Abstract
Background
The diuretic effect of tolvaptan, a vasopressin V2 receptor antagonist, in patients with severe renal dysfunction remains poorly characterized. Thiazide diuretics reduce urinary volume (UV) in patients with nephrogenic diabetes insipidus, which lacks V2 receptor function.
Objective
This retrospective study investigated the acute urinary effects of tolvaptan in patients with stage G5 chronic kidney disease and congestive heart failure (CHF), and the impact of thiazide diuretics on the urinary effects of tolvaptan.
Methods
UVs 24 h before and after tolvaptan administration and 30-day dialysis initiation rate were compared between patients with and without thiazide diuretic administration.
Results
Thiazide diuretics were used in 26 of the 106 recruited patients (age 73.4 ± 13.0 years; estimated glomerular filtration rate 8.07 ± 3.13 mL/min/1.73 m2). The pre- and post-tolvaptan 24-h UVs were significantly higher in patients not administered thiazide diuretics (1043.4 ± 645.6 vs. 1422.2 ± 774.0 mL/day; p < 0.001) than in those administered thiazide diuretics (1177.3 ± 686.5 vs. 1173.1 ± 629.1 mL/day; p = 0.93). In a multivariate regression model, thiazide diuretic use was significantly associated with decreased 24-h UV (β coefficient − 486.7, 95% confidence interval [CI] − 674.5 to − 298.8); increased urine osmolality (β coefficient 37.7, 95% CI 17.1–58.4); increased body weight (β coefficient 0.62, 95% CI 0.31–0.92); and increased 30-day dialysis initiation rate (odds ratio 3.40, 95% CI 1.18–9.82) after tolvaptan administration.
Conclusions
Tolvaptan exhibited significant diuretic effects in patients with CHF, including those with severe renal dysfunction, which were diminished with concomitant thiazide diuretic use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We retrospectively analyzed the acute urinary effects of tolvaptan (TLV) on patients with chronic kidney disease (CKD) stage G5 and congestive heart failure (CHF) without renal replacement therapy, and also assessed the impact of thiazide diuretics on the efficacy of TLV. |
This retrospective study demonstrated the diuretic effects of TLV, including increased UV and decreased urine osmolarity and body weight, in patients with CKD stage G5, suggesting that coupling TLV with loop diuretics to treat CHF is useful even if a patient’s kidney function is compromised. |
Thiazide diuretics significantly attenuated the diuretic effect of TLV in this study; one should exercise caution when prescribing concomitant use of thiazide diuretics with TLV. |
1 Introduction
Tolvaptan (TLV) is a vasopressin V2 receptor (V2R) antagonist that increases electrolyte-free water diuresis. TLV is indicated for treating hyponatremia in the US and Europe and cannot be used for congestive heart failure (CHF) alone. Conversely, along with other Asian countries, including China, Turkey, and Singapore, the Japanese guidelines for heart failure indicate TLV for treating fluid retention associated with CHF [1], mainly based on its demonstrated short-term efficacy in heart failure [2]. This drug may be administered along with other diuretics, mainly loop diuretics. A previous randomized controlled trial (RCT) showed that TLV increased urinary volume (UV) in patients with CHF with residual congestion and renal dysfunction, and contrary to furosemide, it did not worsen renal function [3]. However, how TLV affects patients with advanced chronic kidney disease (CKD), especially stage G5 (estimated glomerular filtration rate [eGFR] < 15 mL/min/1.73 m2), is poorly understood [4,5,6].
Thiazide diuretics have long been known to act paradoxically and improve polyuria (i.e., decrease UV) in patients with nephrogenic diabetes insipidus, in whom the vasopressin V2R function is absent; therefore, they may reduce UV in patients receiving TLV and whose vasopressin V2R is pharmacologically blocked [7]. Previous reports found that concomitant use of thiazide diuretics with TLV reduced UV and increased urine osmolality (UOsm) in patients with autosomal dominant polycystic kidney disease (ADPKD) [8, 9]; however, this phenomenon has not been confirmed among patients with CHF.
We therefore retrospectively analyzed the acute urinary effects of TLV on patients with CKD stage G5 and CHF without renal replacement therapy. We also assessed the impact of thiazide diuretics on the effect of TLV.
