Abstract
Introduction
Hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) is the most frequently diagnosed metastatic breast cancer (mBC) subtype. Combinations of endocrine therapy (ET) with cyclin-dependent kinase 4/6 inhibitors (CDK4 & 6is) improve outcomes compared with ET alone. The efficacy and safety of abemaciclib among patients with HR+/HER2− mBC has been demonstrated in the MONARCH clinical trials; however, there is a paucity of real-world evidence, particularly in older patients.
Methods and Materials
This retrospective cohort study analyzed the electronic medical record data/charts of adult patients with HR+/HER2− mBC receiving abemaciclib in US-based community oncology settings (1 September 2017 to 30 September 2019). Patients with other primary malignancies, clinical trial enrollment, and incomplete charts were excluded. Patient characteristics, treatment attributes and patterns, and real-world outcomes (clinical benefit rate [CBR] and stable disease among patients with response data available, time to chemotherapy [TTC], time to treatment discontinuation [TTD], and progression-free survival [PFS]) were summarized. Multivariable models evaluated the association between demographic/clinical characteristics and outcomes.
Results
Of the 448 final patients, 99% were female, with a median age of 67 years (25% were ≥ 75 years) and median follow-up of 11 months; most (60%) initiated abemaciclib within 2 years of mBC diagnosis. Patients received a median of 1 (P25 = 0, P75 = 3) prior line of therapy for mBC before abemaciclib, including other CDK4 & 6is (48%) and prior chemotherapy (31%); most (57%) had visceral disease. The CBR for the overall population was 53%, with 48% achieving stable disease. The median TTC was not reached; median TTD was 249 days (95% confidence interval [CI]: 202, 304). The median PFS was 329 days (95% CI 266, 386). The discontinuation rate of abemaciclib owing to adverse events (30%) trended higher with age (years) (P = 0.027): 18–49 (n = 42; 19%), 50–64 (n = 155; 25%), 65–74 (n = 138; 32%), 75–84 (n = 82; 37%), ≥ 85 (n = 31; 49%); only 23% of patients overall had a dose hold or reduction prior to discontinuation.
Conclusions
These patients were older than those in the MONARCH studies with substantial visceral disease, and prior chemotherapy and CDK4 & 6i use. Discontinuation rates were higher than in previous real-world studies (11.9%), highlighting the need for proactive management to optimize outcomes, particularly in older patients with mBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with HR+/HER2− mBC treated with abemaciclib in routine clinical practice, in the 2 years following initial FDA approval, were older and sicker than those who participated in the MONARCH clinical trials. |
Abemaciclib treatment discontinuation rates were high, highlighting the need for proactive management of these patients. |
This study is the largest analysis of EMR data for patients treated with abemaciclib for mBC in the USA and it might help inform clinicians with the management of older patients. |
1 Introduction
Breast cancer (BC) is the most commonly diagnosed cancer in women, with an estimated 290,560 cases in the USA to be diagnosed in 2022 [1]. Adults aged ≥ 65 years old account for the majority of patients diagnosed with BC and account for most BC-related deaths, despite significant advances in diagnosis and treatment [2]. Patients with metastatic breast cancer (mBC) have a 5-year survival of < 30% [1]. Hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) is the most frequently diagnosed molecular subtype of mBC, including in older patients, and is associated with better survival rates than other hormone receptor and HER2 subtypes [3, 4].
Treatment goals for mBC include delaying progression, prolonging survival, and providing symptom relief, while minimizing treatment-related side effects [5, 6]. Utilizing endocrine-based treatments as initial treatment and delaying chemotherapy, if appropriate, is preferred owing to effectiveness and avoidance of chemotherapy-related side effects and reduced quality of life [6,7,8,9,10,11]. Managing the benefit of treatment relative to risk is especially relevant for older patients with cancer given the decreased life expectancy and reduced tolerance to side effects [7, 12]. Understanding the effectiveness and safety of new therapies in this population is difficult, as older patients are underrepresented in clinical trials, and those who are included are likely to be a selected population of fitter patients [13,14,15].
Combinations of endocrine therapy with cyclin-dependent kinase 4/6 inhibitors (CDK4 & 6is), including abemaciclib, have led to improved outcomes over endocrine therapy alone [16,17,18,19,20,21,22,23,24]. Guidelines recommend CDK4 & 6is in combination with endocrine therapy as first-line therapy in patients with HR+ mBC who are treatment-naïve and those with progressive disease after endocrine therapy [25].
