido bonds, and the loading efficiency (LE) was detected to be 15.69%. For comparison, the GOX LE was 20.79% for Ti3C2-GOX (TG) and CPO LE was 9.35% for Ti3C2-CPO (TC), respectively (Fig. S3). After loading, the particle size of Ti3C2 nanosheets (115 nm) increased to ~ 145 nm (Fig. 2e), and the zeta potential ( − 28.9 mV) slightly changed to -24.5 mV (Fig. 2f), implying the successful loading of GOX and CPO. Afterward, hypoxia-activated prodrug TPZ was loaded onto Ti3C2-GOX-CPO (TGC) through physical interactions. The ultraviolet–visible (UV–Vis) spectra demonstrated the successful loading of TPZ in the TGC (Fig. S4a), when the ratio of TGC/TPZ was 1:2, the loading efficiency (LE) and entrapment efficiency (EE) were 63.06% and 85.12% (Fig. S4b), respectively. The zeta potential of Ti3C2-GOX-CPO/TPZ (TGCT) increased to − 18.5 mV (Fig. 2f). To obtain meTGCT, TGCT was embedded with 4T1 cell membrane with CD47 high expression by repeated extrusion. As shown in Fig. S5, meTGCT exhibited a transverse dimension (160 nm) and transparent membrane coating, confirming the successful packaging of 4T1 cell membrane. What’s more, the size of meTGCT widened to 180 nm (Fig. 2e), and the zeta charge swelled to ~ -14.6 mV (Fig. 2f). In addition, the protein expression of meTGCT was detected by SDS-PAGE identification, and 4T1 cell membrane proteins were largely retained, and the bands of GOX (80 kDa) and CPO (46 kDa) were also observed in the meTGCT group (Fig. S6), confirming that GOX and CPO were successfully accommodated in meTGCT. These results all verified the successful preparation of meTGCT. Of special note, the hydrodynamic size and PDI of meTGCT were kept constant for at least 7 days, and the good dispersion of meTGCT in PBS was observed after 7 days in contrast to the precipitation occurring in TGCT, confirming the excellent stability of meTGCT in the physiological condition (Fig. S7). Moreover, almost no visible difference was observed in X-ray diffraction (XRD) patterns among meTGCT, Ti3C2 nanosheets and the bulk Ti3C2 (Fig. 2g), indicating that meTGCT reserved the uniform crystal structure and crystallinity as Ti3C2.
a Design principle of the synthesis of meTGCT. b SEM and element mapping of the bulk Ti3C2. c TEM image of Ti3C2 nanosheets. d AFM image of the exfoliated Ti3C2 nanosheets. e The size and f apparent zeta charge of Ti3C2, meT, TG, meTG, TC, meTC, TGC, meTGC, TGCT, meTGCT. g XRD images of the bulk Ti3C2, Ti3C2 nanosheets and meTGCT
3.3 Detection of Cascade Catalytic Reactions and Drug Release
It has been reported that chemical modifications could reduce the catalytic capabilities of enzymes [37]. Thus, the catalytic activities of GOX and CPO in meTGCT were evaluated. GOX catalyzes glucose to generate H2O2 and gluconic acid under O2, leading to pH decrease and O2 consumption [38,39,40], and then, CPO could catalyze H2O2 and Cl− to produce HClO. On the basis of these, the cascaded-enzyme catalytic capacities of meTGCT were measured by O2 consumption, pH decrease and HClO generation. Firstly, the dissolved O2 level of various groups in PBS containing 4 mg mL−1 of glucose and 25 mM of Cl− were measured by an O2 probe. As displayed in Fig. 3a, meTC group exhibited similar results as the control group, indicating that meTC did not consume O2. However, with the addition of GOX, the dissolved O2 level quickly declined and gradually reached a platform. The O2 level of meTG gradually decreased from 8.1 mg L−1 to a balanceable value of 1.4 mg L−1 within 10 min, and the consumption rate of O2 obviously increased in meTGC and meTGCT groups, indicating that the presence of CPO accelerated GOX-catalyzed reaction activities. Importantly, under the 808 + 635 nm lasers irradiation, the O2 level in meTGCT group decreased faster and reached 0.8 mg L−1 within 4 min, which might be because that 635 nm laser irradiation could induce the energy transfer of Ti3C2 that reacts with O2 to produce 1O2, and the increased temperature caused by 808 nm laser could enhance the enzymatic activities of GOX and CPO. Besides, the O2 consumption rate curves in Fig. 3a accorded well with the exponential equation (y = a × e−kx + b), where x and y were the reaction time and O2 concentration, respectively. The constant k interpreted as the O2 consumption rate is shown in Fig. 3b, demonstrating meTGCT could rapidly consume O2 with laser irradiation. Considering that the temperature enhancement by laser irradiation might accelerate enzymatic catalytic rate, the photothermal effect of meTGCT was also evaluated via 808 nm laser. As shown in Fig. S8a, meTGCT displayed a photothermal effect with concentration and time dependence, the temperature of meTGCT (25 μg mL−1) rapidly added up to 18 °C under 808 nm laser irradiation for 10 min, exhibiting a superior photothermal capability, while temperature of solution without meTGCT rose mildly (ΔT≈ 1.7 °C). The photothermal stability is further measured and shown in Fig. S8b, and there was no distinct variation in photothermal capacity of meTGCT during four heating cycles, presenting excellent photothermal stability. Besides, the photothermal conversion efficiency of meTGCT was computed as 53.87% based on the cooling curve (Fig. S8c), which is remarkably higher than most photothermal agents (PTAs) such as black phosphorus (BP) nanoparticles (29.47%), graphene oxide (25%), MoS2 nanosheets (27.6%), PEG modified antimonene quantum dots (PEG-AMQDs, 45.5%) [41, 42]. Therefore, the results proved that meTGCT possessed good photothermal conversion properties, which would benefit for improving the enzyme activity. Next, we further evaluated the enzymatic activity of meTGCT by pH change in PBS containing 4 mg mL−1 of glucose and 25 mM of Cl−, which was monitored by a pH meter. As clarified in Fig. S9, pH values of PBS (containing 4 mg mL−1 of glucose) remained constant at 7.4 without GOX treatment. With the increase in concentration of additional GOX, the pH of glucose solution gradually declined and dropped to 4.24 when the concentration of GOX was 50 μg mL−1, verifying that GOX could improve glucose oxygenolysis in the manner of time and concentration dependence. In addition, the pH values of glucose solution with different treatments were detected to evaluate the catalytic activity of meTGCT (Fig. 3c). The pH values of the control and meTC groups remained constant, while the meTG group exhibited certain pH changes, illustrating the catalytic activities of meTG were retained. Compared with meTG group, meTGC and meTGCT groups exhibited an enhanced drop of pH, demonstrating that CPO could accelerate the GOX-catalyzed reaction process, while the significantly reduced pH values were shown in the meTGCT + L group, which was ascribed to the enhanced catalytic reactivity by hyperthermia induced by laser irradiation.