2 Methods
2.1 Study Population
This single-center retrospective cohort study, which was performed in Keio University Hospital, Tokyo, Japan, was approved by the Research Ethics Committee of Keio University, School of Medicine (approval number: 20211114) and included all hospitalized patients who were older than 20 years of age, had stage 5 CKD, and were newly prescribed TLV for CHF treatment in the Department of Nephrology between 1 January 2011 and 31 March 2021. A diagnosis of CHF is based on the presence of dyspnea and edema, which result from failed compensation of cardiac function accompanied by decreased exercise tolerance [1]; however, in this study, no strict diagnostic criteria were set and the CHF diagnosis was left to the clinician’s judgment due to the retrospective study design.
We excluded patients whose UV was not available or who underwent dialysis on the day of TLV prescription. Informed consent to participate in this study and for publication of the data was obtained in the form of opt-out online.
2.2 Data Collection and Patient Evaluation
The following patient demographics were collected during TLV administration: age, sex, primary CKD status, TLV dose (mg), and type of antihypertensive agent or diuretic concomitantly used. The dose and method of administration of furosemide were also recorded. Azosemide 60 mg was considered equivalent to furosemide 40 mg [10]. Moreover, changes in the dose of loop diuretics, thiazide diuretics, and mineralocorticoid receptor antagonists during the 24-h period before TLV administration were obtained from the records. Furthermore, the length of time between hospitalization due to CHF to TLV administration was evaluated.
Baseline serum parameters obtained from the records within 24 h before beginning TLV treatment included albumin (g/dL); creatinine (mg/dL); eGFR (mL/min/1.73 m2) calculated using serum creatinine based on the following three-variable equation for the Japanese: eGFR (mL/min/1.73 m2) = 194 × serum creatinine − 1.094 × age − 0.287 × 0.739 (if female) [11]; and sodium (mEq/L). Brain natriuretic peptide (BNP; pg/mL) was measured from plasma samples, whereas UOsm and urinary protein-to-creatinine ratio (UPCR; g/gCre) were measured from spot urine samples in the morning. Furthermore, left ventricular ejection fraction (%), left ventricular mass index (g/m2), and the ratio of early diastolic filling velocity to early mitral lateral annulus velocity (E/e′ ratio) were measured using echocardiography.
2.3 Outcome Measures
TLV was administered orally once per day in the morning (8:00–10:00 am). The primary outcomes were the effect of TLV on 24-h UV and the impact of comcomitant thiazide diuretic use on 24-h UV. Briefly, 24-h UV was measured from 6:00 am until 6:00 am the next day, and the 24-h UV on the day before TLV treatment (pre-24-h UV) was compared with that on the day of TLV initiation (post-24-h UV) to assess the acute effects of TLV. Secondary comparisons included baseline UOsm (pre-UOsm) with that of 24 h after administration of TLV (post-UOsm), as well as body weight (BW) in the morning (6:00–8:00 am) just before TLV administration (pre-BW) and that on the following day (post-BW).
All-cause 30-day mortality (defined as death from any cause 30 days following the initiation of TLV), 30-day dialysis initiation (defined as induction of dialysis 30 days following the initiation of TLV), and length of hospital stay (days, defined as the difference between discharge and the day of TLV initiation; patients discharged on death were excluded) were obtained from the records [12].
2.4 Statistical Analysis
Continuous variables were presented as means ± standard deviation or medians (interquartile range [IQR]) based on the Kolmogorov–Smirnov test. Binary variables were expressed as n (%). Differences between patients taking thiazide diuretics and those who were not were analyzed using an unpaired Student’s t-test, the Mann–Whitney U test, and Fisher’s exact test. Pre- and post-values were compared using the paired Student’s t-test. Analysis of covariance (ANCOVA) was used to test for significant differences between patients with and without thiazide diuretics, with pre-values as covariates and post-values as dependent variables.
Multiple linear regression was performed using post-24-h UV, UOsm, and BW as dependent variables and pre-24-h UV, UOsm, and BW as independent variables. Additionally, the variables that might affect UV or diuresis were selected as independent variables. CHF, nephrotic syndrome, and CKD lead to sodium and water retention, with decreased sodium water excretion accompanied by decreased UV [13], and induce resistance to diuretics [14,15,16]. Therefore, as independent variables along with thiazide diuretic use, serum albumin and UPCR were included as indicators of the nephrotic state, BNP was included as a marker of CHF, and eGFR was included as an indicator of CKD. Additionally, multivariate sensitivity analyses were conducted with post-24-h UV as a dependent variable. These analyses were performed by (1) adding the change in the dose of loop diuretics as an independent variable, and (2) excluding those patients in whom the dose of loop diuretics was changed. Additionally, age, sex, pre-24-h UV, eGFR, and thiazide diuretic use were used as candidate independent variables in the logistic regression model for 30-day dialysis initiation because demographics such as age and sex could have a significant influence on the relationship between the timing of dialysis initiation and the subsequent clinical outcomes [17], along with UV, whose decrease directly exacerbates volume overload and eGFR.
