Abstract
Background
The effectiveness of long-acting injectable antipsychotics (LAIAs) has been demonstrated in studies using prescription claims data. However, the validity of claims data for LAIAs has not been established.
Objective
We aimed to validate date dispensed, quantity dispensed and days supplied fields in prescription claims data, and to compare claims- and medical record-derived persistence estimates.
Methods
We evaluated LAIA dispensations in the Drug Programs Information Network prescription claims database from Manitoba, Canada against a random sample of medical records. Adults with one or more LAIA prescription between April 2015 and March 2016 were eligible. Results were stratified by LAIA type (first-generation LAIA, risperidone LAI or paliperidone LAI). Persistence estimates were assessed using Kaplan–Meier survival analysis and proportion of patients covered method.
Results
Claims data had high positive predictive value, ranging from 80.0% (95% CI 51.9–95.7) to 100.0% (95% CI 89.7–100.0), but low negative predictive value, ranging from 0.0% (95% CI 0.0–2.5) to 62.5% (95% CI 40.6–81.2). Quantity dispensed and days supplied exactly matched dose and dosing interval, respectively, for 99.7% and 97.1% of risperidone LAI doses, 100.0% and 76.6% of paliperidone doses, and 8.9% and 28.3% of first-generation LAIA doses. There were no significant differences in claims-derived versus medical record-derived persistence estimates.
Conclusions
Quantity dispensed and days supplied provide valid estimates of dose and dosing interval for second-generation LAIAs, but underestimated these parameters for first-generation LAIAs. However, a large proportion of medical record-confirmed doses were missing from claims data, and dose and dosing interval are underestimated in claims data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Nearly all prescription claims for long-acting injectable antipsychotic medications were linked to a corresponding injection in the medical record. |
Dose and dosing interval for second-generation long-acting injectable antipsychotic medications can be accurately estimated from claims data. |
1 Introduction
Non-adherence to antipsychotic medication contributes to symptom recurrence and relapse, hospitalization, unemployment, homelessness and criminal victimization and perpetration [1,2,3,4] Long-acting injectable antipsychotics (LAIAs) extend the interval between doses, ensuring sustained exposure to a therapeutic dose and entailing regular contact with health care providers for medication administration [5, 6]. LAIAs were shown to reduce hospitalization, health resource use and mortality compared with oral antipsychotics in real-world settings [7,8,9]. Compared with randomized controlled trials, observational studies may be better situated to measure real-world safety and effectiveness of LAIAs, where their primary advantage over oral antipsychotics is to improve adherence [10].
Estimates of drug dose and dosing interval derived from quantity dispensed and days supplied variables in prescription claims data are widely used [11]. However, concerns have been raised about the validity of dose and dosing interval estimated from prescription claims for LAIAs [12, 13]. In particular, comparison of different methods to correct errors in days supplied values for LAIAs in administrative data led to significant differences in adherence and persistence estimates [12], and days supplied values in prescription claims data were shown to be inconsistent with the labelled dosing interval from product monographs [14]. First-generation long-acting injectable antipsychotics (FG-LAIAs), such as flupentixol, fluphenazine, haloperidol and zuclopenthixol are supplied in ampules or multidose vials where only a portion of the quantity dispensed is administered. In the case of ampules, any remaining medication must be discarded as sterility cannot be maintained. In the case of multidose vials, the remainder may be saved for future injections [13]. While second-generation antipsychotics, such as risperidone, paliperidone, aripiprazole and olanzapine have long been preferred over first-generation antipsychotics, the effectiveness of FG-LAIAs and the high cost of second-generation long-acting injectable antipsychotics (SG-LAIAs) have contributed to continued use of FG-LAIAs [9].
The aims of the present study were to assess the validity of quantity dispensed, dispensed date and days supplied fields in prescription claims data to estimate dose, administration date and dosing interval for LAIAs; and to assess validity of persistence estimates obtained from administrative data.
2 Methods
2.1 Validation Data Set
The Drug Programs Information Network (DPIN) is a comprehensive, population-based record of prescription medications dispensed in Manitoba, Canada, excluding in-hospital pharmaceuticals [15]. DPIN contains de-identified person-level prescription and demographic data and is part of the Manitoba Population Data Repository housed at the Manitoba Centre for Health Policy (MCHP). DPIN data can be linked to other administrative health databases through a scrambled personal health identification number (PHIN). All Manitoba residents are eligible for a provincial Pharmacare program, which covers 100% of drug costs above an income-based deductible [16]. Variables for validation included dispensation date, quantity dispensed and days supplied. To allow for direct comparison of the different drugs studied, we also assessed daily dose measured in defined daily dose (DDD) [17, 18]. Dispensations with a days supplied value of 1 (n = 11) were assumed to be pharmacy-level data entry errors and corrected to the median days supplied value [11, 19].
