Abstract
Background
Isotretinoin, indicated for severe acne, is a potent teratogen and therefore contraindicated in pregnancy. Thus, the pregnancy prevention program (PPP) for isotretinoin has been introduced.
Objectives
The aim of this study was to assess the concomitant use of isotretinoin and effective contraception and the rate of potential isotretinoin-exposed pregnancies in females of childbearing age in 2017–2020 in Estonia. In addition, we aimed to evaluate whether compliance with the PPP has improved compared with the previous study conducted in Estonia covering the period of 2012–2016.
Methods
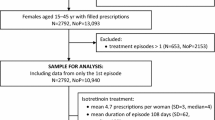
This retrospective, nationwide study using prescription and healthcare claims data included 2575 females aged 15–45 years who started using isotretinoin between 2017 and 2020.
Results
For 64.7% of females of childbearing age, no concurrent use of an effective contraceptive was detected while using isotretinoin. A moderately higher contraceptive coverage (35.3%) was observed compared with the previous study (29.7%) (p < 0.001). Complete contraception coverage was highest in females aged 30–39 years with an adjusted OR of 12.8 (p < 0.001) compared with the age group 15–19 years and 2.47 (p < 0.001) compared with the age group 20–29 years. 17 pregnancies coincided with the isotretinoin treatment-related period. The risk for potential isotretinoin-exposed pregnancy was 6.6 (95% CI 3.9–10.5) per 1000 treated females of childbearing age over the 4-year observation period. The risk for potential isotretinoin-exposed pregnancies per 1000 treated females was 1.0 in females aged 15–19 years, 11.6 in females aged 20–29 years, 8.8 in females aged 30–39 years, and 7.4 in females aged 40–45 years (p = 0.009).
Conclusion
A slight improvement in complete contraceptive coverage during isotretinoin use has not resulted in a decrease in the risk of isotretinoin-exposed pregnancies. The contraceptive usage and risk for pregnancy vary greatly across age groups, suggesting the need for a more targeted approach to improve the effectiveness of the PPP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite the simplification of the isotretinoin pregnancy prevention program (PPP) and educational materials in 2018, our study still refers to the ineffectiveness of current efforts. |
Although the complete contraceptive coverage during isotretinoin use is slightly improved, it remains still very low and has not resulted in a decrease in the risk of potential isotretinoin-exposed pregnancies. |
Differences in contraceptive behavior and risk of potential isotretinoin-exposed pregnancies across age groups suggest that more targeted educational efforts and application of modern assistive technologies are needed to improve the effectiveness of the PPP rather than setting new requirements. |
1 Introduction
Isotretinoin is primarily used for the treatment of severe forms of acne vulgaris, a common cutaneous disorder that can have a profound psychologic impact, contributing to low self-esteem, depression, and anxiety [1]. Although isotretinoin can be highly effective, both prescribers and patients must perceive the serious risks associated with its use. The most crucial safety concern of isotretinoin in females of childbearing age is teratogenicity [2, 3]. Like all retinoids, isotretinoin, even if used for a short period, is associated with a high risk of severe, life-threatening pregnancy outcomes and is therefore contraindicated in pregnancy. The most typical major malformations, in about 25% of infants exposed to isotretinoin during the first 20 weeks of pregnancy, are craniofacial, cardiac, thymic, and central nervous system abnormalities [3].
To ensure that females of childbearing potential are not pregnant when starting isotretinoin therapy and do not become pregnant while using isotretinoin, the pregnancy prevention program (PPP) for Roaccutane was first introduced in 1988 [4]. In 2003, due to the launch of generic formulations of isotretinoin and divergences in the product information across the European Union (EU), the harmonized EU PPP for all oral isotretinoin-containing medicinal products was implemented [5]. In Estonia, measures of the EU PPP were implemented in 2005 after joining the EU. A study conducted in Estonia assessing the compliance with the PPP during isotretinoin treatment in females of childbearing age between 2012 and 2016 showed that 29.7% of females had either full or partial contraceptive coverage [6]. The risk for potential isotretinoin-exposed pregnancy was 3.6 per 1000 treated females during the 5-year observation period [6]. In literature, the estimates of contraceptive coverage while using isotretinoin vary greatly depending on the methods and data used. In studies using administrative data, the contraceptive coverage has ranged from approximately 30% in Canada [7] and the United States [8] to about 50% in the Netherlands [9]. A systematic review of studies and case reports in Europe has shown a pregnancy incidence of 0.2–1.0 per 1000 women of childbearing age using isotretinoin [4].
