Abstract
Background
Adherence to antipsychotic medication and care discontinuity remain a challenge to healthcare practitioners providing care to patients with schizophrenia.
Objective
This study used real-world data from a US hospital-based, all-payer database to examine clinical quality measures among patients with schizophrenia initiated on a long-acting injectable (LAI) or switched to a new oral antipsychotic medication (OAP) following a hospitalization.
Methods
A retrospective cohort study using the PINC AI™ Healthcare Database compared two cohorts of patients with schizophrenia on post-index hospitalization clinical quality and care continuity endpoints. Patients initiated on an LAI (n = 7292) or switched to a new OAP (n = 31,956) during an index hospitalization between April 2017 and April 2020 were included. Propensity score weighting addressed differences in patient, hospital, and clinical characteristics between the two cohorts.
Results
Patients who initiated an LAI experienced significantly greater adjusted 30-day antipsychotic medication continuation to index therapy, higher rate of 30-day outpatient follow-up care, longer mean time to discontinuation of index therapy, and lower risk of discontinuing their index treatment compared to patients who switched to a new OAP (all p values < 0.001). Probability of 30-day antipsychotic medication continuation was significantly higher for LAI initiators than for patients who switched to a new OAP, even after controlling for patient, clinical, and hospital characteristics (adjusted odds ratio = 1.2, 95% CI 1.1–1.3, p < 0.001).
Conclusion
Patients who initiated an LAI in a hospital setting experienced better clinical quality and care continuity outcomes compared to patients who were switched to a new OAP. These findings may be useful in identifying solutions to help improve the quality of medication management post-hospital discharge among patients with schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with schizophrenia who initiated a long-acting injectable (LAI) demonstrated significantly better clinical quality outcomes following a psychiatric hospitalization compared to oral antipsychotic (OAP) switchers. |
Despite the high index medication discontinuation rate observed across both treatment groups, more LAI patients were adherent to their medication, stayed on it longer, and were more likely to receive follow-up care within 30 days of hospital discharge compared to OAP switchers. |
These findings may be useful in identifying solutions to help improve the quality of medication management and care continuity during the critical period between hospitalization and aftercare support. |
1 Introduction
Schizophrenia is a serious, chronic, and debilitating mental illness with prevalence estimates ranging from 0.25 to 0.64% [1]. The economic burden of schizophrenia in the USA is substantial, with total costs estimated by one study at $282 billion in 2020. About one-third of the total excess economic burden of schizophrenia is driven by caregiver costs, with lifetime costs estimated at $3.8 million per individual with schizophrenia [2].
Low adherence to antipsychotic medication among patients with schizophrenia has been well documented and is also tied to care continuity. Innovative interventions, including pharmacological approaches, patient and family education, cognitive-behavioral approaches, motivational interviewing, case management, collaborative care, and combinations of these strategies have been employed to improve medication adherence among patients with schizophrenia [3,4,5]. Despite use of these strategies, adherence to antipsychotic medication is only about 42%, with large variation reported in the published literature [3, 6]. A recent meta-analysis indicated that drug-related factors, disease factors, problem behavior, low income and quality of life, and personal characteristics appear to be risk factors for medication adherence in people with schizophrenia, while support level, positive attitude, and behavior appear to be protective factors [7].
Receiving timely follow-up care after hospital discharge is critical, given that patients with a recent hospitalization and their caregivers are particularly vulnerable during this time [8]. Lack of timely follow-up care when a patient transitions from the inpatient setting to outpatient care can result in medication discontinuation, rehospitalization, and other negative outcomes, such as poor symptom control, relapse, and impacts on families and caregivers [9,10,11,12].
In addition to patient-based strategies to bolster medication adherence and care continuity, larger scale pay-for-performance initiatives for healthcare systems and providers have been implemented regionally and nationally with a focus on adherence to antipsychotic medications and timely follow-up care within 30 days after a psychiatric hospitalization [13,14,15]. Notable national initiatives include the Centers for Medicare and Medicaid Services (CMS) Inpatient Psychiatric Facility Quality Reporting Program and the National Committee on Quality Assurance (NCQA) Healthcare Effectiveness Data and Information Set (HEDIS®) quality measures to improve care for adults with serious mental illness.
Among pharmacological strategies to improve medication adherence, previous studies have shown that patients initiated on long acting injectables (LAIs) experience better health outcomes compared to patients treated with oral antipsychotics (OAPs), including lower odds of hospitalization, fewer ER visits, and higher medication adherence [3, 16,17,18]. Given that low medication adherence among individuals with schizophrenia is a frequent cause of relapse, LAIs have been prescribed to patients with frequent relapses and poor adherence to OAPs. Second-generation (SGA) LAIs have been shown to reduce healthcare resource utilization and costs compared to OAPs in patients with schizophrenia because of reduced dosing and delivery, and improved adherence [19,20,21].
In a recent systematic review of real-world studies on the use of LAIs versus OAPs among patients with schizophrenia, Lin and colleagues [22] found that patients initiated on an LAI compared to patients treated with OAPs had lower odds of hospitalization, fewer hospitalizations, and fewer ER visits. Although initiating an LAI was associated with higher per-patient-per-year pharmacy costs compared to patients treated with OAPs, it was offset by lower medical costs. Patients initiated on an LAI also had higher odds of being adherent to their medication.