All statistical analyses were performed using SPSS software for Macintosh (version 27; IBM Corporation, Armonk, NY, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [18]. A two-tailed p-value <0.05 indicated statistical significance.
3 Results
3.1 Patient Clinical Characteristics
Among the 339 patients assessed for eligibility, 122 patients fulfilled the inclusion criteria. Nine patients receiving maintenance peritoneal dialysis and seven patients whose pre- and/or post-UV data were missing were excluded; therefore, the study included 106 patients (Fig. 1). Thiazide diuretics were concomitantly used in 26 patients (25%), including 23 patients taking trichlormethiazide (1 mg/day for 13 patients and 2 mg/day for 10 patients), 1 patient taking hydrochlorothiazide, and 2 patients taking indapamide. Mineralocorticoid receptor antagonists were prescribed in 13 patients, including 10 patients taking spironolactone (25 mg/day for 9 patients and 50 mg/day for 1 patient), 1 patient taking potassium canrenoate, and 2 patients taking eplerenone. The drug doses were not changed from the previous 24 h before TLV administration. The clinical characteristics of the study population at the time of TLV initiation and its division into two groups according to the use of thiazide diuretics are summarized in Table 1. TLV was administered at a median of 5 days (IQR 3.0–11.8) after hospitalization due to CHF. All patients used loop diuretics at baseline, which were administered intravenously and orally to 69 and 37 patients, respectively, whose median doses were 80 and 40 mg. Among 106 patients using loop diuretics, the dose was reduced from the previous 24 h in 21 patients, whereas the dose was increased at the time of TLV administration in 18 patients; the dose remained the same in the remaining 67 patients. Serum creatinine levels were significantly higher in those patients with thiazide diuretics (7.96 ± 4.91 vs. 6.14 ± 2.44 mg/dL; p = 0.01), but eGFR calculated from creatinine did not significantly differ between the groups (7.38 ± 3.48 vs. 8.29 ± 3.00 mL/min/1.73 m2; p = 0.2). Although the exact cause of this discrepancy is difficult to explain, it could be due to the interaction of serum creatinine with sex and age, as eGFR is calculated by sex and age in addition to serum creatinine. The remaining characteristics, including age (72.1 ± 14.8 vs. 73.9 ± 12.4 years; p = 0.54), male predominance (69 vs. 71%; p = 1), pre-UV (1177.3 ± 686.5 vs. 999.9 ± 630.1 mL/day; p = 0.23), concomitant medications, and other cardiac parameters were not significantly different (Table 1).
3.2 Effect of Tolvaptan (TLV) on Urinary Volume (UV)
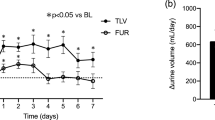
Twenty-four-hour UV significantly increased for all participants upon TLV administration (1043.4 ± 645.6 vs. 1422.2 ± 774.0 mL/day; p < 0.001) (Table 2). UV also significantly increased in patients who were not administered thiazide diuretics (999.9 ± 630.1 vs. 1503.1 ± 802.4 mL/day; p < 0.001), but was almost unchanged in the thiazide diuretics group (1177.3 ± 686.5 vs. 1173.1 ± 629.1 mL/day; p = 0.93). The serial change of UV was significantly smaller in those patients with thiazides than those without (− 508.1 ± 93.2 mL/day; p < 0.001) according to ANCOVA.
The use of thiazide diuretics was significantly associated with reduced post-24-h UV (− 486.7, 95% confidence interval [CI] − 674.5 to − 298.8) in the multivariate linear regression analysis, but the remaining variables, including serum albumin, BNP, and UPCR, were not significantly associated with post-24-h UV (Table 3).
In the multivariate sensitivity analysis where the change in loop diuretic dose was added as an independent variable, thiazide diuretic use remained significantly associated with reduced post-24-h UV (− 503.6, 95% CI − 675.8 to − 331.4), whereas increased loop diuretic dose was associated with increased post-24-h UV (563.9, 95% CI 321.9–806.0) compared with decreased loop diuretic dose (electronic supplementary Table S1). Additionally, in the multivariate sensitivity analysis where only patients receiving the same loop diuretic dose were included, thiazide diuretic use remained significantly associated with reduced post-24-h UV (− 449.5, 95% CI − 616.5 to − 282.5).