The concerns about noncompliance with the PPP in clinical practice, notices of shortcomings in the PPP, and inconsistencies in the educational materials led to a European Medicines Agency’s (EMA) review on measures for pregnancy prevention during retinoid use. As a result of this review, updated PPP measures were implemented in June 2018, including simplified physician and patient educational materials that pointed out more clearly the teratogenicity risk and the need to use at least one highly effective method of contraception (i.e. a user-independent method) or two complementary user-dependent methods [5]. A recent survey conducted in Ireland found that although healthcare professionals were highly aware that isotretinoin should not be prescribed to women of childbearing age unless PPP conditions are met, the application of this knowledge differed significantly in clinical practice [10].
Our study aimed to assess the concomitant use of isotretinoin and effective contraception and the rate of potential isotretinoin-exposed pregnancies in females of childbearing age in Estonia during 2017–2020, and also to evaluate whether compliance with the PPP has improved since the previous study conducted in Estonia covering the period of 2012–2016.
2 Methods
2.1 Study Design and Data
This retrospective, nationwide drug utilization study included females aged 15–45 years who started using isotretinoin between 2017 and 2020. The STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Checklist for cross-sectional studies was used in the reporting of this study [11].
Prescription and healthcare claims data were obtained from the Estonian Health Insurance Fund (EHIF), the national health insurance provider in Estonia. During the study period, about 95% of the Estonian population was covered by the EHIF [12, 13].
To identify females who started using isotretinoin, data on isotretinoin (ATC code D10BA01) prescriptions issued between November 2016 and December 2020 was retrieved. For women receiving isotretinoin, prescription data of dispensed hormonal contraceptives (ATC groups G02B, G03A, and G03DA02) and healthcare claims data of insertion or management of an intrauterine contraceptive device (IUD) (International Classification of Diseases [ICD-10] diagnostic codes Z30.1 and Z30.5) from November 2012 to February 2021 were retrieved. Also, healthcare claims data related to pregnancy (ICD-10 diagnostic codes Z32.1, Z33, Z34, Z35, O00-O08) from November 2016 to March 2021 was obtained.
2.2 Use of Isotretinoin
Females aged 15–45 years filling at least one isotretinoin prescription during the study period were included in the analysis.
To calculate treatment episodes, daily treatment doses indicated on the prescription were used. Dosage regimens and physicians’ comments were reviewed by two researchers. Treatment episodes were calculated based on consecutive purchases and the daily dose, also taking into account the overlap. If the gap between the end of the calculated episode and the new purchase was more than 60 days, it was considered a new treatment episode. Only the first treatment episodes beginning from January 2017 to December 2020 were included in the final analysis (first episodes accounted for about 82% of all treatment episodes calculated). To exclude treatment episodes that started before the year 2017, we used a 2-month wash-out period from November to December 2016. At the time, isotretinoin had a 30-day supply limit, thus this 2-month period was considered sufficient.
Prescribers’ specialties (dermatovenerologists, general practitioners, other) and diagnoses for which isotretinoin was prescribed (acne, rosacea, seborrheic dermatitis, other follicular disorders, or other) were also identified.
2.3 Use of Effective Contraception
Effective contraception was considered as the use of hormonal contraceptives (excluding emergency contraceptives) or non-hormonal IUDs. For estimating the covered time of purchased oral, vaginal, and transdermal hormonal contraceptives, defined daily doses were used [14]. For IUDs and implants containing progestogen, the duration of use was set to 5 or 3 years, depending on how long the specific product was intended to be used [15]. For depot medroxyprogesterone acetate injection, the duration of use was set to 13 weeks [16]. In addition, contraception was also identified by healthcare service indicating an insertion or a surveillance of an IUD with a 5-year duration of coverage since the date of service provided.
