Abstract
Extant research supports the hypothesis that biometric indicators of life history (LH) strategy, such as the timing of sexual debut, are calibrated in response to cues sampled early in development (before age 7). Herein, we theorize that the experience of sexual debut itself may further calibrate women’s LH-related behavioral phenotypes across adolescence by modulating subsequent investment in mating effort, which is associated with faster (vs. slower) LH speed. We tested this hypothesis using longitudinal data, which included Q-Sort LH scores at ages 14, 18, and 23, and a report of whether or not sexual debut occurred between ages 14 and 18. Results demonstrated that women (but not men) who experienced their sexual debut between the ages of 14 and 18 showed greater relative acceleration of LH speed during this period than women who had not debuted by age 18. However, these same effects had weakened by age 23—which underscores the broader idea that LH-related behavioral phenotypes exhibit substantial cue-based plasticity across adolescence and into adulthood. Although alternative explanations for the observed patterns remain unfalsified, these findings are consistent with the hypothesis that developmental LH trajectory is regulated in relation to the timing of sexual debut in adolescence. This possibility could be further investigated in future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Life history (LH) theory originated as an explanation for changes in organisms’ reproductive strategies under conditions of increasing environmental constraints (Pianka 1970) with a rapid or quantity reproductive strategy predominating with loose constraints and a more restrained quality reproductive strategy prevailing as constraints tightened. Promislow and Harvey (1990) proposed that these strategies accounted for a collation of differences between species, finding significant covariance amongst such indices as rate of maturation, litter size, and maternal investment in offspring. Similarly, LH theory was applied to human group (Rushton 1985) and individual differences (Draper and Harpending 1982) such that individual differences in rate of maturation, sexual debut, and parental investment covary forming LH strategies between people. Individual differences in LH strategy are now described as varying in speed along a fast to slow continuum. Faster LH strategies are defined by earlier maturation and sexual debut, higher frequency of sexual intercourse with less stable pair bonds, and reduced parental investment. Slow LH strategies are defined by later maturation and sexual debut, lower frequency of sexual intercourse with more stable pair bonds, and increased parental investment.
The earliest models on the development of LH strategies stressed the critical influence of early childhood experiences that would have predicted LH-relevant environmental parameters across human ancestral environments (Belsky et al. 1991; Draper and Harpending 1982; Ellis et al. 2003). For example, Belsky et al. (1991) proposed that the quality of parental attachment in childhood is the key determinant in calibrating developmental trajectories toward a fast or slow LH strategy. Secure parental attachment guides development down the path of a slow LH strategy, with the corresponding phenotypic traits geared toward investment in future reproduction via delayed maturation and sexual debut. Alternatively, insecure parental attachment leads to a fast LH strategy with early maturation and earlier sexual debut, unstable pair bonds, and low parental investment in offspring. At several points in their article, Belsky et al. (1991) reiterate Draper and Harpending's (1982) position that early childhood experiences (explicitly stated as occurring in the first 5–7 years of life) are critical in setting the fast (called Type I in the article) or slow (called Type II in the article) developmental trajectories. This position is summarized in the following quote from the article (p. 650):
A central tenet of the theory we advance is that a principal evolutionary function of early experience—the first 5–7 years of life—is to induce in the child an understanding of the availability and predictability of resources (broadly defined) in the environment, of the trustworthiness of others, and of the enduringness of close interpersonal relationships, all of which will affect how the developing person apportions reproductive effort.
The position that there is a critical period wherein LH strategy crystalizes in the middle of the first decade may be too strong. While recognizing a significant degree of heritability in LH strategy (Figueredo et al. 2004), additional research and advancing theory on the development of LH strategies in reaction to proximate somatic (Nettle et al. 2013; von Rueden et al. 2015) and environmental (e.g., Brumbach et al. 2009) cues both suggest that significant plasticity in LH strategies remains after the 5–7 years of age period identified by Belsky et al. (1991). On the other hand, the issue of the importance of early versus late developmental experience in helping shape LH strategy remains an open question with some findings pointing to the importance of early experiences relative to those experienced later in life. For example, Simpson et al. (2012) found that the level of environmental predictability experienced in the first 5 years of life had a significant effect on LH strategy at age 23, but the level of environmental predictability experienced in later childhood and adolescence did not.