2.2 Reference Standard Data and Linkage
Medical records from patients attending an outpatient psychiatry clinic in the largest tertiary hospital in Winnipeg, Canada comprised the reference standard. Winnipeg is the capital city of the province of Manitoba, and in the study period, nearly 60% of Manitoba’s population of 1.34 million lived in Winnipeg [20]. Each patient was under the care of a psychiatrist, registered psychiatric nurse and case worker; missed injection appointments were documented in the medical record by the clinic nurse and a follow-up appointment scheduled by the case worker. Adult patients who were dispensed an LAIA from an on-site outpatient pharmacy between April 1, 2015 and March 31, 2016 were eligible for medical record review (n = 264). This time frame was selected because we observed roughly equal numbers of users of first- and second-generation LAIAs in administrative data during this time frame [21]. Eligible records were randomly selected for data extraction until the minimum sample size was achieved. Doses were stratified by type of LAIA used (first-generation LAIA (FG-LAI), risperidone-LAI (R-LAI), paliperidone-LAI (P-LAI)].
We extracted drug name, date of administration, dose and dosing interval for each LAIA dose scheduled during the study period from the medical record. Data were extracted by clinicians specialized in psychiatry (RR) and pharmacy (DJ), and 10% of sampled records were selected for independent two-person data extraction. Subjects who did not attend a scheduled injection were recorded as receiving a 0-mg dose on the scheduled date. Identifying variables for each subject (full name, date of birth, PHIN, sex, study ID number) were recorded separately and submitted to the data provider to create a crosswalk file containing scrambled PHIN and study ID number. The crosswalk file was subsequently transferred to the secure MCHP Repository for analysis. The data extraction form can be viewed in electronic supplementary material (ESM). Ethics and data access approvals were obtained from the Health Research Ethics Board of the University of Manitoba, Health Information Privacy Committee, Winnipeg Regional Health Authority Research Access and Approval Committee, Health Sciences Centre Research Impact Committee and the Manitoba Centre for Health Policy.
2.3 Sample Size Estimation
Minimum sample size was determined using principles for cross-sectional surveys [22], assuming the days supplied value equaled the prescribing dosing interval for at least 15% of FG-LAI [13], 87% of R-LAI [14] and 60% of P-LAI [14]. A minimum of 197 doses of FG-LAI, 174 doses of R-LAI and 369 doses of P-LAI were required. A total of 74 medical records (1233 confirmed doses) were included in the final analysis. Minimum sample size was exceeded for FG-LAI (n = 651) and R-LAI (n = 390) but not for P-LAI (n = 191). However, since both R-LAI and P-LAI are supplied in single-use dosage forms and have fixed manufacturer-recommended dosing intervals, we do not expect findings in the P-LAI stratum to differ significantly from R-LAI [23, 24].
2.4 Data Analysis
2.4.1 Sample Representativeness
DPIN data were linked to hospital discharge abstracts, medical services claims and insurance registry data to obtain demographic characteristics, diagnoses and concomitant medications of patients in the validation sample, and in all Manitoba patients who were dispensed an LAIA during the study period. Income category was determined using the socioeconomic factor index, a summary score that assigns an income category based on the average household income, percent of single-parent households, unemployment rate and high-school education rate within an individual’s dissemination area [25]. Descriptive statistics and standardized differences were used to compare characteristics of patients in the validation sample with all LAIA users in the Manitoba population.
2.4.2 Dispensation Date
Doses were classified as medical record positive (confirmed administered) or negative (scheduled but not administered), and DPIN positive or negative (dispensed or not dispensed within 3 days before injection). Sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) and kappa were calculated based on the number of dispensations determined to be true positive, false positive, false negative or true positive, using medical record classification as the reference standard (Fig. 1). For dispensations with a confirmed injection within the days supplied field in DPIN, the mean and median time between dispensation and administration was determined.
Mean time (days) between DPIN dispensation date and medical record administration date. CI confidence interval, DPIN Drug Programs Information Network, FG-LAI first-generation long-acting injectable antipsychotics, IQR interquartile range, P-LAI paliperidone long-acting injectable, R-LAI risperidone long-acting injectable
2.4.3 Dose and Dosing Interval
Among dispensations with a confirmed injection within the days supplied field, the proportion of dispensations where quantity dispensed and daily dose from DPIN exactly matched administered dose and daily dose, respectively, from the medical record was determined for each drug and stratum. Similarly, the proportion of days supplied values exactly matching the prescribed dosing interval was determined. Mean and median quantity dispensed, daily dose and days supplied from DPIN data were compared with mean and median dose, daily dose and dosing interval, respectively, from the medical record, rounded to the nearest milligram. When the days supplied value was greater than the expected dosing interval, for example prescriptions dispensed in 84 or 90 days supplied, the median days supplied for that drug was used as a proxy.