Complete contraception coverage was defined as consistent use of contraceptives for 30 days before the start of the isotretinoin episode until 30 days after the end of the isotretinoin episode, as described as being mandatory in the summary of product characteristics and the PPP of isotretinoin. Consequently, partial coverage was identified if contraception was missing at some point in time during the period described above. If the isotretinoin episode did not coincide with any use of contraceptive, it was defined as the absence of contraception.
2.4 Potential Isotretinoin-Exposed Pregnancies
Pregnancy was determined based on ICD-10 codes indicating early pregnancy (Z32.1, Z33, Z34, Z35, O00–O08). Potential isotretinoin-exposed pregnancy was detected if a pregnancy-related healthcare service coincided with the period from the beginning of the isotretinoin episode to 30 days after the end of the isotretinoin episode. All detected pregnancy cases were reviewed manually by researchers KK and MI to ensure that none was counted more than once.
2.5 Statistical Analysis
Statistical analysis of the current study was performed similarly to the Uusküla et al. 2018 study [6], to enable comparison of the results. Descriptive statistics were used to summarize patient characteristics and isotretinoin and contraception usage. Females were stratified into the following age groups: 15–19, 20–29, 30–39, and 40–45 years.
Fisher’s exact test was used to test statistical differences between the age groups and contraception coverage. Factors associated with contraception coverage during isotretinoin treatment were assessed using logistic regression analysis, generating univariate and multivariable estimates of odds ratios with 95% confidence intervals.
The risk for potential isotretinoin-exposed pregnancy with a 95% confidence interval was calculated per 1000 treated females, stratified by age groups, year, and contraception coverage.
Statistical analysis was performed using R statistical software, version 4.1.2 [17].
2.6 Ethical Considerations
The study procedures were conducted in accordance with local data protection regulations. The study was approved by the Health Development Institute Ethics Committee (Decision 643 on 23 February, 2021 in Estonia).
3 Results
3.1 Characteristics of Study Subjects and Treatment Courses
During the study period, 2975 females started using isotretinoin in Estonia, and most of them (86.6%, n = 2575) were of childbearing age (15–45 years). The majority of females of childbearing age were young females, 40.4% in the years 15–19 and 36.7% in the years 20–29; 17.6% were 30–39 years old and 5.2% were 40–45 years old at the start of using isotretinoin.
The incidence of isotretinoin use was consistent over the study years, with approximately 2.7 users per 1000 females of childbearing age in the population. However, the number of prescriptions per user decreased significantly in 2020, with only 3.4 prescriptions compared with 4.6–4.8 prescriptions in the years 2017–2019 (p < 0.001). The median duration of an isotretinoin treatment episode was 134 days (range 10–1230 days), the mean daily dose of isotretinoin was 31.1 mg (SD 12.8, range 4.3–140 mg). The majority of prescriptions were issued by dermatovenerologists (94%), rarely by family medicine (3.7%) or other specialties (2.3%). Isotretinoin was mostly prescribed for acne (91.7%), followed by rosacea (6.1%) and other skin disorders (e.g. other follicular disorders, seborrheic dermatitis).
3.2 Effective Contraception Coverage
For the majority of females of childbearing age (64.7%, n = 1667), it was not possible to detect concurrent use of an effective contraceptive with isotretinoin. The absence of contraception was highest among young females aged 15–19 years (83.1%).
In total, 35.3% (n = 908) of females of childbearing age were using effective contraception concurrently with isotretinoin. The most common contraceptive method was combined oral contraceptives (53.3%), followed by IUD (36.7%), progestin-only pills (4.7%), vaginal ring (4.1%), transdermal patch (3.4%), and implant (2.8%). Contraceptive method preferences differed significantly between age groups (p < 0.001). User-dependent methods were prevalent among young females (Fig. 1).
Only 20.6% (n = 531) of females of childbearing age were completely covered and 14.6% (n = 377) were partially covered with contraception. Out of those who had partial coverage, 16.4% (n = 62) started using contraception on the same day as isotretinoin. Complete coverage was highest among females aged 30–39 years (42.8%), partial coverage for females aged 20–29 years (21%) (Fig. 2).