Abemaciclib was first approved by the Food and Drug Administration (FDA) in September of 2017 for women with HR+/HER2− mBC [26]. The efficacy and safety of abemaciclib as monotherapy and in combination with fulvestrant or an aromatase inhibitor (AI) has been demonstrated in this patient population across various treatment lines [17, 27,28,29,30,31]. While older patients were enrolled in the MONARCH trials, they may not be representative of the general older population of patients with mBC. Therefore, there is a data gap regarding the use and effectiveness of abemaciclib in the real world, including understanding the safety and effectiveness in older patients with mBC. To begin to address this gap, this real-world retrospective cohort study aimed to describe patient characteristics, dosing and management, treatment patterns, and real-world outcomes of predominantly older patients with HR+/HER2− mBC treated with abemaciclib in routine clinical practice.
2 Materials and Methods
2.1 Study Design and Data Source
This was a retrospective cohort study of patients with HR+/HER2− mBC receiving abemaciclib in US-based community oncology settings. Data were included from two electronic medical record (EMR) sources: Florida Cancer Specialists (FCS) and Research Institute and the Electronic Medical Office Logistics (EMOL) Health Oncology Warehouse (OWL™) database. FCS is one of the largest independent, privately held, medical oncology/hematology practices in Florida with over 230 physicians in their network. The EMOL Health OWL database contains EMR data from oncology practices spanning 239 locations in 26 states, representing over 600 medical oncologists. Both sources have the OncoEMR® framework, and while slight differences were noted in the data structure, the data were mapped to ensure the data fields used to define the study cohort and create the source variables aligned across both sources (Supplementary Fig. 1). There was no overlap or duplication of available patient data in these EMRs, and the study was approved with a waiver of consent and a full waiver of Health Insurance Portability and Accountability Act authorization by the Advarra Institutional Review Board.
The structured data fields were supplemented with data abstracted from patients’ medical charts. Data from patient charts was prioritized over structured EMR data in case of discrepancies [32]. Interrater reliability testing indicated good agreement (Fleiss’ kappa > 0.8) [33] among the abstracted data across the trained curators.
The patient identification period was from 1 September 2017 to 30 September 2019, and the index date was the first documented start of the abemaciclib regimen. The follow-up period was ≥ 90 days and started from the index date (inclusive) through earliest date of death, loss to follow-up (last structured activity in the EMR), or study end date (31 December 2019).
2.2 Study Population and Groups
The study population included adults (≥ 18 years old) with HR+/HER2− mBC who initiated treatment with abemaciclib, regardless of line of therapy (LOT), during the identification period. Patients were excluded for the following reasons: active treatment (any modality) for primary malignancies other than mBC, treatment with abemaciclib as part of a clinical trial, < 90 days of follow-up from index through earliest loss of follow-up or study end date (patients who died within 90 days of the index date were not excluded), incomplete medical charts, or > 90-day gap between mBC diagnosis date and first activity date.
The population was divided into four groups based on index regimen: abemaciclib + AI (letrozole, anastrozole, or exemestane), abemaciclib + fulvestrant, abemaciclib monotherapy, and abemaciclib + other. Results for the abemaciclib + other group are not presented owing to small sample size.
2.3 Study Variables
Study variables included demographic and clinical characteristics at abemaciclib initiation, treatment attributes (initial abemaciclib dose and schedule; changes from initial dose or schedule including dose holds), treatment patterns, and real-world outcomes (time to chemotherapy [TTC], time to treatment discontinuation [TTD], progression-free survival [PFS], and clinical benefit rate [CBR]). The dosing information, treatment patterns, and outcomes were primarily obtained from patients’ medical charts. Additional details on the operationalization of variables have been noted in the respective results tables where appropriate and in Supplementary Table 1.
2.4 Statistical Analysis
All analyses were conducted using SAS Enterprise Guide version 8.1 (SAS Institute Inc.). Descriptive summary statistics included frequencies and percentages, means, standard deviations (SDs), medians, and 25th and 75th percentiles. Kaplan–Meier methods were used to estimate TTC, TTD, and PFS. Post hoc analyses of select variables by (1) therapy initiation date window (1 September 2017 to 30 September 2018 [time period 1]; 1 October 2018 to 30 September 2019 [time period 2]) (data not shown) and (2) age strata (≤ 49, 50–64, 65–74, 75–84, 85+ years) were completed.