a Changes of dissolved O2 in PBS containing various samples with/without 635 nm (0.5 W cm−2) and 808 nm (1.5 W cm−2) lasers under 4 mg mL−1 of glucose and 25 mM of Cl−. b O2 consumption rates based on a. c Changes of pH in PBS containing different samples. d HClO production content of PBS containing different samples. e HClO production content of PBS containing meTGCT with different concentrations in the presence of 4 mg mL−1 of glucose and 25 mM of Cl−, p values were contrast with the group (concentration: 0 μg mL−1). f A/A0 of the DPBF treated with Ti3C2, meT and meTGCT with or without 635 nm light irradiation. (***p < 0.001, **p < 0.01, *p < 0.05, n = 3)
The cascade catalytic activity of meTGCT was further evaluated by CPO-catalyzed product. CPO could catalyze H2O2 and Cl− into highly cytotoxic HClO, so we detected the generated HClO of the meTG, meTC, meTGC, meTGCT, and meTGCT + L in PBS with the addition of glucose (4 mg mL−1) and Cl− (25 mM) to imitate physiological environment. As revealed in Fig. 3d, meTGC group exhibited enhanced HClO generation capacity compared with the meTG and meTC groups, demonstrating the successful cascaded catalysis of GOX and CPO enzymes. The absence of either GOX or CPO could restrict the catalytic activity and limit the generation of HClO. After laser irradiation, the HClO generation of the meTGCT group further increased, which was attributed to the increase in temperature by laser irradiation. Meanwhile, the HClO generation content of meTGCT performed a concentration-dependent manner (Fig. 3e). To further explore the HClO generation capacity of the CPO catalysis, we evaluated the HClO content in the meTGCT group with additional H2O2, glucose or Cl− in PBS. As shown in Fig. S10, the generated HClO content gradually increased with the incremental concentration of H2O2, glucose or Cl−, demonstrating the significant impacts of H2O2, glucose and Cl− for the cascaded catalysis. Afterward, the relative enzyme activities of GOX, CPO in different samples with or without laser irradiation are assessed in Fig. S11, and meTGC exhibited increased enzymatic activity of GOX and CPO compared to meTG and meTC due to the cascaded-enzyme reaction, and meTGCT with laser irradiation further improved the enzyme activity of both GOX and CPO, which could be ascribed to the rise of temperature. The above results indicated that the cascaded-enzyme nanoreactor possessed excellent enzyme catalytic capacity.
Finally, the 1O2 generation capacity of meTGCT was evaluated by DPBF indicator. As shown in Fig. 3f, compared with DPBF alone group, the rapid downswing of absorbance ratio at 420 nm was observed in Ti3C2 + L and meT + L solution within 30 min, indicating the excellent 1O2 generation capacity of Ti3C2, and cell membrane coating did not influence the ROS generation capacity of Ti3C2. A sharper downtrend was observed in meTGCT + L solution owing to the combination of the cascade enzymatic catalysis and photodynamic effect, and the 1O2 quantum yield of meTGCT increased from 0.18 without laser irradiation to 0.60 with laser irradiation (Fig. S12). Therefore, it could be concluded that meTGCT possessed a significantly superior capacity of 1O2 generation, which is promising for tumor treatment studies in vitro and in vivo.
The TPZ release is determined in Fig. S13. Compared with TPZ-releasing percentage of 26.1% under normal conditions, 808 nm laser irradiation enhanced the TPZ release due to the heat effect produced by Ti3C2. Especially, nearly of 65.9% of TPZ was released from meTGCT under the existence of glucose and Cl−, it could be deduced that meTGCT catalyzed the glucose to generate gluconic acid, which decreased the electrostatic interaction between TPZ and Ti3C2, thus accelerating the drug release [43, 44].