There is limited research investigating clinical quality measures and care continuity outcomes following a recent inpatient hospitalization among patients with schizophrenia. Given the importance of the critical period between hospital discharge and timely aftercare support, this study utilized data from a hospital-based, administrative database to compare medication continuation and clinical quality measures among patients with schizophrenia initiated on an LAI or switched to a new OAP following an inpatient hospitalization. This study also offers the advantage of utilizing data from an all-payer database, which includes commercial, Medicaid, Medicare, and uninsured populations from more than 1100 hospitals and healthcare systems. We also examined predictors of medication continuation across patient, clinical, visit, and hospital characteristics.
2 Methods
2.1 Data Source
Data are derived from the PINC AI™ Healthcare Database (PHD) (formerly known as Premier Healthcare Database). PHD is a commercially available database, which contains data from more than 1.2 billion patient encounters, or one in every five inpatient discharges in the USA. The PHD has been used in more than 1000 publications by researchers in industry and academic institutions. The PHD is tokenized and linked to a closed claims database. The closed claims database covers the complete treatment journey of patients enrolled in the insurance plan—through inpatient, outpatient, and pharmacy settings, and even after switching providers [23].
2.2 Ethical Considerations
The data used were compliant with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule’s deidentification standard. Institutional review board approval for this study was not required, based on US Title 45 Code of Federal Regulations, Part 46, because the study used existing deidentified hospital discharge data, and recorded information cannot be identified directly or through identifiers linked to individuals. Informed consent of study participants was not pursued due to the nature of the deidentified data.
2.3 Study Design and Patient Selection
A retrospective cohort study design was used. The study utilized an active comparator, new user (ACNU) design to help mitigate potential confounding by indication, as well as temporality bias (i.e., immortal time bias) that can be introduced through varying exposure times of treatment initiation [24,25,26]. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline [27].
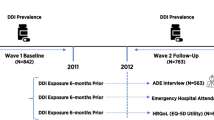
The study population included all adult patients (≥ 18 years of age) with an inpatient discharge date between 1 April 2017 and 30 April 2020 (study index period) and with a diagnosis code for schizophrenia (International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes F20.0–F20.9) or schizoaffective disorder (diagnosis codes F25.0, F25.1, F25.8, F25.9) during the index hospitalization or during two separate outpatient visits in the 6-month pre-index period. Since patients can have multiple hospital admissions during the study index period, the first qualifying hospitalization during the study index period was defined as a patient’s first hospitalization. The pre-index period was defined as the 6-month period preceding the patient’s index hospitalization. Two mutually exclusive cohorts were identified: Patients who initiated an LAI (“LAI Initiators”) or switched to a new OAP (“OAP Switchers”) during their index hospitalization. The medication observed during the index hospitalization (i.e., either initiated an LAI or OAP switch) was defined as the index therapy.
Patients were included in the “LAI Initiator” cohort if they switched from OAPs used during the pre-index period and initiated an LAI during the index hospitalization. Patients were included in the “OAP Switcher” cohort if they used OAPs during the 6-month pre-index period and initiated a new OAP during the index hospitalization without evidence of use of the new OAP or an LAI during the 6-month pre-index period. Only patients who were treated in hospitals that continuously contribute data to the PHD during the study period were included in the study sample.
Patients were excluded from the study if there was evidence of any LAI prescription during the 6-month pre-index period or evidence of a prescription for clozapine during the pre-index or index period. Patients with evidence of service days but with missing cost data also were excluded (Fig. 1).
2.4 Study Covariates
Patient characteristics at index hospitalization included gender, age group, race/ethnicity, marital status, and healthcare payor type. Patient clinical characteristics during index hospitalization included patient comorbidities using the Charlson-Deyo Comorbidity Index [28] and evidence of a principal or secondary discharge diagnosis of COVID-19 (ICD-10-CM code U07.1). Concomitant medications at index visit included other psychiatric co-medications and non-antipsychotic psychiatric co-medications (i.e., anticholinergics/propranolol, antidepressants, anxiolytics/hypnotics, and mood stabilizers). Mental health-related comorbidities at pre-index included cognitive disorders, psychotic disorders other than schizophrenia/schizoaffective disorders, affective disorders, anxiety, stress-related or somatoform disorders, personality disorders, disorders associated with physical or physiological disturbances, substance abuse disorders, developmental disorders or disorders diagnosed in childhood, and unspecified disorders [29]. Pre-index mental health-associated hospitalization was also included as a covariate. Hospital characteristics were assessed based on setting (i.e., rural vs. urban), teaching status, total number of beds, and US geographic region. Antipsychotic medication class was defined as first generation versus second generation. Antipsychotic medications investigated in this study included aripiprazole, asenapine, brexpiprazole, cariprazine, chlorpromazine, fluphenazine, haloperidol, iloperidone, loxapine, lurasidone, olanzapine, paliperidone, perphenazine, prochlorperazine, quetiapine, risperidone, thioridazine, thiothixene, and trifluoperazine. Generic and brand names for all oral and LAI formulations were included.
2.5 Clinical Quality Outcomes
The following clinical quality measures were assessed within 3 months of hospital discharge: (1) medication continuation of index therapy, defined as evidence of a filled prescription for the index therapy between discharge and 30 days post-discharge. (2) Follow-up care at an outpatient clinic within 30 days after index psychiatric hospitalization, defined as evidence of receiving mental health-related care at an outpatient clinic within 30 days of discharge. (3) Discontinuation of index therapy following index hospitalization, defined using the last prescription dispensing date plus the days’ supply of medication plus a “grace period” of 14 days. A patient refilling his prescription for the index medication before the date of discontinuation was classified as a continuous user [24]. Time to discontinuation of index therapy, defined as the time elapsed (in days) before discontinuation of index therapy without subsequent re-initiation. (4) All-cause 30-day hospital readmission, defined as any hospital admission following discharge from the index inpatient visit. (5) All-cause, 30-day emergency room (ER) visit, defined as a visit with an appropriate admission type with charges billed to an ER within 30 days of the index hospitalization.