The complete coverage in this study period was significantly higher (4.9%, 95% CI 2.8–7.0; p < 0.001) compared with the results from the previous study of the years 2012–2016. This improvement was statistically significant in age groups 20–29 years and 30–39 years but not for age groups 15–19 years or 40–45 years (Table 1).
When comparing females with complete coverage with those who were not or were partially covered, age was the most significant factor after adjustment. Complete contraception coverage increased with age and was highest in females aged 30–39 years, whose adjusted odds ratio (OR) for complete coverage was 12.8 (95% CI 9.26–17.91; p < 0.001) compared with females aged 15–19 years and 2.47 (95% CI 1.93–3.16, p < 0.001) compared with those aged 20–29 years) (Fig. 3). During the study period, statistically significant improvement in complete coverage was seen only for 2019 (adjusted OR 1.38, 95% CI 1.03–1.85; p = 0.030) compared with 2017.
3.3 Potential Isotretinoin-Exposed Pregnancies
During the study period, 17 pregnancies coincided with isotretinoin treatment. Of these pregnancies, 11 (64.7%) were terminated by medical abortion, two (11.8%) were spontaneous abortions, and four pregnancies (23.5%) were ongoing (no data on the outcome). On average, these pregnancies were diagnosed 90 days (SD 67, range 14–279) after the start of isotretinoin treatment. The risk for potential isotretinoin-exposed pregnancy was 6.6 (95% CI 3.9–10.5) per 1000 treated females of childbearing age over the 4-year observation period. Compared with the previous study (3.6 per 1000 treated females), the risk was not statistically significantly higher (95% CI −1.19 to 7.23; p = 0.171).
By age group, the risk per 1000 treated females was 1.0 (1/1041; 95% CI 0–5.3) among 15- to 19-year-olds, 11.6 (11/946; 95% CI 5.8–20.7) in women aged 20–29 years, 8.8 (4/453; 95% CI 2.4–22.5) in women aged 30–39 years, and 7.4 (1/160; 95% CI 0.2–40.6) in women aged 40–45 years (p = 0.009). The risk for potential isotretinoin-exposed pregnancy was statistically significantly lower in females aged 15–19 years compared with women in their 20s (p = 0.014).
The risk varied between the study years: 4.6 per 1000 females (95% CI 1.0–13.5) in 2017, 7.8 per 1000 females (95% CI 2.5–18.2) in 2018, 9.5 per 1000 females (95% CI 3.5–20.5) in 2019, 4.6 per 1000 females (95% CI 0.9–13.3) in 2020, but the difference was not statistically significant (p = 0.649).
By contraception coverage, the risk for potential isotretinoin-exposed pregnancy was 2- to 3-fold higher in females with no contraceptive use (6.6 per 1000 females, 95% CI 3.3–11.7) and females with partial coverage (10.8 per 1000 females, 95% CI 2.9–27.3) than in females with complete coverage (3.8 per 1000 females, 95% CI 0.5–13.5). However, it failed to reach statistical significance (p = 0.444).
4 Discussion
In this nationwide repeat study, somewhat higher contraceptive coverage (35.3%) was observed in females of childbearing age using isotretinoin compared with the previous study (29.7%) [6] or compared with the overall prevalence of hormonal contraceptive use among females of childbearing age in Estonia in 2019 (28.5%) [18]. However, considering that isotretinoin is a potent teratogen, this rate is not sufficient to meet the objective of the PPP, as a higher absolute number of potential isotretinoin-exposed pregnancies was detected than in the previous study [6]. Interestingly, the observed risk for potential isotretinoin-exposed pregnancy is much higher in Estonia (6.6 per 1000 treated females) than recently reported in four EU countries (0.1–0.4 per 1000 retinoid users), in an impact study on oral retinoids by the European Medicines Agency (EMA) [19, 20]. This discrepancy may be partially explained by the incompleteness of the registries used and methodological differences in studies. A similar rate of isotretinoin-exposed pregnancies to our study has been found in Canada (6.2 per 1000 treated females) [7]. The risk of potential pregnancies exposed to isotretinoin varied during the study years, with a lower risk reported in 2020. This decline in risk could be partially attributed to a reduction in the number of prescriptions per woman in 2020, possibly due to limited access to medical care during the COVID-19 pandemic. Furthermore, COVID-19 restrictions such as stay-at-home orders and social isolation may have reduced unintended pregnancies by impacting young people’s sexual activity.