3.4 Cellular Uptake Study of meTGC
It had been reported that cancer cell membrane-coated nanocarriers exhibited preferential homologous tumor targeting capacity [45,46,47,48,49]. Thus, the homologous targeting capacity of meTGC on 4T1 cells was measured via flow cytometry and CLSM, and C6 dye was defined as a fluorescence probe to label meTGC. As illustrated in Fig. S14, only tiny amounts of C6 in cells were observed after 10 h incubation with the pure C6, the mean fluorescence intensities of TGC/C6 enhanced over time and achieved the maximum at 8 h. By comparison, meTGC/C6 group exhibited stronger fluorescence intensities at all times, indicating the well homologous targeting ability. Besides, the intracellular locations of TGC and meTGC against 4T1 cells were detected by CLSM (Fig. 4a–-c), the signal (green) of the C6-labeled cascaded-enzyme nanoreactor surrounding the nuclei in the meTGC/C6 group was strongest among all groups, and the quantitative flow cytometry analysis showed the fluorescence intensity of meTGC/C6 group was 1.9 times as high as that of TGC/C6 group (Fig. 4c), further indicating cell membrane coating could improve tumor cell uptake for the cascaded-enzyme nanoreactor.
a CLSM results of 4T1 cells uptake toward TGC/C6 and meTGC/C6 at 4 h. b Flow cytometric results and c quantitative flow analysis of 4T1 cells uptake of C6-labeled different formulations. d CLSM results of 4T1 cells were swallowed by macrophages after different treatments with mwTGC and meTGC. e Flow histogram analysis and f quantitative flow analysis of 4T1 cells swallowed by macrophages after treatments with mwTGC and meTGC. g CLSM results of intracellular ROS level. (***p < 0.001, **p < 0.01, n = 3)
3.5 meTGC Promoted M1 Macrophages Phagocytosis of Cancer Cells
CD47, overexpressed on the surface of meTGC, could interact with SIRPα presented on macrophages and protected meTGC from the phagocytosis by macrophages through sending “don’t eat me” signal. At the same time, CD47-overexpressed meTGC would block the interaction between macrophages and tumor cells through competitively occupying SIRPα, which could enhance the macrophage-mediated phagocytosis for tumor cells [33, 34]. Therefore, we firstly constructed cellular uptake experiments to confirm the immune escape capacity of the meTGC by flow cytometry. As shown in Fig. S15, an obviously decreased fluorescence intensity of cells treated with meTGC/C6 was found as compared to the TGC/C6 group, and the cell uptake of TGC/C6 was three times as strong as that of meTGC/C6 group, indicating that coating TGC with CD47 high-expressed 4T1 cell membrane could effectively inhibit the immune recognition to achieve immune escape. To evaluate whether meTGC could induce M1 macrophages-mediated 4T1 cells phagocytosis, we pretreated M1 macrophages with meTGC for 2 h and then explored the co-culture of M1 macrophages and mCherry-labeled 4T1 cells to investigate the phagocytosis effect of macrophages by CLSM and flow cytometry. As clarified in Fig. 4d, the red signal (mCherry-labeled tumor cells) in the meTGC group was obviously stronger than that of in control and mwTGC (wild-type cell membrane-coated TGC) groups, indicating that high-expressed CD47 on the surface of the meTGC could promote macrophages-mediated 4T1 cells phagocytosis, which was because that CD47 on meTGCT could competitively combine with SIRPα, thus increasing the interaction between macrophages and 4T1 cells [50,51,52,53]. Similar results were confirmed in flow cytometry analysis (Fig. 4e, f), the fluorescence signal in meTGC group was three times as strong as that in mwTGC group, further clarifying the meTGC could preoccupy SIRPα, leading to the saturation of SIRPα, then enhancing macrophage-mediated phagocytosis of tumor cells [35].
3.6 Cellular ROS Detection
The intracellular ROS level was also measured on 4T1 cells after treatment with meTGCT by flow cytometer and CLSM. As revealed in Fig. 4g, the fluorescence signal was nearly detected in the control group regardless of 635 nm light irradiation, and that of the Ti3C2 group slightly enhanced, indicating the ROS generation capacity of Ti3C2 under laser irradiation. The fluorescence signal in meT group was as strong as that of Ti3C2 treatment, which could be ascribed to the increased uptake by tumor cells owing to homologous targeting mediated by the cell membrane (Fig. 4a–-c). Further enhanced DCF fluorescence was found in the meTG and meTC groups, which was because meTG could catalyze glucose and O2 into H2O2, and meTC could catalyze endogenous H2O2 into HClO, and meTGC group exhibited more intensive fluorescence signal than the meTG and meTC groups, indicating that the cascaded catalytic reaction could effectively generate ROS. The fluorescence intensities of the meTGCT group were further strengthened, which might be because that the secondary electrons derived from the irradiated Ti3C2 could be captured by TPZ to further generate hydroxyl radicals by a reductive reaction [54]. Most importantly, the strongest DCF signal was observed in meTGCT + L group, suggesting meTGCT could effectively generate ROS in living cells under 635 nm light. The results of the flow cytometry also verified that meTGCT + L group exhibited the strongest ROS production among all the treatment groups, which was almost 13-fold higher than that of meT group (Fig. S16). These results confirmed that meTGCT could generate sufficient ROS via several different mechanisms, thereby achieving promising overall therapeutic effects in vitro.
3.7 Intracellular Hypoxia Detection of 4T1 Cells
The intracellular O2 consumption effects were evaluated by incubating 4T1 cells with different samples and detecting the changes of the dissolved O2 in the DMEM medium. As shown in Fig. 5a, a quick depletion of O2 was found in the meTGC and meTGCT groups, and the most rapid O2 depletion rate was exhibited in the meTGCT group with 635 nm laser irradiation, indicating that the cascaded-enzyme could increase intracellular O2 consumption. In addition, the cellular hypoxia level was further assessed by CLSM and flow cytometry using a FITC-Mab1 antibody (Fig. S17). It could find that the fluorescence intensities of all groups with 635 nm light irradiation were significantly higher than those of groups without laser irradiation, attributing to the consumption of O2 caused by the photodynamic effect. Besides, the merely slight green fluorescence of hypoxia-positive signal was found in the meT group with 635 nm laser irradiation, attributing to the small amount of O2 consumption caused by PDT effect. 4T1 cells treated with meTG exhibited enhanced fluorescence intensity, whereas the strongest fluorescence intensity was observed in the meTGC group, which ascribed to the more O2 depletion by cascaded-enzyme (GOX and CPO) and PDT effect of Ti3C2 (Fig. 5b, c). The aforementioned results indicated that the cascaded-enzyme catalytic reaction could effectively consume cellular O2 level for the potential activation of TPZ in treatment studies.