2.6 Statistical Analysis
Descriptive statistics were used to compare the demographic, hospital, visit, and clinical characteristics of patients who initiated an LAI versus those who were switched to a new OAP. A chi-square test was used to test for statistical differences between groups for categorical variables. Two-sample comparisons were evaluated using Wilcoxon Rank Sum test for continuous variables. For all comparisons, a two-sided statistical significance level of 0.05 was used to determine whether to accept or reject the null hypotheses.
Propensity score (PS) weighting using generalized boosted modeling (GBM), a machine-learning technique, was used to address imbalances in pretreatment patient, clinical, visit, and hospital characteristics. GBM can adjust for many covariates and allows for greater model complexity, including nonlinear relationships. The GBM model fits a piecewise constant function by iteratively combining simple regression trees with each additional tree to improve the overall fit of the model. To avoid model overfitting, a stopping rule is utilized to decide on the optimal number of trees for estimating propensity scores (i.e., fine-tune the model) [30]. It has been noted that when GBM is used in this way, it can provide weights that yield the best covariate balance and treatment effects compared to other propensity score estimation approaches [31, 32]. Covariates for LAI and OAP pre-treatment balance included all patient, clinical, visit, and hospital covariates described above. In general, patients are weighted up or down to match the covariate distribution of the target population in a way that optimizes covariate balance. Covariate balance was assessed pre- and post-weighting by calculating absolute standardized mean differences (ASMD), with values approaching zero indicating that balance has been achieved. An ASMD conservative threshold value of 0.05 was used (standardized mean differences < 0.20 are considered small [31]). The target estimand of interest was the average treatment effect on the treated (ATT), which weights the comparison cases (the LAI Initiator cohort served as the treatment cohort and the OAP Switcher cohort served as the comparison cohort). Once balance was achieved, propensity score weights were extracted for further analysis.
Multivariable analysis with forward selection using weighted logistic regression with all patient, clinical, visit, and hospital covariates was used to determine a final fitted model of 30-day continuation. The weighted regression along with covariates in the multivariable model provides doubly robust estimates of treatment effects [30, 31]. The Akaike information criterion (AIC) was used to determine the relative fit of the final model. Adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs) were calculated. Multicollinearity was assessed by computing the variance inflation factor (VIF) for each coefficient using a threshold < 2. Time to discontinuation of index therapy (in days, including 95% CIs) was assessed by the Kaplan-Meier estimator and log-rank test was performed to test for the difference between the two treatment cohorts. In addition, a Cox proportional hazards model was used to estimate the impact of LAI and OAP cohorts on time to medication discontinuation. All statistical analyses were conducted using R version 4.1.3 [33].
3 Results
3.1 Patient Selection and Baseline Characteristics
The final sample included 7292 (18.6%) “LAI Initiators” and 31,956 (81.4%) “OAP Switchers” for a total of 39,248 patients who met the study selection criteria. In the overall sample, 58.7% were male, 65.5% were 35+ years, 52.2% were White, and 30.1% African American. Most patients (47.0%) were covered by Medicaid, followed by Medicare (35.9%), commercial insurance (9.7%) or other payer type (7.3%). About 16% of patients had evidence of a pre-index period mental health-related hospitalization, while 23.4% of patients had one or more comorbid conditions.
LAI Initiators and OAP Switchers showed significant differences across several baseline demographic, clinical, visit, and hospital characteristics. Propensity score weighting was successful in addressing imbalances across all baseline characteristics. The table in Online Resource 1 (Online Supplemental Material, OSM) shows the balance achieved across covariates between the LAI Initiators and OAP Switchers cohorts along with ASMDs before and after propensity score weighting. All ASMDs following PS weighting were < 0.02 and all p values were > 0.10.
3.2 Clinical Quality Measures and Other Endpoints
Overall, 30-day antipsychotic medication continuation rate was 27.2% (95% CI 26.4–28.0) and was significantly higher for LAI Initiators (29.0%, 95% CI 27.9–30.0) than for OAP Switchers (25.4%, 95% CI 24.3–26.5) with a difference of 3.6 percentage points (95% CI 2.0–5.1 percentage points, p < 0.0001). In addition, only 9.1% (95% CI 7.3–11.0) of patients had evidence of mental health-related outpatient care within 30 days after hospitalization. Although low, outpatient care within 30 days after hospitalization was significantly higher (p < 0.01) for LAI Initiators (10.9%, 95% CI 8.5–13.4) than for OAP Switchers (5.5%, 95% CI 3.0–7.9) with a difference of 5.4 percentage points (95% CI 2.0–8.9 percentage points, p < 0.0001).
Mean time to discontinuation of index therapy was 67.5 days (95% CI 65.8–69.2) overall, 62.0 days (95% CI 59.8–64.3) for OAP Switchers and 72.2 days (95% CI 69.7–74.6) for LAI Initiators (log-rank test, χ2 = 11.3, p < 0.001). Median time for discontinuation was 66 days (interquartile range (IQR): 63–73) for LAI Initiators and 60 days (IQR: 59–61) for OAP Switchers. Cox regression was used to compare the risk of medication discontinuation between the LAI and OAP cohorts after controlling for covariates. LAI Initiators had an 18% lower risk of discontinuing their index treatment than OAP Switchers (hazard ratio (HR): 0.82, 95% CI 0.76–0.89, p < 0.001) (Fig. 2).