Cox proportional hazards (PH) models were used to explore the association between outcomes (TTD and PFS) and patient/clinical characteristics at abemaciclib initiation; covariates included age; abemaciclib initiation year; smoking status; BC-adapted Elixhauser comorbidity index [34]; stage at initial BC diagnosis; presence of visceral, brain, and/or bone metastases; Eastern Cooperative Oncology Group (ECOG) performance status score; treatment with prior chemotherapy; prior CDK4 & 6i therapy; abemaciclib LOT; and whether the patient had a dose reduction or dose hold. Dose reduction/hold was included as a time-varying covariate. Abemaciclib is indicated in combination with an AI or fulvestrant for different patient profiles, hence the abemaciclib regimen-based treatment group was specified as a strata variable to allow the model to specify different baseline hazard functions across each stratum.
Similarly, a logistic regression model was used to explore the association between patient and clinical characteristics and the likelihood of obtaining clinical benefit. Treatment group was included as a covariate in addition to the covariates listed above with the exception of dose reduction/hold.
3 Results
3.1 Patient Characteristics (Table 1)
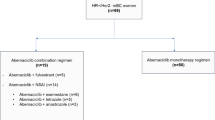
A total of 448 patients were included in the study (Fig. 1). Almost all patients were female (99%), with a median age of 67 years. Notably, 56% of patients were ≥ 65 years, 25% of patients were ≥ 75 years, and 7% were ≥ 85 years. More than 70% of patients were White and treated in the southern USA, respectively. Median follow-up time was 11 months.
Patient attrition. aPatients who died within 90 days of index date were not excluded. bReviewers were asked to stop data abstraction for the patient after any one exclusion criterion was met. CDK4 & 6i cyclin-dependent kinase 4/6 inhibitor, ER estrogen receptor, HER2 human epidermal growth receptor-2, PR progesterone receptor
Approximately half (48%) of the patients had a score of 3+ on the BC-adapted Elixhauser comorbidity index, 12% had a history of other primary cancers, and 42% were past or current smokers. The top three comorbidities were hypertension (52%), depression (25%), and anemia (23%). One-fourth of the patients had mBC at initial diagnosis, and, of the remaining patients, 86% had ≥ 2 years between initial diagnosis and mBC diagnosis.
Most patients (60%) initiated abemaciclib within 2 years of mBC diagnosis. At the time of initiation, 39% of patients had at least three metastatic sites, with the most common sites being bone (81%), visceral (57%), and lung (30%); 66% of patients had an ECOG score of ≤ 1 and 13% had an ECOG score of ≥ 2 at abemaciclib initiation. ECOG scores were unknown for one-fifth of the study sample.
All patients had estrogen receptor-positive tumors; progesterone receptor status was available for 99% (n = 443) and was positive in 78% (n = 344). Overall, 41% of the patients’ tumors were deemed to have developed endocrine resistance; 17% and 24% had primary versus secondary resistance, respectively. The remainder of the patients’ tumors were either endocrine sensitive or had missing data.
3.2 Treatment Attributes and Patterns (Table 2)
The most common starting dose and schedule of abemaciclib was 150 mg twice daily (72%). In the abemaciclib monotherapy group, the most common starting doses and schedules included 150 mg twice daily (41%) and 200 mg twice daily (41%); in the abemaciclib + AI and + fulvestrant groups, the majority of patients started 150 mg twice daily (77% in both groups). In the overall cohort, dose reduction was observed in 26% of patients, 27% had a dose hold, and 9% had a schedule change. The highest frequency of dose reductions (36%) occurred in the oldest age strata (≥ 85 years) (Fig. 2).
Abemaciclib + fulvestrant was the most common regimen (55%), followed by abemaciclib + AI (31%), abemaciclib monotherapy (12%), and abemaciclib + other (2%). Overall, abemaciclib was used in first-line therapy in 33% of patients, second line in 23%, third line in 14%, and fourth line and later in 30% of patients.