a Change of dissolved O2 in 4T1 cells treated with different formulations. b CLSM images and c flow histogram of cellular hypoxia of 4T1 cells incubated with different formulations using a hypoxia probe. d Cell viability of 4T1 cells treated with meTG, meTC, meTGC, and meTGCT with various GOX/CPO concentrations under 808 + 635 nm lasers irradiation. Cell viability of 4T1 cells incubated with various formulations in e 21% O2 condition and f 2% O2 condition with/without laser irradiation. g Live/dead staining images and h cell apoptosis of 4T1 cells after various treatments for 4 h (***p < 0.001, **p < 0.01, *p < 0.05, n = 5)
3.8 In vitro Antitumor Effects of meTGCT
Before measuring the antitumor efficiency of cascaded-enzyme nanoreactor, we estimated the real-time temperature variation in cells with 808 nm light irradiation. As clarified in Fig. S18, the temperature in both of experiment groups showed rapid temperature rise, while control group merely rose to 29.6 °C within 3 min of 808 nm laser irradiation. Particularly, the temperature of meTGC group reached to mild hypothermia condition (44.6 °C), which was beneficial to improve the EDT and PDT effects. Afterward, the remarkable capacities of the cascaded catalysis and the therapeutic effects of meTGCT were evaluated by MTT assays in vitro. As displayed in Fig. S19a, b, almost no distinct cytotoxicity of Ti3C2 and meT indicated their satisfactory biocompatibility. The cell viability decreased with the increment concentration of meT under 808 + 635 nm lasers irradiation, indicating the phototherapy effects of meT. The therapeutic effects of meTG, meTC, meTGC, and meTGCT with various concentrations are shown in Fig. 5d, and meTGCT displayed the highest cytotoxicity against 4T1 cells comparing to other groups, owing to the cancer cell membrane coating prompting more cellular uptake of TGCT, and the cascaded catalytic reaction achieving efficient EDT, as well as hypoxic-activation of TPZ for chemotherapy. Considering that cascaded-enzyme nanoreactor could induce the tumor cell deoxygenation, we evaluated the therapeutic effects of meTGCT in normoxic or in hypoxic environments with/without 808 + 635 nm laser irradiation. Firstly, the cytotoxicity of chemotherapeutic effects on 4T1 cells is evaluated in Fig. S19c, free TPZ exhibited almost no cytotoxicity on 4T1 cells owing to the unactivated status of TPZ in normoxic environment. Besides, Fig. 5e, f shows that cell viability of TPZ free groups (meT, meTG, meTC, and meTGC) in hypoxic environment was similar to that in normoxic environment, while the anticancer effects of the meTGCT and TGCT groups in hypoxic environment were enhanced compared to those in the normoxic environment, which could be attributed to the deoxygenation-activated chemotherapy effect of TPZ [27]. To statistically confirm the combination effects of PTT, PDT, EDT and chemotherapy on 4T1 cells inhibition, the combination index (CI) was calculated on the basis of the Chou-Talalay assay [55]. The 50% inhibition concentration (IC50) of TPZ group in hypoxia environment was 26.92 μg mL−1, and the IC50 of meTGC under laser irradiation group was 51.21 μg mL−1. The IC50 of meTGCT under laser irradiation and hypoxia environment was 19.27 μg mL−1, and the CI was calculated as 0.824, indicating there were synergistic effects between phototherapy and chemotherapy. In addition, we further measured the therapeutic effects of meTGCT in media with high glucose level or low glucose level (Fig. S19d). Compared with the high cell viability of meTG group in high glucose level media, the meTGCT group exhibited the enhanced cell cytotoxicity on account of the cascaded EDT effects. Besides, in low glucose level media, the cell viability of the meTGCT group also reached 12.5% at 24 h under laser irradiation, owing to EDT effect and cell starvation induced by the complete glucose decomposition, indicating the exclusive effect of the meTGCT nanoreactor in tumor cell growth suppression with or without glucose supply. These results strongly indicated that the combined phototherapy/EDT/chemotherapy with meTGCT treatment under laser irradiation could effectively kill tumor cells.
On purpose of evaluating visually anticancer effects of meTGCT in vitro, Calcein-AM/PI staining fluorescence imaging was further conducted to observe the cell live/dead states (Fig. 5g). Briefly, a small number of cells turned into orange or red (dead) in the meTG and meTC groups, indicating meTG and meTC could induce slight cells death. Under laser irradiation, the number of red cells increased, ascribing to the phototherapy effect of Ti3C2. Specially, almost all cells were stained red in meTGCT group with laser irradiation, which further confirmed the best anticancer efficacy of meTGCT treatment under laser irradiation toward 4T1 cells. In addition, the apoptosis experiment confirmed similar results (Fig. 5h). The proportions of early and late apoptosis cells were 11.69%, 34.02%, 37.8%, and 47.57% after treatment with meT, meTG, meTC, and meTGC, respectively, which increased to 38.42%, 46.75%, 50.8%, and 67.23% after being exposed to laser irradiation, respectively, while that of meTGCT with laser irradiation (82.5%) was far higher than the above groups, demonstrating definite superiority for tumor therapy comparing to single EDT, phototherapy or chemotherapy. Therefore, the combination of tumor phototherapy, EDT and chemotherapy was a safe and effective synergistic strategy for tumor treatment.