Our study illustrates well the differences in contraception use and risk for pregnancy between age groups. Among females aged 15–19 years, the lowest use of effective contraception and at the same time the lowest risk for pregnancy was observed. These young females are likely to be predominantly not yet sexually active and thus contraceptive use is not relevant. According to a study conducted in Estonia on sexual behavior, the average age of women during their first sexual intercourse was 18.7 years [21]. Inconsistent contraception coverage among young females has also been demonstrated in a Belgium study, where 63.8% of females aged 12–21 years taking isotretinoin were prescribed at least one contraceptive, but only 15.7% used it as recommended during treatment [22]. Providing better education on the effectiveness of various contraceptive methods and knowledge of the teratogenic risks associated with certain medicines could have a significant impact on individuals in this age group later in life.
According to the findings of our study, the most problematic age group seems to be the 20-year-old. A relatively large proportion of partial contraception coverage and the highest risk for exposed pregnancies indicates the volatile sex life of this age group. In Estonia, young females prefer mainly user-dependent methods, most commonly condoms, hormonal pills, and ineffective methods like withdrawal [18, 23]. Young females may have a knowledge gap in recognizing effective contraception methods and overestimate the effectiveness of contraceptives in typical use [23]. For example, one missed dose can lead to pregnancy in contraceptive pills. Considering this, patient education is important not only to avoid a missed dose but also to teach women what to do in such circumstances. Promoting the use of user-independent methods, which do not depend on user adherence, in young females could minimize the risk of contraceptive failure and unintended pregnancy. While IUD usage increased among isotretinoin users in Estonia between 2012–2016 and 2017–2020 (21.9% to 36.7%, respectively) [6], it is still a less preferred contraceptive method among young females. Better education about the effectiveness and advantages of different contraceptive methods seems essential to avoid unintended pregnancies. In addition, referring young patients for contraceptive counseling could be considered.
Some of the non-compliance with PPP requirements may be attributed to prescribers. Although the awareness of the teratogenic risks of oral retinoids among healthcare professionals is high in some European countries, only a small proportion of them comply with aspects of the PPP [24]. This may be due to several reasons, including time constraints in patient counseling [24], information overload, PPP materials in impractical format, reluctance to read information sent by marketing authorization holders, etc. According to our knowledge, the awareness of healthcare professionals about the isotretinoin PPP has not been investigated in Estonia, indicating a need for further research on this topic. A study conducted in Belgium showed that most healthcare professionals (among respondents, 100.0% GPs and dermatologists, 98% pharmacists) and female patients (87.9% of respondents) were aware of the teratogenic risk of isotretinoin, but only 41.7% of dermatologists, 4.2% of GPs, 24.1% of pharmacists, and 15.2% of female patients indicated that they knew of the existence of the PPP [25]. Following the implementation of the revised isotretinoin PPP, the awareness, knowledge, and experience of implementing the PPP in clinical practice has been studied in Ireland. Hughes et al. [10] showed that healthcare professionals were highly aware (≥ 87%) that isotretinoin should not be prescribed to women of childbearing age unless PPP conditions are met. However, the implementation of this knowledge varied greatly in clinical practice. Specialists (dermatology, obstetrics, and gynecology) were more likely (71.4%) to request a pregnancy test before initiating treatment with isotretinoin than general practitioners (31.6%). Similarly, 47.6% of specialists provided patients with a reminder card when initiating the treatment, while only 11.8% of general practitioners did the same. Only 26.1% of community pharmacists provided patients with a reminder card at each dispensing [10]. It is questionable whether sending hard copies or emailing safety information to physicians is an efficient way to implement risk minimization measures in daily practice. Discussions are ongoing in Estonia regarding incorporating the automated alert system for risk minimization measures into prescription and pharmacy dispensing software, which could raise awareness of the risk and its minimization measures and improve the monitoring of continuous compliance with measures between prescribers and pharmacists. Similar discussions are taking place about enhancing patient access to risk minimization materials through the Estonian Health Portal.