A dose–response relationship could be evaluated in patients receiving trichlormethiazide, a thiazide diuretic. Pre- and post-24-h UV in patients using trichlormethiazide 1 mg/day (n = 13) were 1068.8 ± 556.9 vs. 1117.3 ± 594.3 mL/day (p = 0.28). In contrast, pre- and post-24-h UV in patients using 2 mg/day (n = 10) were 1400.5 ± 834.6 vs. 1338.6 ± 687.1 mL/day (p = 0.56), respectively. There was no clear dose–response relationship in the serial change of UV between the two doses using ANCOVA (p = 0.52).
3.3 Effect of TLV on Urine Osmolality (UOsm) and Body Weight (BW)
UOsm and BW significantly decreased following TLV administration (313.4 ± 63.5 vs. 260.9 ± 59.1 mOsm, p < 0.001; and 63.4 ± 15.3 vs. 62.8 ± 15.1 kg, p < 0.001, respectively) for all participants (Table 2). UOsm significantly decreased in both groups with and without thiazide diuretics (304.2 ± 74.4 vs. 281.1 ± 75.2 mOsm, p = 0.01; and 317.3 ± 59.7 vs. 254.1 ± 51.4 mOsm, p < 0.001, respectively), whereas BW significantly decreased only in those patients who did not take thiazide diuretics (63.1 ± 16.3 vs. 62.4 ± 16.2 kg; p < 0.001). Differences in the serial changes of UOsm (35.4 ± 10.3 mOsm; p < 0.001) and BW (0.61 ± 0.15 kg; p < 0.001) were significant in patients with thiazides compared with those without, according to ANCOVA. The use of thiazide diuretics was significantly associated with the increase in post-UOsm values (37.7, 95% CI 17.1–58.4) and BW (0.62, 95% CI 0.31–0.92) in multivariate linear regression analyses (Table 3).
3.4 Effect of TLV on 30-Day Mortality and Dialysis Initiation and Length of Hospital Stay
Overall 30-day mortality and dialysis initiation were 5 (5%) and 41 (39%) among 106 participants, respectively. All the causes of dialysis induction were failure to control volume status. While mortality was not significantly different between groups with and without thiazide diuretics (1/26 [4%] vs. 4/80 [5%]; p = 1), the rate of 30-day dialysis initiation was significantly higher in patients with thiazide diuretics compared with those without (15/26 [58%] vs. 26/80 [33%]; p = 0.03). In the multivariate logistic regression model, the use of thiazide diuretics was associated with 30-day dialysis initiation (odds ratio [OR] 3.40, 95% CI 1.18–9.82), whereas high eGFR was associated with reduced dialysis initiation (OR 0.78, 95% CI 0.66–0.93) (Table 4). The area under the receiver operating characteristic curve of the logistic regression model showed a sufficiently high value of 0.77. The length of hospital stay among all participants who survived at discharge (n = 100) was 15 days (9–34), which was not significantly different between patients who did and did not receive thiazide diuretics (16 [8–31] vs. 15 [9–36] days; p = 0.73).
4 Discussion
This retrospective cohort study showed the diuretic effects of TLV, including increased UV and decreased UOsm and BW, in patients with CKD stage G5. This result suggests that coupling TLV with loop diuretics to treat CHF is useful even if a patient’s kidney function is compromised. However, as previously assumed [7,8,9], thiazide diuretics significantly attenuated the diuretic effect of TLV in this study; one should exercise caution when prescribing concomitant use of thiazide diuretics with TLV.
