Abstract
This study has applied a geostatistical approach to analyzing and interpreting the distribution coefficient (K d) in soils collected around Hamedan, west of Iran. The samples collected from the agricultural area (about 100 ha) affected by waste water from Shahid Mofatteh power plant. Sorption experiments were carried out using solution of desired concentration of cadmium (Cd), copper (Cu), lead (Pb) and zinc (Zn) (10 and 100 mg l−1). Desorption experiments with CaCl2 0.01 M, were performed immediately following the completion of sorption experiments. Results showed that in all treatments K d100 values is lower than K d10 values. The range of K d10 (l kg−1) were as follows: Cd (253–1656), Cu (545–100000), Pb (1841–100000) and Zn (198–3115). Median K d values showed the following order of decreasing affinity in both 10 and 100 mg l−1 metal concentrations: Pb > Cu > Zn > Cd. Desorption analysis indicated that only trace amounts of heavy metal adsorbed was released. Irreversibility was highest for Pb and Cu, which the K d values were greatest. The resulting variograms of log-transformed K d10 data for Cu indicated the existence of strong spatial dependence. The variograms for K d10 Cd revealed moderate spatial structure and variograms for K d10 Pb and Zn show weak spatial dependence. For K d100, the variogram of Cd show weak spatial dependence and variograms of Cu, Pb and Zn indicate moderate spatial structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, increasing deposition of heavy metals on land [e.g. in fertilizers, pesticides, manure, sewage sludge or industrial emissions (Sparks 1995)] has given rise to considerable concern about its impact on the environment in general and human health in particular (Qin et al. 2004), particularly as regards groundwater contamination (Alloway 1995).
The sorption of deposited heavy metals by soil particles can minimize their passage into surface and subterranean waters, but at the same time creates the possibility that alteration of soil conditions may result in release of the accumulated load into the soil solution, thereby causing pollution of groundwater and/or contamination of plants (Karathanasis 1999). Thus the fate of heavy metals, and the toxic risk they pose, depend crucially on their sorption–desorption equilibria and dynamics in the soils on which they are deposited, and on how these equilibria change in response to changing environmental conditions. As sorption and its reversibility are the main processes that control the fate of heavy metals, it is necessary to examine both processes with routine laboratory tests, which need to be robust and capable of providing information on heavy metal interaction in soils without the high cost associated with field experiments and soil monitoring campaigns.
The solid–solution distribution or partition coefficient (K d) expresses the ratio of the concentrations of adsorbate adsorbed by the mass of solids to the concentrations of adsorbate remaining in the equilibrium solution, and plays a key role in many models used to define guideline values of these metals in soils and to assess related environmental risks. Distribution coefficient is often used to characterize the mobility of heavy metals in environments (Anderson and Christensen 1988).
The numerical value of K d is dependent on the specific characteristics of each soil and on the physicochemical characteristics of the sorbents investigated. Adsorbents with high K d are more strongly adsorbed to soils and are less likely to leach due to lower availability for transport in the soil solution. In contrast, adsorbents with low K d are only weakly adsorbed to soils and are more likely to leach as they are more water soluble and hence exhibit greater mobility in the soil solution. While it has been recognized that laboratory studies may give different estimates of partitioning in comparison with field conditions (Walker et al. 2001), standard batch sorption procedures are still of value for initial estimations of sorption behaviour, and in some cases have been shown to provide values comparable to field studies (Wauchope et al. 2002). Furthermore, batch studies are simple and easy to carry out and thus provide a useful means of inter-laboratory comparison for quality control purposes (Ronnefahrat et al. 1997).
Geostatistics is extensively used to assess the level of soil pollution and calculate the risk in polluted areas, by preserving the spatial distribution and uncertainty of estimates and facilitates quantification of the spatial pattern and distribution of pollutants and enables spatial interpolation and mapping (Komnitsas and Modis 2006).
In this study we determined the sorption and desorption of cadmium (Cd), copper (Cu), lead (Pb) and zinc (Zn) by samples of soils collected in Hamedan (west of Iran). For each soil, and for both sorption and desorption, we ordered the metals by the K d values obtained with 10 and 100 mg l−1concentrationof each metal in single solution. Then geostatistical methods for spatial interpolation were used to assess the heavy metals pollution in unsampled area by creating kriging map showing the K d of Cd, Cu, Pb and Zn in the study area. Finally, sorption–desorption data were used to assess the risk derived from a contamination event in the soils considered here.
