Abstract
Introduction
Hydroxychloroquine can induce QT/QTc interval prolongation for some patients; however, little is known about its interactions with other QT-prolonging drugs.
Objective
The purpose of this retrospective electronic health records study was to evaluate changes in the QTc interval in patients taking hydroxychloroquine with or without concomitant QT-prolonging medications.
Methods
De-identified health records were obtained from the Cerner Health Facts® database. Variables of interest included demographics, diagnoses, clinical procedures, laboratory tests, and medications. Patients were categorized into six cohorts based on exposure to hydroxychloroquine, methotrexate, or sulfasalazine alone, or the combination of any those drugs with any concomitant drug known to prolong the QT interval. Tisdale QTc risk score was calculated for each patient cohort. Two-sample paired t-tests were used to test differences between the mean before and after QTc measurements within each group and ANOVA was used to test for significant differences across the cohort means.
Results
A statistically significant increase in QTc interval from the last measurement prior to concomitant exposure of 18.0 ms (95% CI 3.5–32.5; p < 0.05) was found in the hydroxychloroquine monotherapy cohort. QTc changes varied considerably across cohorts, with standard deviations ranging from 40.9 (hydroxychloroquine monotherapy) to 57.8 (hydroxychloroquine + sulfasalazine). There was no difference in QTc measurements among cohorts. The hydroxychloroquine + QTc-prolonging agent cohort had the highest average Tisdale Risk Score compared with those without concomitant exposure (p < 0.05).
Conclusion
Our analysis of retrospective electronic health records found hydroxychloroquine to be associated with a moderate increase in the QTc interval compared with sulfasalazine or methotrexate. However, the QTc was not significantly increased with concomitant exposure to other drugs known to increase QTc interval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hydroxychloroquine can induce QT/QTc interval prolongation, and little is known about its interactions with other QT-prolonging drugs. |
Exposure to hydroxychloroquine is associated with a moderate increase in QTc interval. There was no evidence that this effect is further increased when hydroxychloroquine is given concomitantly with other drugs known to increase QTc interval. |
Clinicians should cautiously consider risks and benefits if considering use of hydroxychloroquine with other QTc-prolonging agents and should monitor QTc and concomitant medication utilization. |
1 Introduction
Hydroxychloroquine was developed by adding a β-hydroxy chain to chloroquine to reduce toxicity and is commonly used as an antimalarial [1]. Nowadays, because of its anti-inflammatory, immunomodulating, and metabolic properties, hydroxychloroquine has been increasingly used for the treatment of chronic conditions such as systemic lupus erythematosus and rheumatoid arthritis [2], particularly due to its antiproliferative effect on T cells and its reduction of several pro-inflammatory cytokines (i.e., interferon-γ, tumor necrosis factor, interleukin-1) [3].
Hydroxychloroquine is fully absorbed after oral administration and is partly bound to a protein in plasma and metabolized via CYP enzymes in the liver to a major metabolite, N-desethylhydroxychloroquine. The half-life of hydroxychloroquine ranges from 22 days to > 120 days depending on dose and route of administration [4]. Hydroxychloroquine is eliminated by the kidneys and also metabolized by the liver; thus, patients with dysfunctions in these organs might have serious adverse events when taking hydroxychloroquine [1, 5]. Adverse events associated with hydroxychloroquine include gastrointestinal distress such as nausea, vomiting, diarrhea, and weight loss [1], as well as skin rash and itching [6]. Retinopathy is also one of the serious and irreversible adverse events, particularly in patients receiving high doses [7]. Heart failure, cardiac conduction disorders, and cardiomyopathy have also been reported, and though cardiac toxicity is rare, if it occurs, it could be lethal [8, 9].