There has been a lot of debate about the burden of PPPs on the health system and patients. Various studies conducted before and after the update of pregnancy prevention programs demonstrate that pregnancies continue to occur even with comprehensive measures in place [26,27,28]. In the United States, where a stringent risk management program (iPLEDGE) is in place, approximately 30% of clinicians who regularly prescribe isotretinoin have at times chosen not to prescribe isotretinoin to patients with severe acne because of the burden of the iPLEDGE program [29]. Therefore, it is essential that risk minimization measures are balanced to achieve the expected added value and not to limit the patients’ access to effective treatment. However in the EU, during the update of the PPP for retinoids in 2018, requirements for pregnancy testing and contraception were a bit loosened, and some requirements were waived (e.g. limitation of prescription to 7-day validity and a 30-day supply) [5], thus the PPP does not seem to have redundant elements. Pregnancy testing and the use of effective contraception are essential measures in women of childbearing potential to avoid fetal exposure to a teratogen. To highlight this, some pregnancies in our study were diagnosed early in isotretinoin treatment, which could have been avoided with prior pregnancy testing. Furthermore, women should be advised that hormonal contraceptives are not effective immediately upon initiation, and thus there is a requirement that contraception should be started before isotretinoin initiation.
Limitations of this study are mainly associated with using administrative data. It was not possible to ascertain if a female is not sexually active, thus not needing contraception. Also, some females do not have childbearing potential, for example, due to sterilization. However, the proportion of those females is rather marginal in Estonia [23]. In addition, there is no data about condom use, which is considered an effective, yet user-dependent method. The complete contraception coverage may be slightly overestimated as the diagnostic code Z30.5 includes checking, reinsertion, or removal of the IUD. Therefore, for some women, it may have meant removal of the IUD, but was counted as covered. This may also apply for two pregnancies detected in women with complete contraceptive coverage in whom this diagnostic code was present. Thus, there is the possibility that the IUD was actually removed in these women. Another limitation is that the cohort of females of childbearing age using isotretinoin was perhaps too small to reach statistical significance in statistical tests assessing pregnancy risk. A limitation specific to the prescription data also applies here; it cannot be entirely known whether the patient actually consumed the dispensed medicine.
5 Conclusion
This nationwide study provides additional information on compliance with pregnancy prevention recommendations for isotretinoin. Despite the simplification of the PPP and educational materials in 2018, our study still demonstrates the ineffectiveness of current efforts. Although complete coverage with contraception during isotretinoin use is slightly improved, it remains very low, and it has not resulted in a decrease in the risk of isotretinoin-exposed pregnancies. The proportion of partial contraception coverage and the risk for exposed pregnancies was especially pronounced amongst females aged 20–29 years—the most problematic age group according to our study. Attempts to improve the effectiveness of the PPP are necessary. This could be done by improving education of different target groups and the application of modern assistive technologies rather than setting new requirements. Future studies are needed to assess whether the interventions planned would have the desired outcome.
References
Graber E. Acne vulgaris: overview of management. UpToDate, Post TW (Ed), UpToDate, Waltham, MA.
Rosa FW. Teratogenicity of Isotretinoin. Lancet. 1983;322:513.
Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, et al. Retinoic acid embryopathy. N Engl J Med. 1985;313:837–41.
Crijns HJMJ, Straus SM, Gispen-de Wied C, de Jong-van den Berg LTW. Compliance with pregnancy prevention programmes of isotretinoin in Europe: a systematic review. Br J Dermatol [Internet]. 2011;164:238–44. https://doi.org/10.1111/j.1365-2133.2010.09976.x.