Volume control is crucial in CKD stage G5 patients to avoid cardiopulmonary decompensation, which warrants aggressive ultrafiltration or dialysis therapy. Sodium and fluid restriction as well as loop diuretics have been used to promote negative fluid balance [19]; however, loop diuretics are only 10–20% effective in advanced CKD, especially in CKD stage G5, relative to normal subjects [20]. High-dose loop diuretics are required to elicit diuretic effects in patients with advanced CKD; ceiling doses often prove ineffective in CKD stage five patients. Nonetheless, concomitant use of thiazide and loop diuretics has been used to effectively control fluid status in such patients [21, 22]. Coadministration of loop and thiazide diuretics is reasonable because loop diuretics increase NaCl reabsorption in more distal tubules than the loop of Henle, including the site of thiazide action [23]. Thiazide diuretics alone are widely assumed to be ineffective in advanced CKD because the sodium-chloride symporter in the distal convoluted tubule, the primary site of action for thiazide diuretics, is responsible for only 5% of the total filtered sodium reabsorption. Inhibiting such a small fraction of total sodium reabsorption in advanced CKD, which is characterized by reduced sodium filtration, has been considered to be clinically insignificant [24]. However, a recent clinical trial showed that thiazide diuretics were noninferior to dietary sodium restriction in reducing extracellular volume in CKD stage G3 and G4 [24, 25]. Furthermore, another clinical trial demonstrated that compared with placebo, chlorthalidone, a thiazide-like diuretic, improved blood pressure control in patients with CKD stage G4 [25, 26], which suggests that diuretic sensitivity in CKD is maintained despite lower diuretic renal clearance.
TLV is a relatively new diuretic whose mechanism differs from that of loop and thiazide diuretics, and it has shed new light on the treatment of CHF. Severe renal dysfunction is associated with CHF resistant to diuretics, including loop diuretics. While the severity of CHF was proportional to the elevation in vasopressin, loop diuretics could directly induce vasopressin elevation, sometimes associated with solute-free water retention, thereby leading to hyponatremia [27, 28]. In this regard, the addition of TLV, a V2R antagonist, is reasonable to effectively increase UV with enhanced excretion of solute-free water by blocking solute-free water reabsorption from the renal-collecting duct. Indeed, in an RCT of patients with CHF diagnosed with nondialysis CKD stage G3b–G5 (eGFR < 45 mL/min/1.73 m2), TLV significantly increased UV without increasing serum creatinine compared with intervention by increasing the dose of furosemide [3]. Several studies have shown the diuretic effectiveness of TLV in patients with CKD stage G5, although their sample sizes were small with only 8–21 patients [4,5,6]. Our study is the first to describe the efficacy of TLV as a diuretic in over 100 nondialysis-dependent CKD stage G5 patients. Serum albumin and UPCR did not affect the efficacy of TLV, contrary to previous results using furosemide [19]. The efficacy of TLV as a diuretic may be even higher given its success in patients undergoing peritoneal dialysis [29, 30] and hemodialysis [31].
However, using thiazide diuretics with TLV attenuated TLV’s diuretic effects of increasing UV and decreasing UOsm and BW. In addition to treating CHF, TLV delays the increase in total kidney volume and decline in the kidney functions of patients with near-normal kidney functions and late-stage ADPKD [32, 33]. Many discontinue TLV because of polyuria, a serious symptom resulting from high-dose TLV of 60–120 mg compared with < 15 mg. A previous RCT suggested that thiazide reduces the aquaresis caused by TLV in ADPKD patients as well as those with nephrogenic diabetes insipidus [7, 9]. Unfortunately, this reduction may compromise CHF treatment. Indeed, the rate of 30-day dialysis initiation, indicating treatment failure of CHF, was significantly higher in patients with thiazide diuretics than those without in this cohort. Further studies are needed to conclude whether or not thiazide diuretics should be discontinued when treating CHF with TLV for CHF or if they should be replaced with loop diuretics.
This study has several limitations. First, we could not completely exclude other potential confounders because of the retrospective observational design of this study. In particular, the thiazide diuretics group showed significantly higher serum creatinine with lower eGFR than the group without thiazide diuretics, which might indicate the resistance to diuretics. Although this result did not rule out the possibility that there was a difference in patient background characteristics between the two groups, the difference might only reflect the acute effect of thiazide diuretics in combination with loop diuretics, considering higher pre-UV in the thiazide diuretics group [19]. However, our analyses were adjusted for parameters considered to be associated with diuresis, and the multivariate model had a relatively high adjusted R2 of 0.50–0.99, which was enough for goodness-of-fit. The inclusion of serum creatinine instead of eGFR as an independent variable did not change the statistical significance at all (data not shown). Additionally, parameters including UOsm, BW, and cardiac parameters could not be measured in several patients, therefore selection biases were not completely excluded either. Second, we could only analyze the acute effects within 24 h of TLV administration. Some data after 24 h were not available for analysis because they were missing due to the retrospective design or because of immediate dialysis initiation. Simultaneously, we analyzed other longitudinal outcomes, including 30-day mortality and dialysis initiation rate. Although we clarified the significant difference in the rate of 30-day dialysis initiation between patients with and without thiazide diuretics, whether TLV itself could delay dialysis initiation and death or shorten the length of hospital stay was unclear. Additionally, the number of patients included (n = 106) and the number of deaths in 30 days (n = 5) were too small to draw conclusions regarding 30-day mortality in this study. Finally, the generalizability of our results to other patients with CHF remains unclear. The cause of fluid overload was attributed to sodium retention from reduced kidney function rather than cardiac function, because the participants were mainly seen in the Nephrology Department. Additionally, this study did not clarify how thiazide affected the diuretic effects of TLV in patients with CHF with CKD stages other than G5. Due to a single-arm analysis without a control group not using TLV, this study could not provide definite evidence suggesting that TLV was effective in CKD stage G5.