Hydroxychloroquine is structurally similar to class IA antiarrhythmic quinidine, which blocks sodium and potassium channels, prolonging the QT interval and increasing the risk of Torsades de Pointes (TdP), a polymorphic ventricular tachycardia that could, in some cases, incite ventricular fibrillation and sudden cardiac death [10, 11]. In past years, more than half of the medications removed from the market were due to serious cardiac arrhythmias resulting from their ability to cause QT interval prolongation [12, 13]. The association of hydroxychloroquine with QT prolongation is well known and is reported in product labeling, warning clinicians not to administer it in combination with other QT-prolonging drugs [4, 12]. Chatre et al. conducted a review of 86 individual cases and short case series of cardiac complications related to long-term use of chloroquine and hydroxychloroquine and found conduction disorders reported in the majority of patient cases (85%) [14]. At the beginning of the COVID-19 pandemic, several studies reported the use of hydroxychloroquine and the cardiovascular adverse events. One study reported the pooled incidence of discontinuation due to prolonged QTC or arrythmias when exposed to chloroquine or hydroxychloroquine was 5% (95% CI 1–11; I2 = 98%) and the pooled incidence in QTc change from baseline of 60 milliseconds (ms) or > 500 ms QTc was 9% (95% CI 3–17; I2 = 97%) [15].
Antimalarial drugs, such as hydroxychloroquine, that can induce QT/QTc interval prolongation should be used with caution in individuals with identified risk factors including advanced age, bradycardia, electrolyte disturbances, previous rhythm and conduction defects, female sex, congenital prolonged QTc, a family history of sudden unexplained death consistent with cardiac arrhythmias, or concomitant treatment with medication that can prolong the QT interval such as antiarrhythmic drugs, antihistamines, antipsychotics, antidepressants, antibiotics, and those drug combinations that share additive or potentiating effects [16,17,18]. Normal values of QTc in males are 440 ms or less, while in females it is 460 ms or less [19]. Females with either congenital or acquired long-QT syndrome (LQTS) have a greater risk of adverse cardiac events [20]. It has been estimated in patients with congenital LTQS that every 10-ms increase in QTc correlates to a 5–7% increase in the risk of TdP [12, 21]. Compared with a patient with a QTc of 440 ms, a patient with a QTc of 540 ms has a 63–97% higher risk of developing TdP [12].
An important question is whether concomitant administration of hydroxychloroquine with other QT-prolonging drugs significantly increases the risk of cardiovascular complications. Recently, evidence about the risk and degree of QT prolongation in using hydroxychloroquine, with and without concomitant QT-prolonging medications, has been reported among individuals infected with COVID-19 [9, 11, 22,23,24,25,26]. Padilla et al. found that in hospitalized COVID patients, concomitant exposure to hydroxychloroquine and azithromycin was significantly associated (HR 11.28, 95% CI 1.08–117.41) with prolongation of the QTc [27].
1.1 Objective
The purpose of this study was to determine changes in the QTc interval in patients taking hydroxychloroquine with or without concomitant QT-prolonging medications, analyzing data from a large-scale retrospective health records database.
2 Methods
2.1 Data Source
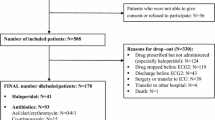
De-identified patient health records were obtained from the Cerner Health Facts® database. This database contained electronic health records collected from over 700 healthcare facilities in the United States between 2002 and 2018, including data for over 60 million patients. The datasets generated during the current study are available from the corresponding author on reasonable request.
The data were loaded into a relational database and queried using SQL language. Data elements of interest included QTc measurements, demographics, diagnoses, medical and surgical procedures, laboratory tests, medications, microbiology records, and clinical events. Because the discrete capture of QTc data has relatively recently occurred with electronic health records, patients with two or more QTc measurements were included in the study.