Patients received a median of 1 (P25 = 0, P75 = 3) prior line systemic treatment in the metastatic setting before receiving abemaciclib, including chemotherapy (31%), hormone therapy (65%), and prior CDK4 & 6is (48%). Among the patients who received palbociclib and/or ribociclib-based regimens, 61% of patients discontinued these regimens owing to disease progression and 36% owing to toxicity (data not shown). Numerically higher proportions of patients in the abemaciclib monotherapy group received prior chemotherapy, hormone therapy, and targeted or immunotherapy than patients in the abemaciclib + AI and + fulvestrant groups, respectively (Table 2).
Overall, 302 (67%) discontinued abemaciclib; some of them (n = 33) continued the concomitant agent. Most patients either discontinued abemaciclib owing to intolerability (30%) or disease progression (25%) (Table 2). A numerically higher proportion of patients initiating abemaciclib within the first year of FDA approval discontinued owing to an AE compared with patients initiating abemaciclib in year 2 (34% [71 of 211] versus 27% [65 of 237]; P = 0.153) (data not shown). There was a higher trend in discontinuations due to AEs with increasing age strata (P = 0.027) (Fig. 2).
Of the 136 patients who discontinued owing to intolerability, 105 (77%) did not have a dose hold or reduction of ≥ 50 mg per day prior to discontinuation (data not shown). Of those who discontinued the abemaciclib-based regimen (n = 269), approximately 34% switched to chemotherapy, 7% to hormone therapy alone, 9% to hormone + non-CDK4 & 6i therapy, and 22% to other CDK4 & 6i therapy, and 3% continued abemaciclib but switched the hormone therapy agents in the regimen.
3.3 Clinical Outcomes (Table 3)
The CBR was 53% (31% in abemaciclib monotherapy, 59% in abemaciclib + AI, and 54% in abemaciclib + fulvestrant groups). Real-world stable disease was reported for 48% among patients with a response assessment. Of patients with stable disease, 43% had stable disease lasting for ≥ 24 weeks. None of the abemaciclib monotherapy patients had stable disease for ≥ 24 weeks.
The median TTC was not reached in the overall study population, in the abemaciclib + AI group, and abemaciclib + fulvestrant groups. The median TTC in the abemaciclib monotherapy group was 121 days (95% confidence interval [CI]: 85, 221). Overall, the median TTD was 249 days (95% CI 202, 304) and varied by the treatment groups.
A total of 42% of patients progressed during the study period; the median PFS was 329 days (95% CI 266, 386) overall. In an unadjusted analysis, the abemaciclib monotherapy group had the shortest median PFS (days; 95% CI) (123; 79, 271), followed by the abemaciclib + fulvestrant group (305; 245, 383) and the abemaciclib + AI group (539; 336, not estimable).
3.4 Survival Models and Logistic Regression (Fig. 3)
Multivariable modeling results. (A) Time to treatment discontinuation. Results from a Cox PH model exploring the association between patient/clinical characteristics and time to treatment discontinuation. aMehta et al. [34]. bDose reduction/hold was included as a time-varying (monthly) covariate. BC breast cancer, CDK4 & 6i cyclin-dependent kinase 4/6 inhibitor, ECI Elixhauser comorbidity index, ECOG Eastern Cooperative Oncology Group, LOT line of therapy. (B) Progression-free survival. Results from a Cox PH model exploring the association between patient/clinical characteristics and progression-free survival time. aMehta et al. [34]. bDose reduction/hold was included as a time-varying (monthly) covariate. Key: BC breast cancer, CDK4 & 6i cyclin-dependent kinase 4/6 inhibitor, ECI Elixhauser comorbidity index, ECOG Eastern Cooperative Oncology Group, LOT line of therapy. (C) Clinical benefit rate. Results from a logistic regression model exploring the association between patient/clinical characteristics and clinical benefit rate. aMehta et al. [34]. BC breast cancer, CDK4 & 6i cyclin-dependent kinase 4/6 inhibitor, ECI Elixhauser comorbidity index, ECOG Eastern Cooperative Oncology Group, LOT line of therapy
The Cox PH model for TTD (hazard ratio [HR]; 95% CI) demonstrated that patients with visceral metastases (1.41; 1.08, 1.84; P = 0.013) and patients with ECOG score ≥ 2 at abemaciclib initiation (1.68; 1.09, 2.61; P = 0.019) had a higher risk of treatment discontinuation. Patients who had a dose reduction or hold had a 57% lower risk of discontinuation in that month (0.43; 0.26, 0.72; P = 0.001).