5 Conclusion
We showed that TLV increased UV in patients with CHF and CKD stage G5, although the concomitant use of thiazide diuretics should be considered with caution because they may reduce the effect of TLV. Further prospective trials should investigate the long-term effects of TLV and concomitant use of thiazide diuretics on patients with CHF with CKD.
References
Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group, et al. JCS 2017/JHFS 2017 Guideline on Diagnosis and Treatment of Acute and Chronic Heart Failure - Digest Version. Circ J. 2019;83:2084–184.
Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–31.
Inomata T, Ikeda Y, Kida K, Shibagaki Y, Sato N, Kumagai Y, Kanagawa Aquaresis Investigators, et al. Effects of additive tolvaptan vs increased furosemide on heart failure with diuretic resistance and renal impairment: results from the K-STAR study. Circ J. 2017;82:159–67.
Otsuka T, Sakai Y, Ohno D, Murasawa T, Sato N, Tsuruoka S. The effects of tolvaptan on patients with severe chronic kidney disease complicated by congestive heart failure. Clin Exp Nephrol. 2013;17:834–8.
Katsumata M, Hirawa N, Sumida K, Kagimoto M, Ehara Y, Okuyama Y, et al. Effects of tolvaptan in patients with chronic kidney disease and chronic heart failure. Clin Exp Nephrol. 2017;21:858–65.
Iwatani H, Kawabata H, Sakaguchi Y, Yamamoto R, Hamano T, Rakugi H, et al. Urine osmolarity predicts the body weight-reduction response to tolvaptan in chronic kidney disease patients: a retrospective, observational study. Nephron. 2015;130:8–12.
Crawford JD, Kennedy GC, Hill LE. Clinical results of treatment of diabetes insipidus with drugs of the chlorothiazide series. N Engl J Med. 1960;262:737–43.
Kramers BJ, van Gastel MDA, Meijer E, Gansevoort RT. Case report: a thiazide diuretic to treat polyuria induced by tolvaptan. BMC Nephrol. 2018;19:157.
Uchiyama K, Kitayama C, Yanai A, Ishibashi Y. The effect of trichlormethiazide in autosomal dominant polycystic kidney disease patients receiving tolvaptan: a randomized crossover controlled trial. Sci Rep. 2021;11:17666.
Kuzuya F, Sugino N, Yoshizumi K, Goto U, Oshima K. Clinical investigations on the pharmacology of azosemide (SK-110) in comparison with furosemide in healthy volunteers. Int J Clin Pharmacol Ther Toxicol. 1984;22:291–9.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Bueno H, Ross JS, Wang Y, Chen J, Vidán MT, Normand SL, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303:2141–7.
Abraham WT, Schrier RW. Edematous disorders: pathophysiology of renal sodium and water retention and treatment with diuretics. Curr Opin Nephrol Hypertens. 1993;2:798–805.
Inoue M, Okajima K, Itoh K, Ando Y, Watanabe N, Yasaka T, et al. Mechanism of furosemide resistance in analbuminemic rats and hypoalbuminemic patients. Kidney Int. 1987;32:198–203.
Brater DC. Update in diuretic therapy: clinical pharmacology. Semin Nephrol. 2011;31:483–94.
Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology. 2001;96:132–43.
Streja E, Nicholas SB, Norris KC. Controversies in timing of dialysis initiation and the role of race and demographics. Semin Dial. 2013;26:658–66.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Sica DA, Gehr TW. Diuretic use in stage 5 chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2003;12:483–90.