3.9 In vivo Fluorescence Imaging
Inspired by the excellent capacity of homologous targeting in vitro, the tumor targeting and accumulation performance of meTGCT were determined in vivo. As shown in Fig. 6a, b, the meTGC/Cy5.5 fluorescence intensity increased persistently and achieved a peak at 24 h. Although the fluorescence intensity decreased gradually after 72 h, it was still detected at 120 h, demonstrating the continued retention of meTGC in tumor tissue. mwTGC/Cy5.5 group showed similar fluorescence changes within 24 h, while the fluorescence signal decreased quickly after that. The dramatic distinction was attributed to the enhanced immune escape and long circulation effect of meTGC due to high-expressed CD47. In contrast, the fluorescence signal of TGC/Cy5.5 was weaker at the tumor site and faded away quickly after 12 h post-injection. Moreover, the fluorescence intensity of meTGC/Cy5.5 was always 7 to 34 times as high as that of TGC/Cy5.5 at different time intervals, which clearly exhibited the long-term tumor duration property. Besides, all tumors and organs were obtained after 24 or 120 h post-injection (Fig. 6c, e), semiquantitative analysis of the fluorescence intensity is shown in Fig. 6d, f. In the TGC/Cy5.5 group, Cy5.5 mainly accumulated in liver and kidney after 24 h, while that of meTGC/Cy5.5 group was obviously observed in tumor, 15.6-fold as high as that of TGC/Cy5.5 group, exhibiting the enhanced tumor accumulation and prolonged blood circulation after coating with the homologous tumor cell membrane with high CD47 expression. Such long circulation time would effectively enhance the therapeutic efficiency of nanoreactor. Most importantly, this highly specific tumor recognition capacity of meTGC could significantly strengthen the therapeutic effects, as well as reduce the side effects.
a Biodistribution of TGC/Cy5.5, mwTGC/Cy5.5, and meTGC/Cy5.5 in 4T1 tumor-bearing mice. b Corresponding average fluorescence intensity based on a. Fluorescence imaging of organs and tumor c at 24 h and e at 120 h post-injection. Corresponding average fluorescence intensity of major organs and tumor at 24 h d based on c and 120 h f based on e. g Temperature changes of tumor region under 808 nm light (1.5 W cm−2) within 5 min (***p < 0.001, **p < 0.01, n = 3)
3.10 3.10. In vivo Photothermal Imaging and Tumor Hypoxia Detection
To evaluate the in vivo photothermal effect, the temperature changes of the tumor region were detected (Figs. 6g and S20). In control group, the temperature showed negligible change within 2 min, while that in meTGC treated group rapidly rose from 36.3 to 46.4 °C within 2 min, which could provide a mild hypothermia condition for the enhancement of EDT and PDT effects.
After proving the tumor accumulation of meTGCT, we explored the intratumoral hypoxia status after 48 h intravenous administration of meTGCT in vivo. As displayed in Fig. S21, there was almost no fluorescence signal of hypoxia (green) in PBS and meT groups, while the increased fluorescence intensity in the treatment groups with GOX indicated the aggravation of tumor hypoxia status due to O2 consumption after GOX catalyzing the glucose and O2. Besides, the treatment groups with GOX and CPO showed the greatly enhanced fluorescence intensity, which was ascribed to the cascaded EDT effect increasing O2 consumption. After laser irradiation, the intratumoral O2 level was further exhausted due to PDT and EDT effects, leading to the enhanced tumor hypoxia. Such an O2 consumption dynamic in tumor would be conducive to activate chemotherapeutic effect of TPZ for the enhanced antitumor efficiency.
3.11 3.11. In vivo Antitumor Therapy
Finally, 4T1 tumor-bearing me systemic antitumor activity of meTGCT. The therapy process and therapeutic effects of meTGCT are shown in Fig. 7a, b. The tumor sizes in control and meT groups increased quickly, and the slight tumor growth was inhibited by meTG via tumor starvation. The meTGC exhibited moderate tumor growth inhibition with V/V0 value of 8.1 on day 31, due to the generation of plentiful HClO caused by cascaded-enzyme reaction. Comparing to TGCT and meTGC groups, the mice treated with meTGCT presented a slow growth of tumors and the final V/V0 was 4.5, due to the efficient homologous targeting capacities and starvation/EDT/chemotherapy effects. Besides, under laser irradiation, meTGCT exhibited the strongest therapeutic outcomes (V/V0 = 0.003) among all the groups through the tumor targeting phototherapy and EDT magnified chemotherapy, indicating that the biomimetic cascaded-enzyme nanoreactor offered an optimal strategy for tumor treatment.
a Therapy process of meTGCT. b The variation in V/V0 in different groups. c Variations in body weight and d tumor weight during various treatments. e Photographs of mice and tumor tissues on day 31. f TUNEL staining of tumors were harvested from the mice of different treatments. (G1) Control, (G2) meT, (G3) meTG, (G4) TGC, (G5) meTGC, (G6) TGCT, (G7) meTGCT, (G8) TGCT+laser, (G9) meTGCT+laser (***p < 0.001, **p < 0.01 and *p < 0.05 as contrast with Control group, ###p < 0.001, ##p < 0.01, #p < 0.05, n = 5)
Similarly, the weight (Fig. 7d) and photographs (Fig. 7e) of tumors harvested from the mice on day 31 further attested the superior tumor suppression effect of meTGCT with laser irradiation. It was noted that there were two mice treated with meTGCT and laser irradiation got completely tumor remission at the end of the treatment. Meanwhile, compared to the control group where all mice died, the mice of meTGCT + laser treatment all lived for 31 days and there were two mice got complete elimination of tumor until they were dissected, demonstrating this nanoreactor could extend the survival period of tumor-bearing mice (Fig. S22). And the body weights of the mice showed no distinct variations within 31 days (Fig. 7c), indicating the low systemic toxicity of meTGCT.