The most comprehensive model on how factors beyond the age of seven may impact LH strategies has been proposed by Del Giudice (2009), who suggested that early adolescence, specifically adrenarche, acts as an additional switch point in the development of LH strategies. This idea has been advanced further by Ellis et al. (2012) and Del Giudice and Belsky (2011), both of whom extended the putative period of cue-based plasticity through puberty and into later adolescence. In support of the idea that LH strategy may be continually adjusted across development, well past the age 5 to 7 period, Dunkel et al. (2015) recently found that maternal authoritative parenting received during adolescence uniquely predicted later LH strategy above and beyond the calibration effects of maternal behavior in early childhood.
The current investigation further explores the idea of continued plasticity in the development of LH strategy in adolescence. To that end, we examined another possible contributing factor in developing LH strategies during adolescence: sexual debut (i.e., age of first sex). While sexual debut has most often been used as a type of dependent variable, the timing of which is indicative of LH strategy (see Ellis et al. 2003), there is precedence indicating that it may also serve as an LH cue acting to calibrate LH speed. For example, Vigil et al. (2005) tested the possibility that childhood sexual abuse accelerates LH speed. Vigil et al. (2005) surveyed over 600 women between the ages of 18 and 56 from the Midwestern and Southwestern USA concerning whether or not they experienced childhood sexual abuse, assessed by the single item “I was sexually abused before age 14,” and the subsequent ages at which they experienced a number of indicators of LH strategy (e.g., sexual debut). While, as recognized by Vigil et al. (2005), childhood sexual abuse differs from consensual intercourse later in life in many clear and important ways, the authors proposed that the act of intercourse itself may be one reason why childhood sexual abuse accelerates LH speed. Indeed, they found that women who reported being victims of childhood sexual abuse also reported earlier menarche, earlier timing of first consensual intercourse, and giving birth to their first child earlier in life.
Thus, the primary hypothesis of the present study is that sexual debut accelerates LH speed. The general prediction that LH strategy is facultatively calibrated in ontogeny only holds to the degree that particular developmental cues were ancestrally valid predictors of variable world states (Frankenhius and Panchanathan 2011). Although certain aspects of early environment may contain some valid LH-relevant information, recent theoretical work suggests that internal (e.g., somatic) cues may in many cases have much higher cue validities than external cues (Nettle et al. 2013). This would be especially true for internal cues associated with sexual debut (e.g., psychological experience of having sex; “breaking” of the hymen), which provide highly valid information regarding one’s actual progression into a sexually active developmental stage wherein it would have been ancestrally functional to invest in reproductive effort (at the expense of investment in growth, etc.). If so, selection may have favored facultative adaptations designed to accelerate LH speed in response to sexual debut in adolescence, such that the loss of virginity promotes investment in current reproduction via increased allocation of resources toward mating effort. There is one addendum to this primary hypothesis. Given that the transition from virginity to sexual activity entails much greater potential costs for females than males (Trivers 1972), the tradeoff between growth and reproduction as a function of virginity loss would have been especially pronounced among ancestral women (Vigil et al. 2005). We correspondingly predict that sexual debut will accelerate LH speed to a greater degree in adolescent females than males. We examine these predictions using data from a longitudinal study conducted by Block and Block (2006a) which includes both repeated measures of LH strategy from adolescence and into adulthood based on the California Adult Q-sort (Block 1978; Sherman et al. 2013) and information concerning the timing of sexual debut.
Method
The Block and Block data (2006b) and documentation files were downloaded via the internet from the Henry A. Murray Research Archive. The Block and Block longitudinal study lasted 30 years with multiple waves of data collection and extensive testing taking place at each wave. Participants were initially recruited from two preschools in Berkeley, CA, when they were 3 to 4 years of age. Demographic information including participant sex, ethnicity, and socioeconomic status was obtained from data files in the first wave of data collection.
Data from waves at ages 14, 18, and 23 were also used in the analyses. Because the investigation concerned the transition in LH strategy from age 14 to ages 18 and 23, age 14 serves as the base year for the analyses. At age 14, the sample included 106 participants, of which 54 were female (51 %). Of the 106 participants, 71 were White, 27 were Black, 5 were Asian-American, and 4 were classified as “Other.” Due to the small number of non-Black minorities, participants were grouped as White (67 %) and non-White (33 %).