2.2 Study Population
Hydroxychloroquine is classified as a disease-modifying anti-rheumatic drug (DMARD) and has been frequently used to treat patients with autoimmune disorders such as rheumatoid arthritis and systemic lupus erythematosus. Consequently, we selected two other DMARD agents (methotrexate and sulfasalazine) to identify controls. Six cohorts were formed based on exposure to hydroxychloroquine, methotrexate, sulfasalazine, and hydroxychloroquine, methotrexate, or sulfasalazine in addition to any drug that, according to the CredibleMeds database (https://www.crediblemeds.org/) [12], is a known risk of Torsades de Pointes.
2.3 QTc-Prolonging Agents and QTc Measurements
There are several drugs that could increase these values, either by themselves or through a drug–drug interaction with other drugs known to prolong QT. This study used the following list of QTc-prolonging agents from the CredibleMeds database [12]: amiodarone, anagrelide, azithromycin, bepridil, chlorpromazine, cilostazol, ciprofloxacin, citalopram, clarithromycin, disopyramide, dofetilide, donepezil, dronedarone, droperidol, erythromycin, escitalopram, flecainide, fluconazole, haloperidol, levofloxacin, methadone, moxifloxacin, ondansetron, oxaliplatin, procainamide, propofol, sevoflurane, sotalol, and thioridazine.
The Health Facts® dataset contains one or more encounters for each patient. For this study, the recorded start time of the individual drug or drug combination was considered the index timestamp. We allowed for only one index timestamp per encounter per patient. All included patients had one or more valid QTc measurement before and after the index timestamp but within the same clinical encounter (i.e., hospitalization). To our knowledge, there is no other analysis of a real-world dataset that has this large of a sample where measurements could be compared before and after concomitant exposure within a single encounter.
We counted the number of patients in each cohort who had at least one especially high (≥ 500 ms) QTc measurement before or after the index timestamps. We also evaluated risk factors for prolonged QTc for each subject at the index timestamp using the Tisdale QTc risk score [28]. The Tisdale score identifies the following criteria to be associated with prolonged QTc: age ≥ 68 years; female sex; use of loop diuretic; serum K+ ≤3.5 mEq/L; admission QTc ≥ 450 ms; acute myocardial infarction; exposure to two or more QTc-prolonging drugs; sepsis; heart failure; and exposure to one QTc-prolonging drug. Weights were assigned to each attribute based on the odds ratio and then summed to obtain an overall score. Fewer than 7 points was considered low risk; 7–10 points was considered moderate risk, and scores > 10 were considered high risk for QTc prolongation.
2.4 Ethics
This study was approved as exempt of human subject research requirements by the University of Pittsburgh Institutional Review Board because the utilized data consisted of deidentified electronic health records.
2.5 Statistical Analyses
All statistical analyses were done using R version 3.6. The Pearson's Chi-square test with Yates' continuity correction was used to test for differences (p < 0.05) in the presence of Tisdale risk factors. We also tested for differences in the presence of Tisdale risk factors between patients with concomitant exposure compared with no concomitant exposure for each of the cohorts of interest. For the total Tisdale score, the Welch modified two-sample t-test was used to test for statistically significant difference (p < 0.05). For descriptive purposes, the last QTc before an index date was compared with the first QTc after the index timestamp for each cohort. Two-sample paired t tests were used to test statistically significant differences between the mean before and after QTc measurements within each group and ANOVA was used to test for significant differences across the cohort means.
3 Results
After an exhaustive search, we identified 321 unique patients whose records indicate an exposure to hydroxychloroquine and a QT-prolonging agent. Table 1 shows the sample size for each cohort and the differences in QTc measurements before and after medication exposures across cohorts. Differences were calculated using the first QTc measurement after the index timestamp and the last QTc measurement before the index timestamp. Only the hydroxychloroquine alone cohort had a significant QTc measurement increase of 18.0 ms (p < 0.05) from pre- to post-exposure. Variability in QTc differences was large across all of the cohorts as evidenced by the standard deviations, which ranged from 40.9 ms (hydroxychloroquine) to 57.8 ms (sulfasalazine). ANOVA analysis revealed no statistical differences between cohort QTc measurements.