In addition, the major physiological and biochemical parameters in serum of the mice after treatments were also evaluated. As illustrated in Fig. S23, liver function (ALT, AST, ALB, ALP, TP, γ-GT), kidney function indexes (BUN, CR) and GLU level were in the normal range after treatment with meTGCT, confirming the biosafety of the cascaded-enzyme nanoreactor. Furthermore, H&E staining analysis of the main organs also demonstrated the biosafety of meTGCT during the treatment (Fig. S24). The results indicated that meTGCT could significantly eliminate the tumor with satisfying biosecurity.
To further evaluate the antitumor effects of meTGCT, we dissected the tumors of all groups for terminal transferase dUTP (TUNEL) apoptosis staining (Fig. 7f), H&E and Ki67 antigen results (Figs. S25 and S26), which all suggested that treatment with meTGCT under laser irradiation caused the most significant cell damage and apoptosis in all groups. The superiority of meTGCT treatment further demonstrated the advantages of combined therapy on the basis of cascaded-enzyme nanoreactor-mediated tumor phototherapy, enzyme dynamic therapy and the subsequent deoxygenation-activated chemotherapy.
4 Conclusions
Summarily, an intelligent bionic cascaded-enzyme nanoreactor (meTGCT) was performed to achieve amplified antitumor efficiency via phototherapy/enzyme dynamic therapy/chemotherapy. In vitro and in vivo results exhibited that meTGCT nanoreactor was stable during long blood circulation and was able to achieve immune escape and homologous targeting capacities. Besides, the cascaded-enzyme nanoreactor could readily consume glucose and molecular O2 at tumor sites under laser irradiation to achieve tumor phototherapy/enzyme dynamic therapy and then created a more hypoxic TME to motivate chemotherapy. Significantly, this bionic cascaded-enzyme nanoreactor provided an innovative insight for the development of complementary patterns for efficient antitumor therapy.
References
R.L. Siegel, K.D. Miller, H.E. Fuchs, A. Jemal, Cancer statistics, 2021. Ca-Cancer J. Clin. 71, 7–33 (2021). https://doi.org/10.3322/caac.21654
A. Bergamo, G. Sava, Linking the future of anticancer metal-complexes to the therapy of tumour metastases. Chem. Soc. Rev. 44, 8818–8835 (2015). https://doi.org/10.1039/c5cs00134j
Y.P. Wang, W.Q. Gao, X.Y. Shi, J.J. Ding, W. Liu et al., Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature (2017). https://doi.org/10.1038/nature22393
Y. Shamay, J. Shah, M. Isik, A. Mizrachi, J. Leibold et al., Quantitative self-assembly prediction yields targeted nanomedicines. Nat. Mater. 17, 361 (2018). https://doi.org/10.1038/s41563-017-0007-z
Q. Wu, Z.G. He, X. Wang, Q. Zhang, Q.C. Wei et al., Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat. Commun. 10, 240 (2019). https://doi.org/10.1038/s41467-018-08234-2
Q. Zhang, J.J. Wu, J.J. Wang, X. Wang, C. Wu et al., A neutrophil-inspired supramolecular nanogel for magnetocaloric-enzymatic tandem therapy. Angew Chem. Int. Ed. 59, 3732–3738 (2020). https://doi.org/10.1002/anie.201915118
C. Gorrini, I.S. Harris, T.W. Mak, Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 12, 931–947 (2013). https://doi.org/10.1038/nrd4002
C. Wang, J.Q. Wang, X.D. Zhang, S.J. Yu, D. Wen et al., In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci. Transl. Med. (2018). https://doi.org/10.1126/scitranslmed.aan3682
Z.J. Zhou, J.B. Song, R. Tian, Z. Yang, G.C. Yu et al., Activatable singlet oxygen generation from lipid hydroperoxide nanoparticles for cancer therapy. Angew. Chem. Int. Ed. 56, 6492–6496 (2017). https://doi.org/10.1002/anie.201701181
D. Trachootham, J. Alexandre, P. Huang, Targeting cancer cells by ros-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8, 579–591 (2009). https://doi.org/10.1038/nrd2803
G. Chen, Y.Y. Yang, Q. Xu, M.J. Ling, H.M. Lin et al., Self-amplification of tumor oxidative stress with degradable metallic complexes for synergistic cascade tumor therapy. Nano Lett. 20, 8141–8150 (2020). https://doi.org/10.1021/acs.nanolett.0c03127
B.M. Babior, C. Takeuchi, J. Ruedi, A. Gutierrez, P. Wentworth, Investigating antibody-catalyzed ozone generation by human neutrophils. Proc. Natl. Acad. Sci. USA 100, 3031–3034 (2003). https://doi.org/10.1073/pnas.0530251100
R.J. Reiter, Oxidative processes and antioxidative defense-mechanisms in the aging brain. Faseb J. 9, 526–533 (1995)
S. Colonna, N. Gaggero, C. Richelmi, P. Pasta, Recent biotechnological developments in the use of peroxidases. Trends Biotechnol. 17, 163–168 (1999). https://doi.org/10.1016/S0167-7799(98)01288-8
E.J. Allain, L.P. Hager, L. Deng, E.N. Jacobsen, Highly enantioselective epoxidation of disubstituted alkenes with hydrogen-peroxide catalyzed by chloroperoxidase. J. Am. Chem. Soc. 115, 4415–4416 (1993). https://doi.org/10.1021/Ja00063a091