Measures
LH Strategy
LH strategy was measured using the California Q-sort (CAQ; Block 1978). The CAQ includes a set of 100 items, and the items are to be Q-sorted or arranged in piles based upon the degree to which they describe an individual. Sherman et al. (2013) developed an LH strategy template allowing LH strategy to be measured using the CAQ, and this measure was recently validated and slightly modified by Dunkel et al. (2014) using the Block and Block data.
At ages 14, 18, and 23 between four and six, trained raters independently used the CAQ to rate each participant’s personality. The aggregate of these ratings was then correlated with the LH strategy template. The correlation of the participants’ sort with the template is their score. Higher scores (i.e., stronger correlations) represent a slower LH strategy. A sample item that is reflective of slow LH speed is “sympathetic/considerate.” A sample item reflective of fast LH speed is “unable to delay gratification.”
Sexual Debut
At age 18, participants responded either in the affirmative or negative as to whether they had “lost their virginity” within the last 3 years. Forty-two participants indicated that they had “lost their virginity” in the last 3 years and 60 participants reported that they had not.
Covariates
Sex, ethnicity (dichotomized as White and non-White due to small sample size), and socioeconomic status (SES), measured by Warner’s Index of SES (1949) when participants were 4 years of age, were demographic covariates.
In addition to the demographic covariates, we included four other covariates: intelligence, maternal and paternal parenting style, and the relationship to the person with whom the participants had intercourse for the first time. Intelligence was measured at age 18 by the Wechsler Adult Intelligence Scale (WAIS). Intelligence was included as a covariate to control for the influence of the participants’ cognitive ability, which could plausibly limit one’s ability to reason about the potential consequences of consenting to intercourse. Maternal and paternal authoritative parenting was measured by the Child Rearing Practices Report (CRPR; Block 1965; Kochanska et al. 1989). The CRPR is a 91-item Q-set that participants used at age 18 to rate both their mother’s and father’s parenting style. Authoritative parenting was included as a covariate because it has been found to influence the development of LH strategies during adolescence (Dunkel et al. 2015). The last covariate was the level of intimacy the participants had with whom they reported having lost their virginity (knew each other a little, friend, going steady, or engaged). This covariate was included to test the possibility that the level of partner intimacy played a role in the effect of losing one’s virginity.
Results
Preliminary Analyses
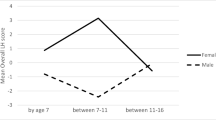
Descriptive statistics (means and standard deviations for the LH scores) by age, sex, and sexual debut are seen in Table 1. LH scores appeared to increase after age 14, which presumably reflects a normative developmental trajectory in the transition from adolescence to adulthood. In addition, males exhibit a faster LH strategy at all ages. These trends were confirmed in a repeated measures analysis of variance (ANOVA) with the within-subjects variable of LH strategy at ages (14, 18, and 23) and between subjects variable of participant sex. The within-subjects effect was significant, F(2,99) = 9.16, p < .001, η p 2 = .16, and so was the between-subjects effect for participant sex, F(1, 100) = 5.52, p < .05, η p 2 = .05. The interaction between variables was not significant and explained little variance, η p 2 = .005. Correlations between sexual debut and LH strategy at the three focal ages were as follows: age 14 r pb (102) = −.06; age 18 r pb (100) = −.24, p < .05; and age 23 r pb (99) = −.14.
Hypothesis Testing
To test the hypothesis that sexual debut occurring in adolescence accelerates LH speed and that this effect is more pronounced in females, a series of partial correlations was conducted looking at the association between sexual debut and LH strategy at ages 18 and 23. Note that partial correlation is used to control for variance in both the criterion (e.g., LH strategy at age 18) and the predictor variable (e.g., sexual debut). Thus, partial correlation is more fitting for testing the hypotheses than an alternatives such as repeated measures ANOVA or regression (i.e., semi-partial correlation) in which the criterion (e.g., LH strategy at age 18) is not altered.