Materials and methods
Studied area and sampling
The research focused on the agricultural area located about 45 km from Hamedan (west of Iran). This area covers about 100 ha. This area is affected by waste water from Shahid Mofatteh power plant. An area of 1000 × 1000 m was marked, and a grid of 100 × 100 m was established within this area. Thus, the site had 100 grid cells, 10 cells in both the x- and 1y-directions. The sampling sites were at the cell centres of the regular 100-m grid. In addition, 18 samples were taken at random in between the grid points. Figure 1 shows locations of the soil sampling sites. At each sampling point, four cores of soil were taken at a depth of 0–30 cm. Then, samples stored in plastic bags prior to chemical analysis. The soil samples were air-dried and passed through a 2-mm sieve for laboratory analyses.
Soil pH (soil:H2O ratio 1:5) was measured using a pH-meter with a glass electrode. Electrical conductivity (EC; soil:H2O ratio 1:5) was measured using an EC-meter. Textural fractions (sand, silt, clay) were determined using hydrometer method (Gee and Bauder 1986). Calcium carbonate equivalent was determined by neutralization with HCl (Rowell 1994).
Batch sorption–desorption studies
Sorption experiments were carried out using 50 ml of metal ions (Cd, Cu, Pb and Zn) solution of desired concentration (10 and 100 mg l−1). In each experiment, 2.5 g of soil sample was suspended in 25 ml of sorption solution, and after equilibration by shaking for 24 h at 25 °C in an orbital shaker this suspension was centrifuged at 4000 g for 10 min. Metal concentrations in the supernatants were determined by atomic absorption spectrophotometer and the amount of each metal sorbed by the soil was calculated by difference.
Desorption experiments were performed immediately following the completion of sorption experiments. After removal of the supernatant, 0.01 M CaCl2 solutions were added to the centrifuge tubes. The centrifuge tubes were vortexed to disperse the absorbent pellets, and the suspensions were shakes for 24 h. The suspensions were then centrifuged for 10 min at 4000 g. The Cd, Cu, Pb and Zn concentrations in the supernatant solutions were measured after desorption process and the quantities of metals retained by each soil were calculated by difference with respect to the amounts sorbed in the sorption stage.
The distribution of each metal i between soil and solution was calculated with the equation:
where C i,soil is the concentration of metal i on the soil (mg kg−1) and C i,solu is the concentration of metal i in solution (mg l−1) (Covelo et al. 2007).
Geostatistical methods
Geostatistics uses the technique of variography, i.e., calculating variogram or semi-variogram, to measure the spatial variability and dependency of a regionalized variable. Variography provides the input parameters for the spatial interpolation of kriging. The variogram function is expressed as:
where γ(h) is the semivariance (variogram), Z(x i ) is the value of the variable Z at location of x i , and N(h) is the number of pairs of sample points separated by the lag distance of h.
In order to evaluate the possible anisotropic spatial variability, surface variogram was calculated in accordance with the symmetrical property of variogram function for all variables (Pannatier 1996). Variogram plots (experimental variograms) were acquired by calculating variogram at different lags. Spherical, exponential and Gaussian models were selected in order to model experimental variograms and acquire information about the spatial structure as well as the input parameters for kriging estimation. Information generated through variography step was used to calculate sample weighting factors for spatial interpolation by an ordinary block kriging procedure.
Result and discussion
Sorption and desorption of Cd, Cu, Pb and Zn
Summary statistics of selected chemical and physical properties of the soil from the field studied are given in Table 1. The soil is neutral to slightly alkaline and has a high EC. The equivalent calcium carbonate contents varied from 3.0 to 24.0 %. The average clay content is 18 %.
Relation between heavy metals K d and soil properties was analyzed (Table 2). Close correlation existed between some soil properties and the K d. The negative correlations were observed between K d and sand content. Significant positive correlations were also found between the K d and clay content.
The dependence of K d and CaCO3 content was observed in Table 2. According to negative correlation, the K d value decreased with increasing CaCO3 content. Maybe it was due to competitive adsorption between heavy metals and Ca (Bowman et al. 1981; O’Connor et al. 1983). There may also be some effect of ionic strength on exchange selectivities. It is not possible to explain the different sensitivities of the soils to salt concentration, but they must arise from the initial soluble salt concentration and composition and the nature of the exchange phase.
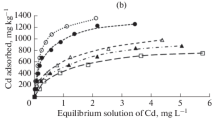
Table 3 show the value of K d measured for studied soils. Median K d values showed the following order of decreasing affinity in both 10 and 100 mg l−1 metal concentrations: Pb > Cu > Zn > Cd.