R. Renirie, C. Pierlot, J.M. Aubry, A.F. Hartog, H.E. Schoemaker et al., Vanadium chloroperoxidase as a catalyst for hydrogen peroxide disproportionation to singlet oxygen in mildly acidic aqueous environment. Adv. Synth. Catal (2003). https://doi.org/10.1002/adsc.200303008
C. Zhang, L. Zhang, W. Wu, F. Gao, R.Q. Li et al., Artificial super neutrophils for inflammation targeting and hclo generation against tumors and infections. Adv. Mater. 31, 1901179 (2019). https://doi.org/10.1002/Adma.201901179
O. Soehnlein, S. Steffens, A. Hidalgo, C. Weber, Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 17, 248–261 (2017). https://doi.org/10.1038/nri.2017.10
D. Roos, C.C. Winterbourn, Immunology - lethal weapons. Science 296, 669–671 (2002). https://doi.org/10.1126/science.1071271
Y. Liu, W.H. Zhang, Y.Y. Cao, Y. Liu, S. Bergmeier et al., Small compound inhibitors of basal glucose transport inhibit cell proliferation and induce apoptosis in cancer cells via glucose-deprivation-like mechanisms. Cancer Lett. 298, 176–185 (2010). https://doi.org/10.1016/j.canlet.2010.07.002
S.M. Kim, S.G. Roy, B. Chen, T.M. Nguyen, R.J. McMonigle et al., Targeting cancer metabolism by simultaneously disrupting parallel nutrient access pathways. J. Clin. Invest. 126, 4088–4102 (2016). https://doi.org/10.1172/JCI87148
H. Cheng, X.Y. Jiang, R.R. Zheng, S.J. Zuo, L.P. Zhao et al., A biomimetic cascade nanoreactor for tumor targeted starvation therapy-amplified chemotherapy. Biomaterials 195, 75–85 (2019). https://doi.org/10.1016/j.biomaterials.2019.01.003
W.P. Fan, N. Lu, P. Huang, Y. Liu, Z. Yang et al., Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving-like/gas therapy. Angew. Chem. Int. Ed. 56, 1229–1233 (2017). https://doi.org/10.1002/anie.201610682
C. Zhang, D.L. Ni, Y.Y. Liu, H.L. Yao, W.B. Bu et al., Magnesium silicide nanoparticles as a deoxygenation agent for cancer starvation therapy. Nat. Nanotechnol. 12, 378–386 (2017). https://doi.org/10.1038/Nnano.2016.280
J.J. Liu, Q. Chen, L.Z. Feng, Z. Liu, Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today 21, 55–73 (2018). https://doi.org/10.1016/j.nantod.2018.06.008
Y.C. Ma, Y.Y. Zhao, N.K. Bejjanki, X.F. Tang, W. Jiang et al., Nanoclustered cascaded enzymes for targeted tumor starvation and deoxygenation-activated chemotherapy without systemic toxicity. ACS Nano 13, 8890–8902 (2019). https://doi.org/10.1021/acsnano.9b02466
J. Chen, L. Liu, S.M. Motevalli, X.L. Wu, X.H. Yang et al., Light-triggered retention and cascaded therapy of albumin-based theranostic nanomedicines to alleviate tumor adaptive treatment tolerance. Adv. Funct. Mater. 28, 1707291 (2018). https://doi.org/10.1002/Adfm.201707291
T.C. Qin, Z.G. Wang, Y.Q. Wang, F. Besenbacher, M. Otyepka et al., Recent progress in emerging two-dimensional transition metal carbides. Nano-Micro Lett. 13, 183 (2021). https://doi.org/10.1007/s40820-021-00710-7
W.Q. Qian, S.W. Xu, X.M. Zhang, C.B. Li, W.Y. Yang et al., Differences and similarities of photocatalysis and electrocatalysis in two-dimensional nanomaterials: strategies, traps, applications and challenges. Nano-Micro Lett. (2021). https://doi.org/10.1007/s40820-021-00681-9
D. Wang, B. Xue, T.Y. Ohulchanskyy, Y.B. Liu, A. Yakovliev et al., Inhibiting tumor oxygen metabolism and simultaneously generating oxygen by intelligent upconversion nanotherapeutics for enhanced photodynamic therapy. Biomaterials 251, 120088 (2020). https://doi.org/10.1016/j.biomaterials.2020.120088
X. Zhou, H.D. Li, C. Shi, F. Xu, Z. Zhang et al., An apn-activated nir photosensitizer for cancer photodynamic therapy and fluorescence imaging. Biomaterials 253, 120089 (2020). https://doi.org/10.1016/j.biomaterials.2020.120089
M. Wang, M.Y. Chang, Q. Chen, D.M. Wang, C.X. Li et al., Au2Pt-PEG-Ce6 nanoformulation with dual nanozyme activities for synergistic chemodynamic therapy/phototherapy. Biomaterials 252, 120093 (2020). https://doi.org/10.1016/j.biomaterials.2020.120093
C.H. Takimoto, M.P. Chao, C. Gibbs, M.A. McCamish, J. Liu et al., The macrophage "do not eat me’ signal, cd47, is a clinically validated cancer immunotherapy target. Ann. Oncol. 30, 486–489 (2019). https://doi.org/10.1093/annonc/mdz006
X.J. Liu, Y. Pu, K. Cron, L.F. Deng, J. Kline et al., Cd47 blockade triggers t cell-mediated destruction of immunogenic tumors. Nat. Med. 21, 1209 (2015). https://doi.org/10.1038/nm.3931
Q.J. Lv, L.L. Cheng, Y. Lu, X.G. Zhang, Y.Z. Wang et al., Thermosensitive exosome-liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer. Adv. Sci. 7, 2000515 (2020). https://doi.org/10.1002/Advs.202000515
J.W. Tian, L. Ding, H.J. Xu, Z. Shen, H.X. Ju et al., Cell-specific and ph-activatable rubyrin-loaded nanoparticles for highly selective near-infrared photodynamic therapy against cancer. J. Am. Chem. Soc. 135, 18850–18858 (2013). https://doi.org/10.1021/ja408286k