In the PFS model, patients with visceral metastases (1.60; 1.14, 2.25; P = 0.007) and patients with ECOG score ≥ 2 (compared with 0) at abemaciclib initiation (2.49; 1.41, 4.41; P = 0.002) were at a higher risk for progression (definition included death).
Finally, in the logistic regression model for CBR (odds ratio [OR]; 95% CI), patients with brain metastases were less likely to achieve clinical benefit (0.25; 0.09, 0.71; P = 0.009). Patients in the abemaciclib + AI (2.66; 1.1, 6.43; P = 0.029) and abemaciclib + fulvestrant groups (2.73; 1.19, 6.24; P = 0.018) both had > 2.5 times higher odds of achieving clinical benefit when compared with those receiving abemaciclib monotherapy.
4 Discussion
This study is the largest analysis of EMR data published to date for patients treated with abemaciclib for mBC in the USA, and it represents real-world experience in the 2 years following initial FDA approval. The characteristics of patients in this study were similar to those from prior studies of abemaciclib in mBC from routine clinical practice [35, 36]. Most patients in this cohort were ≥ 65 years of age and had a heavy cancer and noncancer comorbidity burden when they initiated abemaciclib. Our observations are consistent with prior literature demonstrating that new drugs are typically channeled to patients with poor prognostic features in the early periods following regulatory approval [37]. This study demonstrated a higher discontinuation rate due to AEs (30%) than previously reported real-world studies of abemaciclib (11.9%) [35], with an observation that most patients in the current study discontinued therapy before having a dose hold or dose reduction. Overall, this study supports the body of evidence demonstrating anticancer activity for patients with mBC treated with abemaciclib, including those with characteristics not typical of patients who participated in MONARCH trials [31, 38].
While the incidence rate of HR+/HER2− BC is highest among older women, they are underrepresented in clinical trials [3, 15, 39, 40]; analyses of real-world data can add breadth to the understanding of a treatment. In the current study population, patients were older than those who participated in the MONARCH trials [31, 38] and had a higher comorbidity burden, as evidenced by 48% of patients with a Elixhauser comorbidity score of 3+. Additionally, greater than 50% of patients had evidence of visceral disease and ≥ 3 metastatic sites. An exploratory analysis of PFS and safety in patients participating in MONARCH 2 and 3 was performed for three age groups (< 65, 65–74, and ≥ 75 years) [28]. Abemaciclib + endocrine therapy improved PFS compared with placebo + endocrine therapy, independent of patient age, although select AE rates were found to be higher in older patients, highlighting the importance of appropriate management across all groups, to maintain treatment benefit [28].
Dose holds and reductions may be of particular importance in an older adult population where barriers to adherence and persistence of oral anticancer therapy are especially prevalent [41]. These can include, but are not limited to, cognitive deficits, visual/hearing/functional impairment, comorbidity burden, higher treatment toxicity, polypharmacy, and financial burden, all of which could lead to self-discontinuation or nonadherence, which can be detrimental to outcomes [41]. A meta-analysis of CDK4 & 6i use by Omarini et al. assessed combined endocrine therapy with CDK4 & 6i as first line in older patients with HR+/HER2− mBC. Their results showed a PFS advantage in older patients receiving the combination therapy compared with endocrine therapy alone (HR: 0.57; P < 0.0001), supporting abemaciclib benefit in this population. However, hematological AEs (and diarrhea with abemaciclib) were higher in the older population compared with younger patients [42]. Additionally, a pooled study by the FDA concluded that patients aged ≥ 75 years who received CDK4 & 6i + AI had improved PFS versus AI alone (HR: 0.49; 95% CI 0.31, 0.76) [43]. Notably, > 50% of patients older than 70 years required ≥ 2 dose reductions and interruptions, generally within the first three cycles of therapy; and 32% of patients older than 75 years (versus 12% of patients < 75 years old) discontinued therapy owing to an AE. However, patients with dose reductions had similar or better outcomes than older patients with mBC who did not experience dose reductions and interruptions, reinforcing the importance of proactive management of treatment for these patients.