Retinoid-containing medicinal products | European Medicines Agency [Internet]. https://www.ema.europa.eu/en/medicines/human/referrals/retinoid-containing-medicinal-products. Accessed 28 Nov 2022.
Uusküla A, Pisarev H, Kurvits K, Laius O, Laanpere M, Uusküla M. Compliance with pregnancy prevention recommendations for isotretinoin in Estonia in 2012–2016. Drugs Real World Outcomes [Internet]. 2018;5:129–36. https://doi.org/10.1007/s40801-018-0135-z.
Henry D, Dormuth C, Winquist B, Carney G, Bugden S, Teare G, et al. Occurrence of pregnancy and pregnancy outcomes during isotretinoin therapy. Can Med Assoc J [Internet]. 2016;188:723–30. https://doi.org/10.1503/cmaj.151243.
Pinheiro SP, Kang EM, Kim CY, Governale LA, Zhou EH, Hammad TA. Concomitant use of isotretinoin and contraceptives before and after iPledge in the United States. Pharmacoepidemiol Drug Saf [Internet]. 2013;22:1251–7. https://doi.org/10.1002/pds.3481.
Teichert M, Visser LE, Dufour M, Rodenburg E, Straus SMJM, De Smet PAGM, et al. Isotretinoin use and compliance with the Dutch pregnancy prevention programme: a retrospective cohort study in females of reproductive age using pharmacy dispensing data. Drug Saf [Internet]. 2010;33:315–26. https://doi.org/10.2165/11319190-000000000-00000.
Hughes JE, Buckley N, Looney Y, Kirwan G, Mullooly M, Bennett KE. Evaluating awareness, knowledge and practice of healthcare professionals following implementation of a revised pregnancy prevention programme for isotretinoin in Ireland: a multi-stakeholder cross-sectional study. Pharmacoepidemiol Drug Saf. 2023;32:137–47.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9.
Estonian Health Insurance Fund (2022) [Internet]. https://statistika.haigekassa.ee/PXWeb/pxweb/et/kindlustatu/kindlustatu__Kindlustus/KN05.px/table/tableViewLayout2/?rxid=edfa6b1b-be27-4b15-86ef-1f0228a0af43. Accessed 15 Mar 2022.
Rahvaarv | Statistikaamet [Internet]. https://www.stat.ee/et/avasta-statistikat/valdkonnad/rahvastik/rahvaarv. Accessed 15 Mar 2022.
WHOCC—Home [Internet]. https://www.whocc.no/. Accessed 16 Apr 2022.
Estonian State Agency of Medicines. Register of Medicinal Products. [Internet]. https://ravimiregister.ee/en/publichomepage.aspx. Accessed 16 Apr 2022.
Health Products Regulatory Authority. Depo-Provera 150 mg/ml Suspension for injection. Summary of product characteristics. 2021. http://www.hpra.ie/img/uploaded/swedocuments/Licence_PA0822-124-001_21062021164149.pdf. Accessed 16 Apr 2022.
R Core Team (2021). R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing [Internet]. Vienna, Austria; https://www.r-project.org/.
Kurvits K, Laius O, Uusküla M, Laanpere M. Trends in the use of hormonal contraception in Estonia 2005–2019 and the risk of arterial and venous thromboembolism: a population-based study. Eur J Contracept Reprod Heal Care [Internet]. 2021;26:413–20. https://doi.org/10.1080/13625187.2021.1931839.
21st ISoP Annual Meeting "A New Era of Pharmacovigilance: Challenges and Opportunities” 20–23 September 2022 Verona, Italy. Drug Saf [Internet]. 2022;45:1111–327. https://doi.org/10.1007/s40264-022-01219-7
The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. Impact of EU label changes and revised pregnancy prevention programme for oral retinoid containing medicinal products: utilization and prescribing trends [Internet]. https://www.encepp.eu/encepp/viewResource.htm?id=45887. Accessed 4 Dec 2022.