Overall, all-cause 30-day hospital readmission rate was 10.8% (95% CI 10.2–11.3). In addition, the overall mean number of all-cause 30-day hospital readmissions was 1.18 (95% CI 0.23–2.12). The overall percentage of all-cause 30-day ER visits was 11.7% (95% CI 11.1–12.3) (Table 1).
Results of the multivariable analysis indicated that various patient, clinical, visit, and hospital characteristics were significantly associated with post-hospitalization 30-day antipsychotic medication continuation. African Americans were 20% less likely than Whites to maintain index medication continuation at 30 days. Patients receiving their health insurance through Medicaid were two times more likely than Medicare recipients to continue their medication at 30 days post hospitalization, while patients with other types of payors were less likely than Medicare recipients to continue their medication at 30 days post hospitalization. Patients receiving medical care in the Northeast or West were up to 1.4 times more likely than patients receiving medical care in the South to be adherent at 30 days post hospitalization.
Patients with comorbid conditions were up to 1.8 times more likely to be adherent to their index medication at 30 days than patients without any comorbid conditions. Patients with any mental health-related hospitalization during the 6-month pre-index period also were more likely to maintain their index medication at 30 days compared to patients without any pre-index period hospitalization. Patients receiving second generation antipsychotics were 8% less likely than patients receiving first generation antipsychotics to be adherent. Patients with evidence of mental and behavioral disorders due to psychoactive substance use were 10% less likely than patients without these mental and behavioral disorders to be adherent to their index therapy at 30 days.
Finally, after controlling for all other patient, clinical, visit, and hospital characteristics, the probability of maintaining index therapy at 30 days post hospitalization was significantly higher for LAI Initiators compared to OAP Switchers (aOR: 1.21, 95% CI 1.12–1.30, p < 0.001). Using analysis of deviance, the addition of antipsychotic product administration type (i.e., LAI or OAP) significantly contributed to the fitted logistic model after controlling for all other covariates (Likelihood Ratio χ2 = 24.2, p < 0.0001) (Table 2).
4 Discussion
This retrospective study used a large, US hospital-based, all-payer database along with a robust ACNU design to compare post-hospitalization clinical quality of care and care continuity measures between patients receiving LAIs or OAPs. Patients who initiated an LAI experienced more positive outcomes after a psychiatric hospitalization compared to OAP Switchers. Although these differences are relatively small, our findings build upon prior studies and reinforce the clinical advantages of LAI formulations over OAPs given their more stable pharmacokinetics and longer dosing intervals [20, 22, 34, 35].
The findings from this study are consistent with a prior study by Green et al. examining medication adherence and discontinuation of LAIs versus OAPs among patients with schizophrenia. Although the Green et al. study was limited to Medicaid patients, patients using LAIs experienced a 5% higher adjusted medication adherence rate and were 20% less likely to discontinue their medication during the 1-year post-index period compared to the OAP cohort. The median time to discontinuation of index LAI was 196 days compared to 123 days for oral users [3]. In a similar study, adherence (proportion of days covered (PDC) > 0.8) of 33.0% versus 21.7% at 1 year was observed in the LAI versus OAP cohorts, respectively, and usage of OAPs compared to LAIs was associated with a greater risk of discontinuation (HR = 1.19) in the complete switch cohort. The median time to end of therapy was 211 days for the LAI cohort and 120 days for OAP users [36]. Finally, in a retrospective study of Medicaid beneficiaries, 27.2% of second-generation LAI patients and 15.8% of first-generation LAI patients were adherent to the index medication (PDC ≥ 80%) at 1 year compared with 24.6% of OAP patients [37].
It is important to note that the difference in adherence rates in the current study is relatively smaller (3.6 percentage points) and reflects a short-term endpoint at 30 days post-discharge compared to prior studies documenting longer-term (1-year) endpoints. In addition, prior studies have found larger differences in time to discontinuation between LAI and OAP groups compared to our study, which found only a 5-day difference. It is possible that these differences are due to differences in the underlying patient populations. Two of the three studies described here utilized Medicaid databases, representing enrollees from a selected number of states across the USA [3, 37], while the third study utilized a claims database of German patients with schizophrenia [36]. The current study utilized a broader mix of patients from all payer types across the USA.
The smaller differences in endpoints between the LAI and OAP cohorts found in our study also may reflect differences in the types of antipsychotics used and prescribing practices across patient populations, including the possibility of limiting LAI use to selected patients (e.g., patients who are highly nonadherent). Other contributing factors may include symptom management, adverse events experienced when initially switching medications, and care continuity. For example, prior studies have documented differences in prescribing practice and clinical practice guidelines, particularly regarding oral antipsychotic polypharmacy. While use of concurrent OAPs is not uncommon, concurrent and longer-term use of LAIs and OAPs has been documented, with patients typically receiving oral formulations of their LAI [38, 39]. A prior study using therapeutic drug monitoring concentration measurements from patients treated with oral antipsychotics found increasing adjusted rates of nonadherence to polypharmacy versus monotherapy among patients receiving up to four or more co-prescribed antipsychotics [40]. Despite this practice, there is limited evidence and guidance available in real-world clinical practice settings, particularly for patients who do not respond well to antipsychotic monotherapy [41, 42].