Gomes et al. (2001) and Fontes and Gomes (2003) observed that Cu and Pb were more strongly adsorbed than Cd and Zn in Brazilian soils. In Spanish soils, Vega et al. (2006) studied the adsorption and desorption of Cd, Cr, Cu, Ni, Pb and Zn and concluded that Pb, Cu and Cr had the greatest affinity in both cases. The selectivity sequences based on the K d were in line with the values of the first hydrolysis constant of the elements. Low value of K d for Cd and Zn indicate that most of the metals present in the system remain in the solution and are available for transport, chemical processes and plant uptake; on the other hand, large value of K d for Pb and Cu reflect a large affinity of solid soil components for the metals.
In all treatments K d100 values is lower than K d10 values (Table 3).The range of K d10 (l kg−1) were as follows: Cd (253–1656), Cu (545–100000), Pb (1841–100000) and Zn (198–3115).
The K d quantification was extremely dependent on the initial metal concentration, with decreases of various orders of magnitude in the K d values, especially for Pb and Zn, when the initial metal concentration was increased (Mesquita and Vieira e Silva 1996). This indicates that heavy metal sorption at low metal load is controlled by high selectivity sorption sites, in which adsorption is the main process governing sorption, and that when the metal load increases sorption sites become saturated and metal sorption takes place in low selectivity cation exchange sites. For high initial metal concentrations, accumulation in the solid phases by precipitation should not be disregarded either, especially for metals with low solubility, such as Cu (Sastre et al. 2002).
Extraction of adsorbed heavy metals with CaCl2 allowed consideration of the reversibility of heavy metal soil adsorption. Analysis indicated that only trace amounts (0.01–1.9 %) of heavy metal adsorbed was released by the 0.01 M CaCl2 solution, reflecting the highly irreversible nature of heavy metal adsorption to soils, in agreement with the high soil K d estimated in the batch experiments (Table 3). Irreversibility was highest for Pb and Cu, which the K d values were greatest, demonstrating the strong nature of the chemical binding.
Descriptive statistics
According to statistical summary of the K d for heavy metals is given in Table 3, almost the medians of all treatments were much lower than means, which is consistent with the high skewness, showing there were some very high values. Also Figs. 2 and 3 revealed that K d data was highly skewed. A distribution is considered highly positively skewed when the coefficient of skewness is much higher than 1 (Webster and Oliver 2001). A Kolmogorove–Smirnov test was computed and indicated that all K d data were significantly lognormal (p < 0.001). As in conventional statistics, a normal distribution for a variable under study is desirable in linear geostatistics (McGrath et al. 2004). Serious violation of normality, such as too high skewness, can impair the variogram structure and the kriging results. It is often observed that environmental variables are lognormal or positively skewed, and data transformation is necessary to normalize such data sets. Taking logarithms of the K d data removed most of the skewness (Figs. 2, 3).
Geostatistical analysis
Experimental variograms were computed on the log-transformed data. The directional variograms suggest a fairly isotropic spatial distribution of the log-adsorption heavy metals values, so that the omnidirectional variograms with the fitted models were considered. Linear, Gaussian, spherical and exponential functions were fitted to the experimental variograms.
The selection of appropriate model was based on qualitative interpretation of which model best represented the overall behavior of the experimental variogram. The numerical results are given in Table 4. To define the degree of spatial dependency, spatial class ratios similar to those presented by Cambardella et al. (1994) were adopted. That is the ratio of nugget variance (noise) to total variance (sill) multiplied by 100. If the ratio of spatial class was < 25 % then the variable would be considered to be strongly spatially dependent; if the ratio was between 25 and 75 %, the variable was regarded as moderately spatially dependent; and if the ratio was more than 75 %, the variable was considered weakly spatially dependent.
The resulting variograms of log-transformed K d10 data for Cu indicated the existence of strong spatial dependence. The variograms for K d10 Cd revealed moderate spatial structure and variograms for K d10 Pb and Zn show weak spatial dependence. For K d100, the variogram of Cd show weak spatial dependence and variograms of Cu, Pb and Zn indicate moderate spatial structure.
For mapping the K d data, ordinary kriging system was used along with isotropic variograms to estimate K d values at unobserved locations. For all treatments, log-transformed data used for kriging interpolation and then the estimated kriged values were back-transformed. Optimal kriging parameters were found based on the results from the cross validation procedure.
Figures 4 and 5, respectively, presents the spatial patterns of the K d10 and K d100 of heavy metals adsorption values generated from their variograms and corresponding kriging systems. The kriged maps show the spatial variation of soil K d for heavy metal adsorption estimates.