The cohort demographics and prevalence of the Tisdale QTc-prolonging risk factors for each cohort are shown in Table 2. Patients’ rheumatologic common conditions are reported in Table 3. Because these medications are not common and the study required two QTc measurements, the cohorts were relatively small. The cohort receiving hydroxychloroquine + QTc-prolonging agents had the most patients (n = 171), while the cohort receiving sulfasalazine without a QTc-prolonging agent had the least (n = 10). The sulfasalazine + QTc-prolonging agents and methotrexate + QTc-prolonging agents cohorts had a lower proportion of females and a lower percentage of patients exposed to two or more QTc-prolonging drugs than the hydroxychloroquine + QTc-prolonging agents cohort.
Descriptively, the sulfasalazine cohort included the largest proportion (70%) of older patients (≥ 68 years). The three cohorts with the highest proportion of patients with an admission QTc ≥ 450 ms were methotrexate + QTc-prolonging agents (85%), hydroxychloroquine + QT-prolonging agents (82%), and sulfasalazine + QTc-prolonging agents (78%). The hydroxychloroquine + QTc-prolonging agents cohort also had the highest average Tisdale Risk Score compared with other cohorts. This suggests that patients in this group were at the highest risk of QTc prolongation. The three cohorts without the combination of QTc-prolonging agents generally had a lower overall Tisdale score than the three cohorts with concomitant exposure (p < 0.05).
4 Discussion
This study found that exposure to hydroxychloroquine alone was associated with a significant increase in QTc interval compared with exposure to either sulfasalazine or methotrexate alone. However, there was no evidence of significant additional QTc prolongation when hydroxychloroquine was given concomitantly with other drugs known to increase QTc interval. This finding might be explained by several factors in our study that could modify drug-induced QT-prolonging activity. Our study population was not limited by treatment site and may have had different sensitivity to the effects of QT-prolonging drugs. Because we set the index timestamp as either the start of hydroxychloroquine monotherapy or the start of combination therapy, patients receiving combination therapy may have had a longer exposure to hydroxychloroquine prior to QTc measurement.
Baseline QTc in the hydroxychloroquine combination cohort was similar to that of the treatment QTc in the hydroxychloroquine alone cohort. When two drugs that have the same mechanism of action but different potencies are administered concomitantly, the less potent drug may act as a partial agonist [29]. It is possible that the most common concomitant exposures were with drugs that have limited QT-prolonging activity, either intrinsically or at the doses they were given. Our study was not designed to examine whether the results would be different if focused on concomitant exposure to hydroxychloroquine and specific QT-prolonging drugs instead of any QT-prolonging drugs. Cavalcanti et al. found no difference in QT > 480 ms between patients taking hydroxychloroquine alone and patients taking the drug with azithromycin [9]. However, in a case series analysis conducted by Bessière et al., 6 of 18 (33%) COVID-19 patients treated with hydroxychloroquine and azithromycin developed a prolonged QTc of ≥ 500 ms versus 1 of 22 (5%) of those treated with hydroxychloroquine alone (p = 0.03) [11].
Our analysis found that subjects taking a comparator drug and one or more QTc-prolonging agents had Tisdale scores > 8 (moderate risk), but patients taking hydroxychloroquine and one or more QT-prolonging drug had Tisdale scores of approximately 11 (high risk). Thus, patients receiving hydroxychloroquine plus another QTc-prolonging drug may be at greater risk of prolonged QTc even though our analysis did not show a significant difference across the cohorts. This may be in part due to the high variability in QTc scores in the Health Facts dataset. A Tisdale score of ≥ 11 has a reported QT prolongation prediction sensitivity of 0.74, specificity of 0.77, positive predictive value of 0.79, and negative predictive value of 0.76 [28]. The incorporation of Tisdale scores into clinical decision support systems could allow for prompt identification of subjects at higher risk of developing QTc prolongation, leading to re-evaluation of current treatment and cardiac monitoring [30]. While QTc alone can be used to evaluate the occurrence of ventricular tachyarrhythmias and risk of sudden cardiac arrest, it has limited specificity when used as a surrogate marker [31].