Our study also found that patients receiving second-generation antipsychotics were 8% less likely to be adherent to their index medication at 30 days compared to patients receiving first-generation antipsychotics. Although statistically significant, this association was the weakest compared to other covariates with an upper 95% CI of 0.99 and may not be clinically meaningful. While it is not clear what is driving this association, there may be unaccounted differences that may explain this association, such as changes in prescribing practices from transition from inpatient to outpatient care settings. This finding also might reflect differences in the underlying population mix, access to community services, or even differences in active patient engagement during discharge planning [43].
This study provides further evidence supporting the use of LAIs in patients with schizophrenia. However, adherence to antipsychotic medication and care discontinuity remain a challenge to healthcare practitioners, given the complexity surrounding treatment decisions. Practitioners must weigh treatment options against numerous patient factors, including illness severity, treatment preferences, mental health comorbidities, and the patient’s support network [44]. In addition, interventions to improve medication adherence can include a combination of pharmacological approaches, patient and family support networks, and behavioral approaches. It is important to note that other factors, such as access to mental-healthcare and coordination of outpatient care, have been shown to impact treatment outcomes [45]. While the enactment of the 2010 Affordable Care Act and expansion of Medicaid eligibility has improved care access, some vulnerable populations including those with mental health challenges are disproportionately susceptible to healthcare access barriers [46]. In 2015, the Substance Abuse and Mental Health Services Administration, Center for Mental Health Services funded states to develop Certified Community Behavioral Health Clinics (CCBHCs). The purpose of this program is to increase access to and improve the quality of community mental and substance use disorder treatment services. The CCBHC model has been shown to expand access to care, improve care coordination, add and sustain evidence-based practices, decrease wait times for care, expand access to medication-assisted treatment, as well as address health disparities and social determinants of health [47].
Our study offers several methodological strengths to build on prior publications. First, the PHD is an all-payer database sourced from over 1000 medical facilities across the USA and represents 25% of annual US inpatient admissions. The PHD contains robust information on drug utilization, and patients can be tracked across inpatient and hospital-based outpatient settings within a single hospital, as well as across visits, using a unique identifier. Second, given that the potential differences in patients’ characteristics between the LAI and OAP treatment groups may lead to bias in the direct comparison of treatment effect on outcomes – and especially if there is a strong relationship between these characteristics and the endpoints – propensity score weighting was used to reduce the bias caused by these differences to make the treatment groups comparable. Generalized boosted model estimation, a machine learning technique, also was used to estimate propensity scores and has been shown to determine the best balance between two treatments. And, finally, the study utilized an ACNU design to help mitigate potential confounding by indication, as well as temporality biases (such as immortal time bias) that can be introduced through varying exposure times of treatment initiation.
4.1 Limitations
This study was subject to limitations. The PHD is a hospital administrative database and does not include as many clinical details as electronic health records would. The identification of clinical conditions, procedures, and medications relies on the accuracy of the hospital-reported diagnosis and procedure codes and chargemaster descriptions. While the PHD provides a comprehensive view of inpatient and outpatient visits from geographically diverse hospitals and across all payers and therapeutic areas, it is not a random sample. However, the PHD demonstrates a similar distribution to the American Hospital Association’s (AHA) member hospitals by region, urban versus rural setting, and teaching status, although the PHD does contain data from a greater proportion of larger hospitals. Also, it is possible that patients may have had multiple new medication use periods that may have occurred prior to the 6-month pre-index period, which would not have been documented in the current study [24]. There is also the possibility of confounding due to clinical decisions to use either an LAI or an OAP and/or patient preferences for LAIs versus OAPs [48, 49]. While our study defined medication adherence as adherence to the index therapy during hospitalization, patients may have subsequently switched to other antipsychotic treatment(s) that would not have been documented. Another limitation with claims and other large administrative healthcare databases is loss to follow-up. Our shorter-term endpoints would have minimized loss-to-follow-up rates. It is possible that patients could have been lost to follow-up primarily because they experience adverse events or complications while adjusting to new medications following hospitalization and for other reasons previously described. We also attempted to reduce missing data by including patients from hospitals that contributed data continuously over the follow-up period. Finally, although not investigated in this study, it is possible that state-based formulary restrictions and preferred drug lists may limit the range of drug therapies and access to certain medications, in this case atypical antipsychotics, that are available to control overall costs or pharmacy expenditures [50, 51].
5 Conclusions
LAI antipsychotics have been used to improve medication adherence among patients with schizophrenia. Using real-world data from a US hospital-based, all-payer, administrative database, this study compared clinical quality and care continuity measures among patients with schizophrenia initiated on an LAI or switched to a new OAP during a recent hospitalization and found a positive association between clinical quality outcomes and use of LAI antipsychotics. These findings may be useful in identifying treatment options for certain types of patients with known non-adherence risk to antipsychotic medications. The overall low medication continuation to antipsychotic medication observed with this study population also warrants further investigation using real-world data into factors that may influence both short- and long-term treatment outcomes and quality of care and care continuity measures.
References
National Institute of Mental Health. Prevalence of schizophrenia. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543. Accessed 11 Aug 2023.
Kadakia A, Catillon M, Fan Q, Williams GR, Marden JR, Anderson A, Kirson N, Dembek C. The economic burden of schizophrenia in the United States. J Clin Psychiatry. 2022;83(6):22m14458. https://doi.org/10.4088/JCP.22m14458. (PMID: 36244006).
Greene M, Yan T, Chang E, Hartry A, Touya M, Broder MS. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21(2):127–34. https://doi.org/10.1080/13696998.2017.1379412. (Epub 2017 Sep 29 PMID: 28895758).