Beermann B. Aspects on pharmacokinetics of some diuretics. Acta Pharmacol Toxicol (Copenh). 1984;54:17–29.
Wollam GL, Tarazi RC, Bravo EL, Dustan HP. Diuretic potency of combined hydrochlorothiazide and furosemide therapy in patients with azotemia. Am J Med. 1982;72:929–38.
Fliser D, Schröter M, Neubeck M, Ritz E. Coadministration of thiazides increases the efficacy of loop diuretics even in patients with advanced renal failure. Kidney Int. 1994;46:482–8.
Abdallah JG, Schrier RW, Edelstein C, Jennings SD, Wyse B, Ellison DH. Loop diuretic infusion increases thiazide-sensitive Na(+)/Cl(-)-cotransporter abundance: role of aldosterone. J Am Soc Nephrol. 2001;12:1335–41.
Sinha AD, Agarwal R. Thiazides in advanced chronic kidney disease: time for a randomized controlled trial. Curr Opin Cardiol. 2015;30:366–72.
Bovée DM, Visser WJ, Middel I, De Mik-van EA, Greupink R, Masereeuw R, et al. A randomized trial of distal diuretics versus dietary sodium restriction for hypertension in chronic kidney disease. J Am Soc Nephrol. 2020;31:650–62.
Agarwal R, Sinha AD, Cramer AE, Balmes-Fenwick M, Dickinson JH, Ouyang F, Tu W. Chlorthalidone for hypertension in advanced chronic kidney disease. N Engl J Med. 2021;385:2507–19.
Tominaga N, Kida K, Inomata T, Sato N, Izumi T, Akashi YJ, et al. Effects of tolvaptan addition to furosemide in normo- and hyponatremia patients with heart failure and chronic kidney disease stages G3b–5: a subanalysis of the K-STAR study. Am J Nephrol. 2017;46:417–26.
Gunderson EG, Lillyblad MP, Fine M, Vardeny O, Berei TJ. Tolvaptan for volume management in heart failure. Pharmacotherapy. 2019;39:473–85.
Iwahori T, Esaki M, Hinoue H, Esaki S, Esaki Y. Tolvaptan increases urine and ultrafiltration volume for patients with oliguria undergoing peritoneal dialysis. Clin Exp Nephrol. 2014;18:655–61.
Hiramatsu T, Hobo A, Hayasaki T, Kabu K, Furuta S. A pilot study examining the effects of tolvaptan on residual renal function in peritoneal dialysis for diabetics. Perit Dial Int. 2015;35:552–8.
Ogata H, Shimofurutani N, Okada T, Nagamoto H, Akizawa T. Efficacy and safety of oral tolvaptan in patients undergoing hemodialysis: a Phase 2, double-blind, randomized, placebo-controlled trial. Nephrol Dial Transplant. 2021;36:1088–97.
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, TEMPO 3:4 Trial Investigators, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–18.
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, REPRISE Trial Investigators, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377:1930–42.
Acknowledgments
The authors would like to thank all participants for their dedication to this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Original data are available from the corresponding author on reasonable request.
Conflicts of interest
Kiyotaka Uchiyama, Daiki Kojima, Eriko Yoshida Hama, Tomoki Nagasaka, Takashin Nakayama, Rina Takahashi, Takaya Tajima, Kohkichi Morimoto, Naoki Washida, and Hiroshi Itoh declare no conflicts of interest.
Research involving human participants
This study was reviewed and approved by the Research Ethics Committee of Keio University, School of Medicine (approval number: 20211114) and was performed in accordance with the principles of the Declaration of Helsinki.
Consent to participate
Due to the retrospective design of this study, informed consent for study participation was obtained in the form of an opt-out method on the website.
Consent to publish
Due to the retrospective design of this study, informed consent for publication of study findings was obtained in the form of an opt-out method on the website.
Authors’ contributions
KU designed the study and wrote the initial draft of the manuscript. KU, DK, EYH, TNag, TNak, RT, and TT contributed to data collection. KU, TNak, and KM contributed to the analysis and interpretation of data. NW and HI supervised the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work as well as ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Uchiyama, K., Kojima, D., Hama, E.Y. et al. Effect of Tolvaptan in Patients with Chronic Kidney Disease Stage G5, and Impact of Concomitant Use of Thiazide Diuretics: A Retrospective Cohort Study. Drugs - Real World Outcomes 9, 649–657 (2022). https://doi.org/10.1007/s40801-022-00325-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-022-00325-3