Initially, and most importantly, the partial correlations controlled for LH speed at age 14. Then, in succession, additional partial correlations were calculated; in each case, an additional covariate was added. After LH at age 14, the covariates were added in this order SES, ethnicity, IQ, maternal authoritative parenting, paternal authoritative parenting, and the intimacy level with the partner. The results can be seen in Table 2. For the full sample when controlling just for LH strategy at age 14, sexual debut was correlated with LH strategy at age 18, but not at age 23. Additionally, the relationship between sexual debut and LH strategy at age 18 fluctuated as covariates were added.
When the sample was split by sex, for females, the relationship between sexual debut and LH strategy at age 18 remained as covariates were added. There was an additional trend; the addition of covariates led to the strengthening of the relationship between sexual debut and LH strategy at age 23. To further explore this trend in females, two change scores were computed by subtracting LH strategy scores at age 14 from LH strategy scores at age 18 and by subtracting LH strategy scores at age 14 from LH strategy scores at age 23. Thus, positive scores indicate change in which LH speed slowed. Between ages 14 and 18, those who had their sexual debut during adolescence had a mean change score of (M = 4.80, SD = 16.72), while those who did not have intercourse during adolescence had a mean change score of (M = 13.45, SD = 25.32). Between the ages of 14 and 23, those who had their sexual debut during adolescence had a mean change score of (M = 7.48, SD = 29.05), while those who did not have intercourse during adolescence had a mean change score of (M = 13.20, SD = 28.03). While the differences in the change scores are not statistically significant given the limited statistical power of between-subjects change score comparisons relative to the partial correlation analyses described above, the change scores do assist in interpreting the statistically significant correlations.
Discussion
Recent research findings and theoretical advancements have led to the prediction that plasticity in the development of LH strategies extends beyond the period of early childhood as first envisioned by Belsky et al. (1991). Following these changes in the understanding of LH strategies, it was hypothesized that the timing of sexual debut may impact developing LH strategy, such that sexual debut accelerates LH speed in adolescence. Additionally, it was expected that this effect may be more pronounced in females. To test these hypotheses, the relationship between sexual debut between ages 14 and 18 and LH strategy at ages 14, 18, and 23 was examined.
The results suggest several possibilities. The partial correlations show that, for females, sexual debut may calibrate the developmental trajectory of LH strategy. After controlling for LH strategy in early adolescence and a number of potential confounds, female participants who engaged in sexual intercourse during adolescence had a faster LH speed in late adolescence and possibly into young adulthood. These individual differences are embedded in developmental change wherein raw LH scores increased with age. As such, the difference scores may provide a clue to help interpret these findings. Specifically, the observed age-related increase in LH scores was greater for those who did not engage in intercourse during adolescence.
Given that a fast LH strategy is defined in part by an individual’s degree of investment in mating effort, this pattern is consistent with the idea that sexual debut acts to upregulate women’s engagement in risky tactics of intrasexual competition and/or mate attraction. This facultative response makes functional sense under our hypothesis, which posits that experiencing sexual intercourse would have been an ancestrally valid cue of having progressed into a LH stage during which the acquisition of reproductive benefits (whether via behavioral investment or genetic quality) would have become an adaptive problem of immediate and crucial importance. However, this hypothesis leaves unclear whether we should expect a longer-lasting developmental shift toward a fast LH speed that persists into adulthood. The fact that women’s sexual debut experienced between 14 and 18 is not as strongly associated with LH acceleration by age 23 suggests that virginity loss initiates a shift toward investment in mating effort that is (potentially) temporary and subject to further recalibration as developmental events unfold. For example, some individuals who were temporarily calibrated toward a faster LH profile post-debut may later begin to invest heavily in future reproduction as they enter subsequent LH stages. Likewise, some women who remain abstinent between 14 and 18 may later become sexually active and consequently experience a temporary LH acceleration of their own. In either case, further life stage-linked shifts in LH after age 18 would be expected to attenuate the association of events in early adolescence with later outcomes.
Conclusion and Limitations
The main premise of the current study is that LH variation is regulated over ontogeny not only in response to early cues but also in relation to cues experienced later in development. With few exceptions (e.g., Vigil et al. 2005), “biometric” indices have been viewed as outcome measures that signify individual differences in developing LH strategies (for an overview of biometric measures, see Copping et al. 2014; Figueredo et al. 2014; Figueredo et al. 2015). Although our correlational findings are subject to alternative explanations, they support the hypothesis that the experience of sexual debut may act to calibrate subsequent LH speed via promotion of investment in mating effort. At the very least, the data indicate that the trajectory of LH development in adolescence and the timing sexual debut are correlated phenotypic components.