Cahaya N, Kristina SA, Widayanti AW, Green J. Interventions to improve medication adherence in people with schizophrenia: a systematic review. Patient Prefer Adherence. 2022;16:2431–49. https://doi.org/10.2147/PPA.S378951.PMID:36072918;PMCID:PMC9444034.
Hodson N, Majid M, Vlaev I. Can incentives improve antipsychotic adherence in major mental illness? A mixed-methods systematic review. BMJ Open. 2022;12: e059526. https://doi.org/10.1136/bmjopen-2021-059526.
García S, Martínez-Cengotitabengoa M, López-Zurbano S, Zorrilla I, López P, Vieta E, González-Pinto A. Adherence to antipsychotic medication in bipolar disorder and schizophrenic patients: a systematic review. J Clin Psychopharmacol. 2016;36(4):355–71. https://doi.org/10.1097/JCP.0000000000000523.PMID:27307187;PMCID:PMC4932152.
Guo J, Lv X, Liu Y, et al. Influencing factors of medication adherence in schizophrenic patients: a meta-analysis. Schizophr. 2023;9:31. https://doi.org/10.1038/s41537-023-00356-x.
Hermer L, Nephew T, Southwell K. Follow-up psychiatric care and risk of readmission in patients with serious mental illness in state funded or operated facilities. Psychiatr Q. 2022;93(2):499–511. https://doi.org/10.1007/s11126-021-09957-0. (Epub 2021 Oct 25. PMID: 34694533; PMCID: PMC9046324).
Smithnaraseth A, Seeherunwong A, Panitrat R, et al. Hospital and patient factors influencing the health status among patients with schizophrenia, thirty days after hospital discharge: multi-level analysis. BMC Psychiatry. 2020;20:592. https://doi.org/10.1186/s12888-020-03001-4.
Lee SY, Kim KH, Kim T, Kim SM, Kim JW, Han C, Song JY, Paik JW. Outpatient follow-up visit after hospital discharge lowers risk of rehospitalization in patients with schizophrenia: a nationwide population-based study. Psychiatry Investig. 2015;12(4):425–33. https://doi.org/10.4306/pi.2015.12.4. (Epub 2015 Apr 15. PMID: 26508952; PMCID: PMC4620298).
Balikci A, Erdem M, Zincir S, Bolu A, Zincir SB, Ercan S, Uzun O. Adherence with outpatient appointments and medication: A two-year prospective study of patients with schizophrenia. Klinik Psikofarmakoloji Bülteni/Bull Clin Psychopharmacol. 2013;23(1):57–64. https://doi.org/10.5455/bcp.20121130085931.
Vega D, Acosta FJ, Saavedra P. Nonadherence after hospital discharge in patients with schizophrenia or schizoaffective disorder: a six-month naturalistic follow-up study. Compr Psychiatry. 2021;108: 152240. https://doi.org/10.1016/j.comppsych.2021.152240. (Epub 2021 Apr 17 PMID: 33873014).
Candon M, Girdish C, Walter J, Engel L, Howell B. Implementing multistate behavioral health pay-for-performance initiatives in medicaid managed care. Am J Account Care. 2022;10(3):16–22. https://doi.org/10.37765/ajac.2022.89232.
Centers for Medicare & Medicaid Services. Inpatient Psychiatric Facility Quality Reporting Program. https://qualitynet.cms.gov/ipf/ipfqr. Accessed 14 Mar 2023.
National Committee on Quality Assurance (NCQA) Healthcare Effectiveness Data and Information Set (HEDIS®). https://www.ncqa.org/hedis/measures/. Accessed 14 Mar 2023.
Patel C, Emond B, Morrison L, Lafeuille MH, Lefebvre P, Lin D, Kim E, Joshi K. Risk of subsequent relapses and corresponding healthcare costs among recently relapsed Medicaid patients with schizophrenia: a real-world retrospective cohort study. Curr Med Res Opin. 2021;37(4):665–74. https://doi.org/10.1080/03007995.2021.1882977. (Epub 2021 Feb 14 PMID: 33507831).
Patel C, Emond B, Lafeuille MH, Côté-Sergent A, Lefebvre P, Tandon N, El Khoury AC. Real-world analysis of switching patients with schizophrenia from oral risperidone or oral paliperidone to once-monthly paliperidone palmitate. Drugs Real World Outcomes. 2020;7(1):19–29. https://doi.org/10.1007/s40801-019-00172-9. (PMID:31786737;PMCID:PMC7061019).
Patel C, Khoury AE, Huang A, Wang L, Bashyal R. Healthcare resource utilization and costs among patients with schizophrenia switching from oral risperidone/paliperidone to once-monthly paliperidone palmitate: a veterans health administration claims analysis. Curr Ther Res Clin Exp. 2020;92: 100587. https://doi.org/10.1016/j.curtheres.2020.100587. (PMID:32714469;PMCID:PMC7378858).
Shah A, Xie L, Kariburyo F, Zhang Q, Gore M. Treatment patterns, healthcare resource utilization and costs among schizophrenia patients treated with long-acting injectable versus oral antipsychotics. Adv Ther. 2018;35(11):1994–2014. https://doi.org/10.1007/s12325-018-0786-x.
Pilon D, Tandon N, Lafeuille MH, Kamstra R, Emond B, Lefebvre P, Joshi K. Treatment patterns, health care resource utilization, and spending in medicaid beneficiaries initiating second-generation long-acting injectable agents versus oral atypical antipsychotics. Clin Ther. 2017;39(10):1972-1985.e1972. https://doi.org/10.1016/j.clinthera.2017.08.008.