Lõhmus L, Lemsalu L, Rüütel K, Vals K. Eesti täiskasvanud elanikkonna seksuaalkäitumine. Uuringuraport 2017. [Internet]. Tallinn: Tervise Arengu Instituut; 2018. https://tai.ee/sites/default/files/2021-03/153501440828_Eesti_täiskasvanud_elanikkonna_seksuaalkäitumine_2017.pdf
Biset N, Lelubre M, Amighi K, Bugnon O, Schneider MP, De Vriese C. Assessment of medication adherence and responsible use of isotretinoin and contraception through Belgian community pharmacies by using pharmacy refill data. Patient Prefer Adherence. 2018. https://doi.org/10.2147/PPA.S149355.
Ottep K, Laanpere M, Ringmets I. Rasestumisvastaste meetodite kasutamine 16–44 aastaste naiste hulgas Eestis: levimus, sotsiaal-majanduslikud ja tervishoiuteenustega seotud võimalikud barjäärid. Eesti Arst. 2019;98:135–43.
The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. Study of impact of EU label changes and revised pregnancy prevention programme (PPP) for oral retinoid-containing medicines: risk awareness and adherence [Internet]. https://www.encepp.eu/encepp/viewResource.htm?id=40650. Accessed 4 Dec 2022.
Lelubre M, Hamdani J, Senterre C, Amighi K, Peres M, Schneider MP, et al. Evaluation of compliance with isotretinoin PPP recommendations and exploration of reasons for non-compliance: survey among French-speaking health care professionals and patients in Belgium. Pharmacoepidemiol Drug Saf [Internet]. 2018;27:668–73. https://doi.org/10.1002/pds.4441.
Brinker A, Kornegay C, Nourjah P. Trends in adherence to a revised risk management program designed to decrease or eliminate isotretinoin-exposed pregnancies: evaluation of the accutane SMART program. Arch Dermatol [Internet]. 2005;141:563–9. https://doi.org/10.1001/archderm.141.5.563.
Crijns I, Straus S, Luteijn M, Gispen-de Wied C, Raine J, de Jong-van den Berg L. Implementation of the harmonized EU Isotretinoin Pregnancy Prevention Programme: a questionnaire survey among European Regulatory Agencies. Drug Saf [Internet]. 2012;35:27–32. https://doi.org/10.2165/11595570-000000000-00000.
Tkachenko E, Singer S, Sharma P, Barbieri J, Mostaghimi A. US food and drug administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol [Internet]. 2019;155:1175–9. https://doi.org/10.1001/jamadermatol.2019.1388.
Lee G, Wolf JR, Somers KE. Administrative Burden of iPLEDGE Deters Isotretinoin Prescriptions: Results From a Survey of Dermatologists. Cutis [Internet]. Frontline Medical Communications Inc; 2022;110:44–7. https://www.mdedge.com/dermatology/article/256053/acne/administrative-burden-ipledge-deters-isotretinoin-prescriptions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received to conduct the study. Open access funded by Helsinki University Library.
Conflicts of interest
Maaja Ivask is employed as a Patient Safety Lead Estonia, Roche International Pharmacovigilance, Roche Eesti OÜ. Katrin Kurvits, Maia Uusküla, Anne Juppo, Ott Laius, and Mia Siven report no conflict of interest.
Availability of data and material
Data were used on the basis of the agreement with the EHIF for conducting the current research and will not be shared in a public repository. It is possible to obtain the data for research from EHIF via appropriate data request (https://www.haigekassa.ee/andmeparingud).
Ethics approval
The study procedures were conducted in accordance with local data protection regulations. The study was approved by the Health Development Institute Ethics Committee (Decision 643 on 23 February, 2021 in Estonia).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
The study was designed by Maaja Ivask and Katrin Kurvits who also analyzed the data. The first draft of the manuscript was written by Maaja Ivask and Katrin Kurvits. Maia Uusküla, Ott Laius, Anne Juppo, and Mia Siven commented on the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ivask, M., Kurvits, K., Uusküla, M. et al. Compliance with Pregnancy Prevention Recommendations for Isotretinoin Following the Amendment of the European Union Pregnancy Prevention Program: A Repeat Study in Estonia. Drugs - Real World Outcomes 11, 91–98 (2024). https://doi.org/10.1007/s40801-023-00381-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-023-00381-3