Adverse events associated with hydroxychloroquine use include gastrointestinal intolerance, retinopathy, and myocardial manifestations (e.g., restrictive cardiomyopathy, cardiac insufficiency, conduction disorders with QTc prolongation, and cardiac arrhythmias). However, these cardiovascular anomalies have been reported mostly in case report studies [32,33,34]. Risk factors for hydroxychloroquine-induced arrhythmia include structural heart diseases (i.e., ventricular hypertrophy), history of ventricular arrhythmia or syncope, the use of heart rhythm devices, and the co-administration of other QTc-prolonging drugs [35].
The lack of strong evidence for QTc prolongation with hydroxychloroquine is not a unique finding. Sridhar et al. studied 75 COVID-19 patients treated with hydroxychloroquine monotherapy and given serial ECGs (baseline and follow-up after a second dose of hydroxychloroquine) and found the mean change in QTc was − 2 ms (range − 65 to + 67 ms; p = 0.53) [24]. Despite this finding, case reports for TdP and sudden death have been described for hydroxychloroquine when used for non-malaria indications [35]. In a Japanese study on systemic lupus erythematosus patients treated with hydroxychloroquine, a significant increase on the QTc was found when compared with a control group with no hydroxychloroquine exposure [36].
In a cohort study of 90 hospitalized COVID-19 patients taking hydroxychloroquine with or without azithromycin, those taking hydroxychloroquine with azithromycin had greater QT prolongation than those receiving hydroxychloroquine alone [10]. A recent large-scale retrospective self-controlled case series study of patients taking hydroxychloroquine and azithromycin observed an increased risk of 30-day cardiovascular mortality (HR 2.19 [95% CI 1.22–3.95]), chest pain/angina (HR 1.15 [95% CI 1.05–1.26]), and heart failure (HR 1.22 [95% CI 1.02–1.45]) [37].
Our study has limitations that should be considered when interpreting its findings. While Health Facts® is a large observational retrospective dataset of real-world QTc measurements, the sample size was relatively small due to the infrequency of concomitant exposure to the drugs of interest among patients for whom QTc measurements were recorded. There is generally sparse information about how the QTc measurement was performed, including whether they were manually or automatically recorded. Because the data were derived from an encounter-based record, we could not ascertain exposure to the medications prior to the index date. It is possible that subjects could have been taking the medications prior to their encounter where the QTc was measured. Another limitation is that data may be incomplete. The study population contains a mix of inpatient and outpatient encounters, and certain laboratory measurements, such as serum potassium, magnesium, or calcium, are expected to be less frequently ordered in an outpatient setting. We did not attempt to impute missing values. Furthermore, while there was interest in evaluating the effect of combining azithromycin and hydroxychloroquine, there were too few concomitant exposure events in the data to evaluate that combination. Thus, we pooled all QT-prolonging drugs and examined the effect of combinations at the individual active ingredient level.
Another limitation of our study was that it was not possible to examine a dose–response relationship when examining the effect of combining QTc-prolonging agents and hydroxychloroquine. It remains possible that the true degree of QTc prolongation was either under- or over-estimated, given the quality of data related to medication exposure in the dataset. Lastly, a cause and effect relationship cannot be determined using retrospective databases, though they are convenient for delivering initial data and informing the development of future prospective studies. Despite these limitations, we propose that clinicians remain cautious about the combination and consider specific patient characteristics, comorbidities, and the use of concomitant medications.
5 Conclusion
Exposure to hydroxychloroquine is associated with a moderate increase in QTc interval compared with exposure to sulfasalazine or methotrexate. However, there was no evidence that this effect is further increased when hydroxychloroquine is given concomitantly with other drugs known to increase the QTc interval. Patients on hydroxychloroquine and other medications known to be associated with QTc prolongation are subject to numerous factors that may contribute to risk of QTc prolongation and TdP.