The discontinuation rate due to AEs for the present study increased with increasing patient age, consistent with observed trends from analyses of MONARCH 2 and MONARCH 3 [44]. A numerically higher proportion of patients in this study discontinued abemaciclib owing to an AE in the first year after FDA approval compared with those in the subsequent year, potentially reflecting increased provider and patient experience with treatment. However, contrary to MONARCH experience, only a minority of patients in the present study had a dose hold or dose reduction prior to discontinuation, representing an important opportunity to optimize management of older patients receiving abemaciclib treatment. Results of Cox regression modeling further reinforce that, even after adjusting for patient characteristics, those who had a dose reduction/hold had a 57% lower risk of discontinuation than those without a dose reduction/hold.
Delaying chemotherapy initiation in this population is an important treatment goal for all patients to avoid toxicity and deteriorations in quality of life. In both MONARCH 2 and 3, addition of abemaciclib to an AI or fulvestrant significantly delayed initiation of palliative cytotoxic chemotherapy [31, 38]. Despite our study population being older, being more heavily pretreated, and having a higher comorbidity burden than those in the MONARCH trials, the TTC results were not estimable except in the abemaciclib monotherapy group, where the median TTC was 121 days. CBR and PFS values are not as robust as those demonstrated in MONARCH studies; however, the differences in patient characteristics, utilization across LOTs, and the extent of chemotherapy and prior CDK4 & 6i use must be taken into consideration when interpreting these results [31, 38].
4.1 Limitations
Limitations include those inherent to a retrospective study based on secondary data. External validity may be limited owing to the geographic region of focus and community sites; approximately 70% of patients were treated in the southern part of the USA, with the majority treated within a single large community oncology network. Additionally, data were incomplete for some variables including race/ethnicity, smoking status, stage and tumor grade at initial diagnosis, and ECOG performance status at abemaciclib initiation. Data on specific adverse event type and/or supportive care use were not objectives of the study and are therefore not available. Next, data for the abemaciclib monotherapy group should be interpreted with caution owing to low patient numbers. Outcomes for CBR, PFS, TTD, and TTC could not be evaluated by line of therapy owing to insufficient patient numbers and should be considered when interpreting these data as well as for future research. Further, the differential impact of dose modification by age on clinical outcomes was not explored and warrants investigation. Finally, these data represent patient characteristics, utilization, and outcomes for abemaciclib in patients in the first 2 years following initial FDA approval. Future analyses are warranted to observe any changes in these aspects over time.
5 Conclusions
The results of this analysis complement the registration studies for abemaciclib by representing a community oncology experience, predominantly in the southern USA, in the first 2 years following abemaciclib FDA approval. Notably, patients in this analysis were older than those who participated in the MONARCH studies and had a substantial comorbidity burden, visceral disease, and significant prior chemotherapy and CDK4 & 6i use. The discontinuation rates observed were higher than previously reported in other real-world studies, highlighting the need for proactive management strategies, especially in older patients, to optimize outcomes in mBC. These findings, together with the previous combined safety analysis of MONARCH 2 and MONARCH 3 data might help inform clinicians of the management of older patients treated in the early breast cancer setting as well [44].
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Battisti NML, de Glas N, Sedrak MS, et al. Use of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors in older patients with ER-positive HER2-negative breast cancer: Young International Society of Geriatric Oncology review paper. Ther Adv Med Oncol. 2018;10:1–26.
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106: dju055. https://doi.org/10.1093/jnci/dju055.
Lobbezoo DJA, van Kampen RJW, Voogd AC, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141:507–14.
Brufsky AM. Delaying chemotherapy in the treatment of hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Med Insights Oncol. 2015;9:137–47.
Smith I. Goals of treatment for patients with metastatic breast cancer. Sem Oncol. 2006;33(2):2–5.
Martin M, Zielinski C, Ruiz-Borrego M, et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: a phase III randomized controlled trial-PEARL. Ann Oncol. 2021;32(4):P488–99.
Park YH, Kim TY, Kim GM, et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): a multicentre, open-label, randomized, phase 2 trial. Lancet Oncol. 2019;20(12):1750–9.
Cardoso F, Harbeck N, Fallowfield L, et al. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl_7):vii11–9.
Partridge AH, Rumble RB, Carey LA, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(29):3307–29.