However, some specific limitations should be noted. While a number of covariates were included, genetic influence on both the timing of sexual debut and LH strategy (e.g., Eisenberg et al. 2007) cannot be ruled out. On the other hand, given that one of the controls was LH strategy at age 14, genetic explanations for the findings must move away from basic additive models, and additive effects appear to account for most of the heritability in psychological traits (e.g., Polderman et al. 2015). Another limitation of the finding is that the effect of sexual debut on LH strategy speed appears to dissipate as participants move from late adolescence into adulthood. Data points in later adulthood might shed light on whether this trend continued, but Q-sort data was last collected at the age 23 wave of data collection in the Block and Block (2006a) longitudinal study. As such, we hope that this article stimulates future research that can disambiguate the associations of biometric and psychosocial events with the development of LH strategy from adolescence into later adulthood.
References
Belsky, J., Steinberg, L., & Draper, P. (1991). Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Development, 62, 647–670.
Block, J. H. (1965). The child-rearing practices report (CRPR): a set of Q items for the description of parental socialization attitudes and values. Berkeley: University of California, Institute of Human Development.
Block, J. (1978). The Q-sort method in personality assessment and psychiatric research. Palo Alto: Consulting Psychologists Press. Originally published in 1961.
Block, J., & Block, J. H. (2006a). Venturing a 30-year longitudinal study. American Psychologist, 61, 315–327.
Block, J., & Block, J. H. (2006b). Block and block longitudinal study, 1969 – 1999. Murray Research Archive [Distributor]. V1 [Version]. Retrieved from: http://dvn.iq.harvard.edu/dvn/dv/mra/faces/study/StudyPage.xhtml?globalId=hdl:1902.1/NGQCIPIDUK&studyListingIndex=2_e3e414a92b8ffc99ae5ad3c701fb.
Brumbach, B. H., Figueredo, A. J., & Ellis, B. J. (2009). Effects of harsh and unpredictable environments in adolescence on development of life history strategies: a longitudinal test of an evolutionary model. Human Nature, 20, 25–51.
Copping, L. T., Campbell, A., & Muncer, S. (2014). Psychometrics and life history theory: the structure and validity of the High K Strategy Scale. Evolutionary Psychology, 12, 200–222.
Del Giudice, M. (2009). Sex, attachment, and the development of reproductive strategies. Behavioral and Brain Sciences, 32, 1–67.
Del Giudice, M., & Belsky, J. (2011). The development of life history strategies: toward a multi-stage theory. In D. M. Buss & P. H. Hawley (Eds.), The evolution of personality and individual differences (pp. 154–176). New York: Oxford University Press.
Draper, P., & Harpending, H. (1982). Father absence and reproductive strategy: an evolutionary perspective. Journal of Anthropological Research, 38(3), 255–273.
Dunkel, C. S., Summerville, L. A., Mathes, E. W., & Kesserling, S. N. (2014). Using the California Q-sort measure of life history strategy to predict sexual behavioral outcomes. Archives of Sexual Behavior. doi:10.1007/s10508-014-0445- 5.
Dunkel, C. S., Mathes, E. W., Kesserling, S. N., Decker, M. L., & Kelts, D. J. (2015). Parenting influence on the development of life history strategy. Evolution and Human Behavior. doi:10.1016/j.evolhumbehav.2015.02.006.
Eisenberg, D. T. A., Campbell, B., MacKillop, J., Modi, M., Dang, D., Koji Lum, J., & Wilson, D. S. (2007). Polymorphisms in the dopamine D4 and D2 receptor genes and reproductive and sexual behaviors. Evolutionary Psychology, 5, 696–715.
Ellis, B. J., Bates, J. E., Dodge, K. A., Fergusson, D. M., Horwood, L. J., Pettit, G. S., & Woodward, L. (2003). Does father absence place daughters at special risk for early sexual activity and teen pregnancy? Child Development, 74, 801–821.