References
Shukla AM, Shukla AW. Expanding horizons for clinical applications of chloroquine, hydroxychloroquine, and related structural analogues. Drugs in Context. 2019;8:1–12.
Platone D, Koudriavtseva T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin Drug Investig [Internet]. 2018;38. https://pubmed.ncbi.nlm.nih.gov/29737455/
Jang C-H, Choi J-H, Byun M-S, Jue D-M. Chloroquine inhibits production of TNF-α, IL-1β and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology. 2006;45:703–10.
DailyMed—HYDROXYCHLOROQUINE SULFATE tablet [Internet]. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84b00366-96ef-41e1-bac6-5d24acdb9e1d. Accessed 20 Aug 2020.
Browning DJ. Pharmacology of chloroquine and hydroxychloroquine. Hydroxychloroquine and chloroquine retinopathy. New York: Springer; 2014. p. 35–63.
Kalia S, Dutz J. New concepts in antimalarial use and mode of action in dermatology. Dermatologic therapy [Internet]. 2007;20. https://pubmed.ncbi.nlm.nih.gov/17970883/?dopt=Abstract
Marmor M, Kellner U, Lai T, Lyons J, Mieler W. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology [Internet]. 2011;118. https://pubmed.ncbi.nlm.nih.gov/21292109/?dopt=Abstract
Tönnesmann E, Kandolf R, Lewalter T. Chloroquine cardiomyopathy—a review of the literature. Immunopharmacol Immunotoxicol. 2013;35:434–42.
Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383:2041–52.
Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;1–6
Bessière F, Roccia H, Delinière A, Charrière R, Chevalier P, Argaud L, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020;5(9):1067–9.
Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, et al. Prevention of Torsade de Pointes in Hospital Settings. A Scientific Statement From the American Heart Association and the American College of Cardiology Foundation Endorsed by the American Association of Critical-Care Nurses and the International Society. J Am Coll Cardiol. 2010;55:934–47.
Ninan B, Wertheimer AI. Withdrawing drugs in the U.S. versus other countries. INNOVATIONS in pharmacy. 2012;3.
Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers YM. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41:919–31.
Tleyjeh IM, Kashour Z, AlDosary O, Riaz M, Tlayjeh H, Garbati MA, et al. Cardiac toxicity of chloroquine or hydroxychloroquine in patients with COVID-19: a systematic review and meta-regression analysis. Mayo Clin Proc Innov Qual Outcomes. 2021;5:137–50.
Nachimuthu S, Assar MD, Schussler JM. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012;3:241.
Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharm. 2017;39:16–25.
Trinkley KE, Page RL, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. 2013;29:1719–26.
Farzam K, Tivakaran VS. QT Prolonging Drugs. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. http://www.ncbi.nlm.nih.gov/books/NBK534864/. Accessed 22 Dec 2020.
James AF, Choisy SCM, Hancox JC. Recent advances in understanding sex differences in cardiac repolarization. Prog Biophys Mol Biol. 2007;94:265–319.
Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Robinson JL, Priori SG, et al. Influence of the genotype on the clinical course of the long-QT syndrome. N Engl J Med. 1998;339:960–5.
Maraj I, Hummel JP, Taoutel R, Chamoun R, Workman V, Li C et al. Incidence and determinants of QT interval prolongation in COVID-19 patients treated with hydroxychloroquine and azithromycin. J Cardiovasc Electrophysiol. 2020;31(8):1904–1907.
Das RR, Jaiswal N, Dev N, Jaiswal N, Naik SS, Sankar J. Efficacy and safety of anti-malarial drugs (chloroquine and hydroxy-chloroquine) in treatment of COVID-19 infection: a systematic review and meta-analysis. Front Med (Lausanne). 2020;7:482.