Pritchard KI, Gelmon KA, Rayson D, et al. Endocrine therapy for postmenopausal women with hormone receptor-positive HER2-negative advanced breast cancer after progression or recurrence on nonsteroidal aromatase inhibitor therapy: a Canadian consensus statement. Curr Oncol. 2013;20:48–61.
National Comprehensive Cancer Network (NCCN). Older adult oncology. Version 1.2021. https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf. Accessed 17 Nov 2021.
Unger JM, Barlow WE, Marin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106(3):dju002.
Freedman RA, Gelman RS, Wefel JS, et al. Translational Breast Cancer Research Consortium (TBCRC) 022: a phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2016;34(9):945–52.
Sedrak MS, Freedman RA, Cohen HJ, et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. 2021;71:78–92.
El Sayed R, El Jamal L, El Iskandarani S, Kort J, Salam MA, Assi H. Endocrine and targeted therapy for hormone-receptor-positive, HER2-negative advanced breast cancer: insights to sequencing treatment and overcoming resistance based on clinical trials. Front Oncol. 2019;9:510.
Sledge GW, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–84.
Goetz M, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–46.
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–36. https://doi.org/10.1056/NEJMoa1607303.
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–39. https://doi.org/10.1016/S1470-2045(15)00613-0.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, PaluchShimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29:1541–7. https://doi.org/10.1093/annonc/mdy155.
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–72. https://doi.org/10.1200/JCO.2018.78.9909.
Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19:904–15. https://doi.org/10.1016/S1470-2045(18)30292-4.
Li J, Huo X, Zhao F, Ren D, Ahmad R, Yuan X, Du F, Zhao J. Association of cyclin-dependent Kinases 4 and 6 inhibitors with survival in patients with hormone receptor–positive metastatic breast cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(10):e2020312–e2020312.
Gennari A, Andre F, Barrios CH, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;S0923–7534(21):04498–507. https://doi.org/10.1016/j.annonc.2021.09.019.
Verzenio [package insert]. Eli Lilly and Company; 2021.
Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(−) metastatic breast cancer. Clin Cancer Res. 2017;23:5218–24.
Goetz M, Okera M, Wildiers H, et al. Safety and efficacy of abemaciclib plus endocrine therapy in older patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: an age-specific subgroup analysis of MONARCH 2 and 3 trials. Breast Cancer Res Treat. 2021;186:417–28.
Johnston S, Martin M, di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. npj Breast Cancer. 2019;5:5. https://doi.org/10.1038/s41523-018-0097-z.
Neven P, Johnston SRD, Toi M, et al. MONARCH 2: subgroup analysis of patients receiving abemaciclib + fulvestrant as first- and second-line therapy for HR+, HER2− advanced breast cancer. J Clin Oncol. 2020;38(suppl_15):1061–1061.
Sledge GW, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–24.
Weiskopf NG, Cohen AM, Hannan J, Jarmon T, Dorr DA. Towards augmenting structured EHR data: a comparison of manual chart review and patient self-report. In: AMIA Annual Symposium Proceedings 2019, vol. 2019. American Medical Informatics Association. p. 903.
Fleiss J. Measuring nominal scale agreement amongst many raters. Psychol Bull. 1971;76:378–82.
Mehta HB, Sura SD, Adhikari D, Andersen CR, Williams SB, Senagore AJ, Kuo YF, Goodwin JS. Adapting the Elixhauser comorbidity index for cancer patients. Cancer. 2018;124(9):2018–25.
Carter GC, Sheffield KM, Gossai A, et al. Real-world treatment patterns and outcomes of abemaciclib for the treatment of HR+, Her2− metastatic breast cancer. Curr Med Res Opin. 2021;37(7):1179–87.
Saverno KR, Beyrer J, Nash Smyth E, et al. Real-world patient characteristics, utilization patterns, and outcomes of US patients with HR+/HER2- metastatic breast cancer treated with abemaciclib. Presented at: 2020 San Antonio Breast Cancer Symposium, December 8–11, 2020. Abstract PS10–46.
Rassen JA, Schneeweiss S. Newly marketed medications present unique challenges for nonrandomized comparative effectiveness analyses. J Comp Eff Res. 2012;1(2):109–11.
Martin M, Johnston S, Huober J, et al. MONARCH 3: updated time to chemotherapy and diseases progression following abemaciclib plus aromatase inhibitor (AI) in HR+, HER2− advanced breast cancer (ABC). Ann Oncol. 2019;30(Suppl_5):v113–4.