Conclusion
Laboratory measurements of Cd, Cu, Pb and Zn K d values for 109 soil samples revealed a large range of values: from a few to several thousand l kg−1. The soil distribution coefficient of Cd, Cu, Pb and Zn was found to be related to the some soil properties. The negative correlations were observed between K d and sand content, also negative correlation were between K d and CaCO3 content. Significant positive correlations were found between the K d and clay content.
References
Alloway BJ (1995) Soil processes and the behaviour of metals. In: Alloway BJ (ed) Heavy metals in soils. Blackie Academic & Professional, London, pp 11–37
Anderson PR, Christensen TH (1988) Distribution coefficients of Cd, Co, Ni, and Zn in soils. J Soil Sci 39:15–22
Bowman RS, Essington ME, O’Connor GA (1981) Soil sorption of nickel: influence of solution composition. Soil Sci Soc Am J 45:860–865
Cambardella CA, Moorman TB, Novak JM, Parkin TB, Karlen DL, Turco RF, Konopka AE (1994) Field scale variability of soil properties in central Iowa soils. Soil Sci Soc Am J 58:1501–1511
Covelo EL, Vega FL, Andrade ML (2007) Simultaneous sorption and desorption of Cd, Cr, Cu, Ni, Pb and Zn in acid soils II: soil ranking and influence of soil characteristics. J Hazard Mater 147:862–870
Fontes MPF, Gomes PC (2003) Simultaneous competitive adsorption of heavy metals by the mineral matrix of tropical soils. Appl Geochem 18:795–804
Gee GW, Bauder JW (1986) Particle size analysis. In: Klute A (ed) Methods of soil analysis. Part 1. Physical and mineralogical methods. Agronomy monograph 9, 2nd edn. ASA and SSSA, Madison, pp 404–407
Gomes PC, Fontes MPF, da Silva DG, Mendonc ES, Netto AR (2001) Selectivity sequence and competitive adsorption of heavy metals by Brazilian soils. Soil Sci Soc Am J 65:1115–1121
Karathanasis AD (1999) Subsurface migration of copper and zinc mediated by soil colloids. Soil Sci Soc Am J 63:830–838
Komnitsas K, Modis K (2006) Soil risk assessment of As and Zn contamination in a coal mining region using geostatistics. Sci Tot Environ 371:190–196
McGrath D, Zhang C, Carton CT (2004) Geostatistical analyses and hazard assessment on soil lead in Silvermines area, Ireland. Environ Pollut 127(2):239–248
Mesquita ME, Vieira e Silva JM (1996) Zinc adsorption by calcareous soil. Copper interaction. Geoderma 69:137–146
O’Connor GA, Essington ME, Elrashidi M, Bowman RS (1983) Nickel and zinc sorption in sludge-amended soils. Soil Sci 135:228–235
Pannatier Y (1996) Variowin: software for spatial data analysis in 2D. Springer, New York
Qin F, Shan X, Wei B (2004) Effects of low-molecular-weight organic acids and residence time on desorption of Cu, Cd, and Pb from soils. Chemosphere 57:253–263
Ronnefahrat IU, Traub-Eberhardru U, Kordel W, Stein B (1997) Comparison of the fate of isoproturon in small and large scale water/sediment systems. Chemosphere 35:181–189
Rowell DL (1994) Soil science: methods and applications. Longman Scientific and Technical, Harlow
Sastre J, Sahuquillo A, Vidal M, Rauret G (2002) Determination of Cd, Cu, Pb and Zn in environmental samples: microwave-assisted total digestion versus aqua regia and nitric acid extraction. Anal Chim Acta 462:59–72
Sparks DL (1995) Environmental soil chemistry. Academic Press, New York
Vega FA, Covelo EF, Andrade ML (2006) Competitive sorption and desorption of heavy metals in mine soils: influence of mine soil characteristics. J Colloid Interface Sci 298:582–592
Walker A, Jurado-Exposito M, Bending GD, Smith VJR (2001) Spatial variability in the degradation rate of isoproturon in soil. Environ Pollut 111(3):407–415
Wauchope RD, Yeh S, Linders JBHJ, Kloskowski R, Tanaka K, Rubin B, Katayama A, Kordel W, Gerstl Z, Lane M, Unsworth JB (2002) Pesticide soil sorption parameters: theory, measurement, uses, limitations and reliability. Pest Manag Sci 58(5):419–445
Webster R, Oliver MA (2001) Geostatistics for environmental scientists. Wiley, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merrikhpour, H., Jalali, M. Geostatistical assessment of solid–liquid distribution coefficients (K d) for Cd, Cu, Pb and Zn in surface soils of Hamedan, Iran. Model. Earth Syst. Environ. 1, 47 (2015). https://doi.org/10.1007/s40808-015-0048-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40808-015-0048-8