Sridhar AR, Chatterjee NA, Saour B, Nguyen D, Starnes EA, Johnston C, et al. QT interval and arrhythmic safety of hydroxychloroquine monotherapy in coronavirus disease 2019. Heart Rhythm O2. 2020;1:167–72.
Bakhshaliyev N, Uluganyan M, Enhos A, Karacop E, Ozdemir R. The effect of 5-day course of hydroxychloroquine and azithromycin combination on QT interval in non-ICU COVID19(+) patients. J Electrocardiol. 2020;62:59–64.
Karamchandani K, Quintili A, Landis T, Bose S. Cardiac arrhythmias in critically ill patients with COVID-19: a brief review. J Cardiothorac Vasc Anesth. 2020;35(12):3789–96.
Padilla S, Telenti G, Guillén L, García JA, García-Abellán J, Ding C, et al. Predictive factors for cardiac conduction abnormalities with hydroxychloroquine-containing combinations for COVID-19. Int J Antimicrob Agents. 2020;56(4):106142.
Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–87.
Berg KA, Clarke WP. Making sense of pharmacology: inverse agonism and functional selectivity. Int J Neuropsychopharmacol. 2018;21:962–77.
Tomaselli ME, Tisdale JE. Predictive analytics for identification of patients at risk for QT interval prolongation: a systematic review. Pharmacother J Hum Pharmacol Drug Ther. 2018;38:813–21.
Guan Y, Camm AJ, Yee D, Yap G. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363–72.
Cotroneo J, Sleik KM, Rodriguez ER, Klein AL. Hydroxychloroquine-induced restrictive cardiomyopathy case report. 2006.
Chen C-Y, Wang F-L, Lin C-C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Philadelphia, Pa). 2006;44:173–5.
O’laughlin JP, Mehta PH, Wong BC. Case report life threatening severe QTc prolongation in patient with systemic lupus erythematosus due to hydroxychloroquine. 2016.
Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent Guidance for Navigating and Circumventing the QTc-Prolonging and Torsadogenic Potential of Possible Pharmacotherapies for Coronavirus Disease 19 (COVID-19). Mayo Clinic Proceedings [Internet]. Elsevier; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7141471/
Nishiyama T, Kondo Y, Tsuboi H, Noma H, Tabuchi D, Sugita T, et al. QTc interval prolongation in patients with systemic lupus erythematosus treated with hydroxychloroquine. Mod Rheumatol. 2021;31:1107–12.
Lane JCE, Weaver J, Kostka K, Duarte-Salles T, Abrahao MTF, Alghoul H, et al. Risk of hydroxychloroquine alone and in combination with azithromycin in the treatment of rheumatoid arthritis: a multinational, retrospective study. Lancet Rheumatol. 2020;2:e698–711.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Prior postings and presentations
None.
Funding
This study was funded by the Agency for Healthcare Research and Quality, grant number R01HS025984.
Disclosure
The authors declare no conflict of interest or financial relationships.
Ethical statement and conflict of interest
This study was approved as exempt human subjects research by the University of Pittsburgh Institutional Review Board. The authors have no conflicts of interest to declare.
Consent to participate
Not applicable.
Author contributions
DCM, EC, LVZ, PDH, JRH, SG, VS, and AR designed the research; RDB and EC performed the research; RDB, EC, LVZ, DCM analyzed data; and all authors contributed to writing the manuscript and give consent for publication.
Availability of data and material (data transparency)
The data that support the findings of this study are available from Cerner® but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Cerner®.
Code availability
The SQL and statistical programming code used in this study are available from the corresponding author on reasonable request.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Villa Zapata, L., Boyce, R.D., Chou, E. et al. QTc Prolongation with the Use of Hydroxychloroquine and Concomitant Arrhythmogenic Medications: A Retrospective Study Using Electronic Health Records Data. Drugs - Real World Outcomes 9, 415–423 (2022). https://doi.org/10.1007/s40801-022-00307-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-022-00307-5