Acheampong T, Kehm RD, Terry MG, Argov EL, Tehranifar P. Incidence trends of breast cancer molecular subtypes by age and race/ethnicity in the US from 2010–2016. JAMA Netw Open. 2020;3(8): e2013226.
Clarke CA, Keegen THM, Yang J, et al. Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst. 2012;104(14):1094–101.
Mislang AR, Wildes TM, Kanesvaran R, et al. Adherence to oral cancer therapy in older adults: the International Society of Geriatric oncology (SIOG) taskforce recommendations. Cancer Treat Rev. 2017;57:58–66.
Omarini C, Pacentini F, Sperduti I, et al. Combined endocrine approaches vs endocrine therapy alone as first line treatment in elderly patients with hormone receptor-positive, HER2 negative, advanced breast cancer: to prescribe for the patient or the physician? A meta-analysis of phase II and III randomized clinical trials. BMC Cancer. 2020;20:418.
Howie LJ, Singh H, Bloomquist E, et al. Outcomes of older women with hormone receptor-positive, human epidermal growth factor receptor-negative metastatic breast cancer treated with a CDK4/6 inhibitor and an aromatase inhibitor: an FDA pooled analysis. J Clin Oncol. 2019;37(36):3475–83.
Rugo HS, Duober J, Garcia-Saenz JA, et al. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive human epidermal growth factor receptor 2-negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist. 2021;26(1):e53–65.
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, Barrios CH, Bergh J, Bhattacharyya GS, Biganzoli L, Boyle F, Cardoso MJ, Carey LA, Cortes J, El Saghir NS, Elzayat M, Eniu A, Fallowfield L, Francis PA, Gelmon K, Gligorov J, Haidinger R, Harbeck N, Hu X, Kaufman B, Kaur R, Kiely BE, Kim SB, Lin NU, Mertz SA, Neciosup S, Offersen BV, Ohno S, Pagani O, Prat A, Penault-Llorca F, Rugo HS, Sledge GW, Thomssen C, Vorobiof DA, Wiseman T, Xu B, Norton L, Costa A, Winer EP. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–49. https://doi.org/10.1016/j.annonc.2020.09.010.
Delgado A, Guddati AK. Clinical endpoints in oncology-a primer. Am J Cancer Res. 2021;11(4):1121.
Acknowledgements
We acknowledge Bridgette Kanz Schroader, PharmD, MPA, BCOP, of Xcenda, LLC, for her contributions as a medical writer to this manuscript and Kylie Matthews of Xcenda, LLC, for her contributions as a copy editor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by Eli Lilly and Company. The sponsor participated in the collection (co-creation of data collection form), analysis, and interpretation of the data and decision to submit the article for publication. All authors vouch for the accuracy of the manuscript and data contained within.
Conflict of interest
Xcenda LLC is a consulting firm that received fees for the conduct of this work from Eli Lilly and Company. A.R. and M.K. have received honoraria for educational activities and advisory boards from Eli Lilly. E.S., K.S., F.S., Z.C., and S.R. are employees of Eli Lilly and company and hold company stock. T.L. is an employee of Xcenda, LLC and may own AmerisourceBergen stock. J.W, O.L., and A.C. were employees of Xcenda, LLC at the time of conduct of the study and may own AmerisourceBergen stock.
Ethics approval
The study was approved with a waiver of consent and a full waiver of Health Insurance Portability and Accountability Act authorization by the Advarra Institutional Review Board.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The dataset is not available for publication as the research data are confidential. Please reach out to the corresponding author with any questions.
Code availability
The SAS code is not available for publication. Please reach out to the corresponding author with any questions.
Author contributions
Conception and design: all authors; data collection: T.L., J.W., O.L., AC; analysis and interpretation of the data: all authors; manuscript writing and editing: all authors; approval of the final article: all authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ring, A., Karuturi, M., Smyth, E.N. et al. Real-World Analysis of Clinical and Demographic Characteristics, Treatment Patterns, and Outcomes in Predominantly Older Patients with HR+/HER2− Metastatic Breast Cancer Receiving Abemaciclib in Routine Clinical Practice. Drugs - Real World Outcomes 10, 589–603 (2023). https://doi.org/10.1007/s40801-023-00391-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-023-00391-1