J.J. Li, A. Dirisala, Z.S. Ge, Y.H. Wang, W. Yin et al., Therapeutic vesicular nanoreactors with tumor-specific activation and self-destruction for synergistic tumor ablation. Angew. Chem. Int. Ed. 56, 14025–14030 (2017). https://doi.org/10.1002/anie.201706964
L.H. Fu, C. Qi, Y.R. Hu, J. Lin, P. Huang, Glucose oxidase-instructed multimodal synergistic cancer therapy. Adv. Mater. 31, e1808325 (2019). https://doi.org/10.1002/adma.201808325
L.H. Fu, Y. Wan, C. Qi, J. He, C. Li et al., Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer therapy. Adv. Mater. 33, e2006892 (2021). https://doi.org/10.1002/adma.202006892
L.H. Fu, Y. Wan, C. Li, C. Qi, T. He et al., Biodegradable calcium phosphate nanotheranostics with tumor-specific activatable cascade catalytic reactions-augmented photodynamic therapy. Adv. Funct. Mater. 31, e2009848 (2021). https://doi.org/10.1002/adfm.202009848
X.G. Zhang, J.J. Tang, C. Li, Y. Lu, L.L. Cheng et al., A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact. Mater. 6, 472–489 (2021). https://doi.org/10.1016/j.bioactmat.2020.08.024
W. Tao, X.Y. Ji, X.D. Xu, M.A. Islam, Z.J. Li et al., Antimonene quantum dots: Synthesis and application as near-infrared photothermal agents for effective cancer therapy. Angew. Chem. Int. Ed. 56, 11896–11900 (2017). https://doi.org/10.1002/anie.201703657
Z. Li, H. Zhang, J. Han, Y. Chen, H. Lin et al., Surface nanopore engineering of 2d mxenes for targeted and synergistic multitherapies of hepatocellular carcinoma. Adv. Mater. 30, e1706981 (2018). https://doi.org/10.1002/adma.201706981
X. Han, J. Huang, H. Lin, Z. Wang, P. Li et al., 2d ultrathin mxene-based drug-delivery nanoplatform for synergistic photothermal ablation and chemotherapy of cancer. Adv. Healthcare Mater. 7, e1701394 (2018). https://doi.org/10.1002/adhm.201701394
L. Rao, B. Cai, L.L. Bu, Q.Q. Liao, S.S. Guo et al., Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano 11, 3496–3505 (2017). https://doi.org/10.1021/acsnano.7b00133
H. Cheng, J.Y. Zhu, S.Y. Li, J.Y. Zeng, Q. Lei et al., An O-2 self-sufficient biomimetic nanoplatform for highly specific and efficient photodynamic therapy. Adv. Funct. Mater. 26, 7847–7860 (2016). https://doi.org/10.1002/adfm.201603212
J.Y. Zhu, D.W. Zheng, M.K. Zhang, W.Y. Yu, W.X. Qiu et al., Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 16, 5895–5901 (2016). https://doi.org/10.1021/acs.nanolett.6b02786
W.S. Chen, K. Zeng, H. Liu, J. Ouyang, L.Q. Wang et al., Cell membrane camouflaged hollow prussian blue nanoparticles for synergistic photothermal-/chemotherapy of cancer. Adv. Funct. Mater. 27, 1605795 (2017). https://doi.org/10.1002/Adfm.201605795
J.A. Tang, D.L. Shen, T.G. Caranasos, Z.G. Wang, A.C. Vandergriff et al., Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat Commun (2017). https://doi.org/10.1038/Ncomms13724
Y.R. Zhang, J.Q. Luo, J.Y. Zhang, W.M. Miao, J.S. Wu et al., Nanoparticle-enabled dual modulation of phagocytic signals to improve macrophage-mediated cancer immunotherapy. Small 16, e2004240 (2020). https://doi.org/10.1002/smll.202004240
X. Zhou, X. Liu, L. Huang, Macrophage-mediated tumor cell phagocytosis: Opportunity for nanomedicine intervention. Adv. Funct. Mater. 31, 2006220 (2021). https://doi.org/10.1002/adfm.202006220
A. Ramesh, S. Kumar, A. Nguyen, A. Brouillard, A. Kulkarni, Lipid-based phagocytosis nanoenhancer for macrophage immunotherapy. Nanoscale 12, 1875–1885 (2020). https://doi.org/10.1039/c9nr08670f
L. Cheng, X. Zhang, J. Tang, Q. Lv, J. Liu, Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of cd47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials 275, 120964 (2021). https://doi.org/10.1016/j.biomaterials.2021.120964
X. Liu, Y. Liu, P.C. Zhang, X.D. Jin, X.G. Zheng et al., The synergistic radiosensitizing effect of tirapazamine-conjugated gold nanoparticles on human hepatoma HepG2 cells under x-ray irradiation. Int. J. Nanomed. 11, 3517–3530 (2016). https://doi.org/10.2147/Ijn.S105348
Z. Zhang, P. Oliver, J.R. Lancaster Jr., P.O. Schwarzenberger et al., Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J. 15, 303–305 (2001). https://doi.org/10.1096/fj.00-0371fje
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51773231), Shenzhen Science and Technology Project (JCYJ20190807160801664) and the Project of Key Laboratory of Sensing Technology and Biomedical Instruments of Guangdong Province (2011A060901013).
Funding
Open access funding provided by Shanghai Jiao Tong University.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., Cheng, L., Lu, Y. et al. A MXene-Based Bionic Cascaded-Enzyme Nanoreactor for Tumor Phototherapy/Enzyme Dynamic Therapy and Hypoxia-Activated Chemotherapy. Nano-Micro Lett. 14, 22 (2022). https://doi.org/10.1007/s40820-021-00761-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40820-021-00761-w