Ellis, B.J., Del Giudice, M., Dishion, T.J., … Wilson, D.S. (2012). The evolutionary basis of risky adolescent behavior: implications for science, policy, and practice. Developmental Psychology, 48, 598–623.
Figueredo, A. J., Vásquez, G., Brumbach, B. H., & Schneider, S. M. R. (2004). The heritability of life history strategy: the K-factor, covitality, and personality. Social Biology, 51, 121–143.
Figueredo, A.J., Wolf, P.S.A., Olderbak, S.G., Gladden, P.R., Fernandes, H.B.F., Wenner, C., . . . Rushton, J.P. (2014). The psychometric assessment of human life history strategy: a meta-analytic construct validation. Evolutionary Behavioral Sciences, 8, 148–185.
Figueredo, A. J., Cabeza de Baca, T., Black, C. J., Garcia, R. A., Fernandes, H. B. F., Wolf, P. S. A., & Woodley of Menie, M. A. (2015). Methodologically sound: evaluating the psychometric approach to the assessment of human life history [Reply to Copping, Campbell, and Muncer, 2014]. Evolutionary Psychology, 13, 299–338.
Frankenhius, W. E., & Panchanathan, K. (2011). Balancing sampling and specialization: an adaptationist model of incremental development. Proceedings of the Royal Society of London B, 278, 3558–3565.
Kochanska, G., Kuczynski, L., & Radke-Yarrow, M. (1989). Correspondence between mother’s self-reported and observed child-rearing practices. Child Development, 60, 56–63.
Nettle, D., Frankenhuis, W. E., & Rickard, I. J. (2013). The evolution of predictive adaptive responses in human life history. Proceedings of the Royal Society of London B, 280, 20131343.
Pianka, E. R. (1970). On r- and K- selection. American Naturalist, 104, 592–597.
Polderman, T. J., Benyamin, B., de Leeuw, C. A., Sullivan, P. F., van Bochoven, A., Visscher, P. M., & Posthuma, D. (2015). Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nature Genetics. doi:10.1038/ng.3285.
Promislow, D. E. L., & Harvey, P. H. (1990). Living fast and dying young: a comparative analysis of life-history variation among mammals. Journal of Zoology, 220, 417–437.
Rushton, J. P. (1985). Differential K theory: the sociobiology of individual and group differences. Personality and Individual Differences, 6, 441–452.
Sherman, R. A., Figueredo, A. J., & Funder, D. C. (2013). The behavioral correlates of overall and distinctive life history strategy. Journal of Personality and Social Psychology, 105, 873–888.
Simpson, J. A., Griskevicius, V., Kuo, S. I., Sung, S., & Collins, A. W. (2012). Evolution, stress, and sensitive periods: the influence of unpredictability in early versus late childhood on sex and risky behavior. Developmental Psychology, 48, 674–686.
Trivers, R. L. (1972). Parental investment and sexual selection. In B. Campbell (Ed.), Sexual selection and the descent of man, 1871–1971 (pp. 136–179). Chicago: Aldine.
Vigil, J. M., Geary, D. C., & Byrd-Craven, J. (2005). A life history assessment of early childhood sexual abuse in women. Developmental Psychology, 41, 553–561.
von Rueden, C. V. R., Lukaszewski, A. W., & Gurven, M. (2015). Adaptive personality calibration in a human society: effects of embodied capital on prosocial traits. Behavioral Ecology. doi:10.1093/beheco/arv051.
Warner, W. L. (1949). Social class in America: a manual of procedure for the measurement of social status. Chicago: Science Research Associates.
Acknowledgments
This project was made possible by the Henry A. Murray Research Archive which is housed by the Institute for Quantitative Social Science at Harvard University. The data employed in this study derive from a 30-year longitudinal study begun with 128 3-year-old girls and boys, planned and conducted by Jack and Jeanne H. Block, involving a sequence of nine independent assessments based on personality and cognitive life, observational, test, and self-report (LOTS) measures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dunkel, C.S., Lukaszewski, A.W. Is Sexual Debut in Adolescence an Accelerator of Life History Strategy?. Evolutionary Psychological Science 1, 201–206 (2015). https://doi.org/10.1007/s40806-015-0023-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40806-015-0023-7