Park SC, Choi MY, Choi J, Park E, Tchoe HJ, Suh JK, Kim YH, Won SH, Chung YC, Bae KY, Lee SK, Park CM, Lee SH. Comparative efficacy and safety of long-acting injectable and oral second-generation antipsychotics for the treatment of schizophrenia: a systematic review and meta-analysis. Clin Psychopharmacol Neurosci. 2018;16(4):361–75. https://doi.org/10.9758/cpn.2018.16.4.361.
Lin D, Thompson-Leduc P, Ghelerter I, Nguyen H, Lafeuille MH, Benson C, Mavros P, Lefebvre P. Real-world evidence of the clinical and economic impact of long-acting injectable versus oral antipsychotics among patients with schizophrenia in the United States: a systematic review and meta-analysis. CNS Drugs. 2021;35(5):469–81. https://doi.org/10.1007/s40263-021-00815-y.
PINC AI Applied Sciences, Premier Inc. PINC AI Healthcare Database: Data that informs and performs (White Paper). September 2022. Charlotte, NC: Premier Inc. https://offers.premierinc.com/rs/381-NBB-525/images/Premier-Healthcare-Database-Whitepaper-Final.pdf. Accessed 5 Oct 2023.
Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–8. https://doi.org/10.1007/s40471-015-0053-5.
Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7):437–41. https://doi.org/10.1038/nrrheum.2015.30.
Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–20. https://doi.org/10.1093/aje/kwg231.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9. https://doi.org/10.1016/j.ijsu.2014.07.013.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Sauders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Mahabaleshwarkar R, Lin D, Fishman J, Blair T, Hetherington T, Palmer P, Patel C, Benson C, Joshi K, Krull C, Tcheremissine OV. The impact of once-monthly paliperidone palmitate on healthcare utilization among patients with schizophrenia treated in an integrated healthcare system: a retrospective mirror-image study. Adv Ther. 2021;38(4):1958–74. https://doi.org/10.1007/s12325-021-01626-9.
Ridgeway, G, McCaffrey DF, Morral AR, Cefalu M, Burgette LF, Pane JD, Griffin BA. Toolkit for weighting and analysis of nonequivalent groups: a tutorial for the R TWANG Package. Santa Monica, CA: RAND Corporation, 2022. https://www.rand.org/pubs/tools/TLA570-5.html. Accessed 28 Sept 2023.
McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388–414. https://doi.org/10.1002/sim.5753.
McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9(4):403–25. https://doi.org/10.1037/1082-989X.9.4.403. (PMID: 15598095).
RStudio Team . RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. 2020. http://www.rstudio.com/. Accessed 19 Nov 2023.
Correll CU, Kim E, Sliwa JK, Hamm W, Gopal S, Mathews M, Venkatasubramanian R, Saklad SR. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39–59. https://doi.org/10.1007/s40263-020-00779-5. (Epub 2021 Jan 28. PMID: 33507525; PMCID: PMC7873121).
Zhornitsky S, Stip E. Oral versus long-acting injectable antipsychotics in the treatment of schizophrenia and special populations at risk for treatment nonadherence: a systematic review. Schizophr Res Treatment. 2012. https://doi.org/10.1155/2012/407171. (Epub 2012 Feb 15. PMID: 22966436; PMCID: PMC3420751).
Mahlich J, Olbrich K, Wilk A, Wimmer A, Wolff-Menzler C. Time to treatment discontinuation in german patients with schizophrenia: long-acting injectables versus oral antipsychotics. Clin Drug Investig. 2021;41(1):99–113. https://doi.org/10.1007/s40261-020-00990-8. (Epub 2020 Dec 17. PMID: 33331979; PMCID: PMC7815621).
Pilon D, Joshi K, Tandon N, Lafeuille MH, Kamstra RL, Emond B, Lefebvre P. Treatment patterns in Medicaid patients with schizophrenia initiated on a first- or second-generation long-acting injectable versus oral antipsychotic. Patient Prefer Adherence. 2017;23(11):619–29. https://doi.org/10.2147/PPA.S127623.PMID:28356723;PMCID:PMC5367457.
Doshi JA, Pettit AR, Stoddard JJ, Zummo J, Marcus SC. Concurrent Oral antipsychotic drug use among schizophrenia patients initiated on long-acting injectable antipsychotics post-hospital discharge. J Clin Psychopharmacol. 2015;35(4):442–6. https://doi.org/10.1097/JCP.0000000000000353. (PMID: 26075492).
Shi L, Ascher-Svanum H, Zhu B, Faries D, Montgomery W, Marder SR. Characteristics and use patterns of patients taking first-generation depot antipsychotics or oral antipsychotics for schizophrenia. Psychiatr Serv. 2007;58(4):482–8. https://doi.org/10.1176/ps.2007.58.4.482. (PMID: 17412849).
Smith RL, Tveito M, Kyllesø L, Jukic MM, Ingelman-Sundberg M, Andreassen OA, Molden E. Impact of antipsychotic polypharmacy on nonadherence of oral antipsychotic drugs—a study based on blood sample analyses from 24,239 patients. Eur Neuropsychopharmacol. 2020Aug;37:64–9. https://doi.org/10.1016/j.euroneuro.2020.06.007. (Epub 2020 Jun 25 PMID: 32595082).
Pae CU, Han C, Bahk WM, Lee SJ, Patkar AA, Masand PS. Consideration of long-acting injectable antipsychotics for polypharmacy regimen in the treatment of schizophrenia: put it on the table or not? Clin Psychopharmacol Neurosci. 2021;19(3):434–48. https://doi.org/10.9758/cpn.2021.19.3.434. (PMID:34294613;PMCID:PMC8316655).
Kamei H. Polypharmacy management of antipsychotics in patients with schizophrenia. Medicina (Kaunas). 2022;58(11):1584. https://doi.org/10.3390/medicina58111584. (PMID:36363541;PMCID:PMC9692600).
Patel C, Pilon D, Morrison L, Holiday C, Lafeuille M, Lefebvre P, Carmela B. Continuity of care among patients newly initiated on second-generation oral or long-acting injectable antipsychotics during a schizophrenia-related inpatient stay. Curr Med Res Opin. 2023;39(8):1157–66. https://doi.org/10.1080/03007995.2023.2237833.
American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia. 3rd ed. Arlington: American Psychiatric Publishing; 2020. https://doi.org/10.1176/appi.books.9780890424841.
Smith TE, Haselden M, Corbeil T, Tang F, Radigan M, Essock SM, Wall MM, Dixon LB, Wang R, Frimpong E, Lamberti S, Schneider M, Olfson M. Relationship between continuity of care and discharge planning after hospital psychiatric admission. Psychiatr Serv. 2020;71(1):75–8. https://doi.org/10.1176/appi.ps.201900233. (Epub 2019 Oct 8. PMID: 31590622; PMCID: PMC7008713).
Coombs NC, Meriwether WE, Caringi J, Newcomer SR. Barriers to healthcare access among U.S. adults with mental health challenges: a population-based study. SSM Popul Health. 2021;15:100847. https://doi.org/10.1016/j.ssmph.2021.100847. (PMID: 34179332; PMCID: PMC8214217).
Siegwarth AW, Miller R, Little J, Brown J, Kase C, Breslau J, Dunbar M. Implementation findings from the National Evaluation of the Certified Community Behavioral Health Clinic Demonstration, Sep 11, 2020. https://aspe.hhs.gov/reports/implementation-findings-national-evaluation-certified-community-behavioral-health-clinic-0#main-content. Accessed 1 Mar 2022.
Blackwood C, Sanga P, Nuamah I, Keenan A, Singh A, Mathews M, Gopal S. Patients’ preference for long-acting injectable versus oral antipsychotics in schizophrenia: results from the patient-reported medication preference questionnaire. Patient Prefer Adherence. 2020;14:1093–102. https://doi.org/10.2147/PPA.S251812. (PMID:32753849;PMCID:PMC7342487).
Kane JM, McEvoy JP, Correll CU, et al. Controversies surrounding the use of long-acting injectable antipsychotic medications for the treatment of patients with schizophrenia. CNS Drugs. 2021;35:1189–205. https://doi.org/10.1007/s40263-021-00861-6.
Goldman DP, Dirani R, Fastenau J, Conrad RM. Do strict formularies replicate failure for patients with schizophrenia? Am J Manag Care. 2014;20(3):219–28 (PMID: 24884751).
Robst JM, Constantine R, Boaz T, Andel R, Teague G, Becker M, Howe A. Short-term impact of preferred drug list changes on health care use and Medicaid costs: injectable risperidone. Psychiatr Serv. 2010;61(9):937–9. https://doi.org/10.1176/ps.2010.61.9.937. (PMID: 20810595).
Acknowledgements
Cate Polacek, MLIS, Senior Medical Writer with PINC AI™ Applied Sciences, Premier Inc., provided publication support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Janssen Scientific Affairs, LLC.
Conflict of Interest
SE, ZC, and MT are employees of Premier Inc. Applied Sciences, a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript. CP and CB are employees of Medical Affairs Neuroscience, Johnson & Johnson Innovative Medicine and stockholders of Johnson & Johnson.
Ethics Approval
The data used were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act. Therefore, no institutional review board exemption was needed.
Consent to Participate
Not applicable since patient data were deidentified.
Consent for Publication
Not applicable since patient data were deidentified.
Availability of Data and Material
The data that support the findings of this study are available from PINC AITM Applied Sciences, Premier Inc., but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly or freely available. Any researchers interested in obtaining the data used in this study can access the database through PINC AITM Applied Sciences, Premier Inc., under a license agreement, including the payment of appropriate license fee.
Code Availability
All statistical analyses were conducted using Rstudio (now Posit, PBC, Boston, MA). The programs are proprietary materials of PINC AITM Applied Sciences, Premier Inc.; therefore, restrictions apply to the access of these codes, which cannot be made available publicly. Reasonable requests or questions related to the analysis will be addressed by authors.
Author Contributions
CP, SE, ZC, and CB made substantial contributions to the conception and design of the study. MT, SE, and ZC contributed to the acquisition and analysis of data. CP, CB, SE, and ZC contributed to the interpretation of data, drafting the article, and revising it critically for important intellectual content. All authors have provided their final approval for the manuscript to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Previous Presentations
Part of the material in this manuscript has been presented at the Elevate by Psych Congress 2023 Annual Meeting, 1–4 June 2023.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Patel, C., Emont, S., Cao, Z. et al. Post Hospitalization Clinical Quality Outcomes Among US Patients with Schizophrenia Treated with a Long-Acting Injectable or Switched to a New Oral Antipsychotic: A Retrospective Cohort Study. Drugs - Real World Outcomes 11, 69–79 (2024). https://doi.org/10.1007/s40801-023-00408-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-023-00408-9