2.4.4 Persistence
Algorithms were developed and assessed to evaluate persistence estimates from DPIN data using Kaplan–Meier survival analysis. We also used the proportion of patients covered (PPC) method, allowing subjects to re-start treatment after a gap [26]. All LAIA doses dispensed or administered to eligible subjects within the 2015/16 fiscal year were included. Subjects were classified as exposed from the date of the first dispensation (DPIN data) or administration (medical record data) of an LAIA between April 1, 2015 and March 31, 2016. To account for the extended half-life of LAIAs at steady state, a grace window of 90 days, 180 days, 1.5*days supplied (or dosing interval), or 2*days supplied (or dosing interval) was added to the days supplied or dosing interval for each dispensation or dose administered.
2.4.5 Sensitivity Analyses
Several sensitivity analyses were conducted. We varied grace windows between dispensation and administration, evaluating windows of 0, 1, 7, 30 and 90 days. We evaluated a second algorithm that restricted to users with two or more dispensations in a 90-day period between April 1, 2015 and March 31, 2016; subjects were classified as exposed from the date of the second dispensation. We repeated analyses of FG-LAIs after excluding repeat doses administered from a multidose vial. We also repeated all analyses after allowing dispensations to occur a maximum of 1, 3 or 7 days after administration. Finally, we repeated analyses of time between dispensation and administration, dose, daily dose and dosing interval using true positive dispensations ≤0, 1, 3, 7, 30 and 90 days before administration.
3 Results
3.1 Description of LAIA users
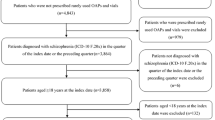
We identified 1145 patients with a dispensation for an LAIA during the study period in DPIN records, 74 of whom were included in the validation sample. Compared with the population of LAIA users in the Manitoba population, patients in the validation sample had similar distributions of age, sex and FG- versus SG-LAI use (Table 1). Patients in the validation sample were higher income, had fewer psychiatric comorbidities and used fewer concomitant medications.
3.2 Medical Record Review
We recorded a total of 1232 LAIA doses from medical records, 1133 of which were confirmed administered and 99 of which were scheduled but not administered. Of these, 651 were for FG-LAIs (610 administered, 41 not administered), 390 were for R-LAI (369 administered, 21 not administered) and 191 were for P-LAI (154 administered, 37 not administered).
3.3 Validity Assessment
Internal validity was assessed by random sample of 11 charts (179 doses) selected for independent data extraction by a second investigator, with >96% agreement for all variables (date administered: 96.6% agreement, dosing interval: 97.2% agreement, dose: 97.8% agreement). Reasons for disagreement included dose recorded by only one investigator (n = 4), disagreement in date administered (n = 2) and disagreement between dosing interval (n = 1).
3.4 Dispensation Date
Sensitivity, specificity, PPV, NPV and kappa results with a 3-day grace window are displayed in Table 2. Overall sensitivity was 32.8%, specificity was 68.7%, PPV was 92.3% and NPV was 5.8%. Compared with FG-LAI, R-LAI and P-LAI had higher PPV and sensitivity, but lower specificity. Results from sensitivity analyses are found in ESM Table 1. Specificity was maximized with a 0-day grace window between dispensation and administration, reaching 100% for R-LAI and P-LAI and 92.7% for FG-LAI. Sensitivity was maximized with a 90-day grace window, reaching 99.7% and 98.1% for R-LAI and P-LAI, respectively, and 80% for FG-LAI.
A total of 652 dispensations were confirmed administered within the days supplied interval (FG-LAIs, n = 158; R-LAI, n = 349; P-LAI, n = 145). The median time between dispensation and administration was 9 days for FG-LAI and 1 day for R-LAI and P-LAI (Fig. 1).
3.5 Dose and Dosing Interval
Quantity dispensed exactly matched administered dose for 99.7% and 100.0% of R-LAI and P-LAI doses, respectively, but only 8.9% of FG-LAI doses. DPIN overestimated dose administered for all FG-LAI drugs, with mean (95% CI) differences ranging from −1550.7 mg (−1694.0 to −1407.4) for zuclopenthixol to −166.9 mg (−89.2 to 244.7) for haloperidol. Daily doses exactly matched for 21.3% of FG-LAI, 97.4% of R-LAI and 89.0% of P-LAI. The mean (95% CI) absolute daily dose differences were −1.0 DDD (−1.1 to −0.9) for FG-LAI, 0.0 DDD (−0.02 to 0.0) for R-LAI and 0.6 DDD (−0.4 to 1.6) for P-LAI (Fig. 2A). The difference in DDD was <1 for all FG-LAIs except fluphenazine (mean difference −2.6 DDD).
(a) Mean absolute difference in defined daily dose (DDD) measured by DPIN versus medical record. (b) Mean absolute difference in dosing interval (days) measured by DPIN versus medical record. CI confidence interval, DPIN Drug Programs Information Network, FG-LAI first-generation LAIAs, LAIA long-acting injectable antipsychotic, SG-LAI second-generation long-acting injectable
Days supplied exactly matched prescribed dosing interval for 28.3% of FG-LAI, 97.1% of R-LAI and 76.6% of P-LAI doses. The mean (95% CI) absolute interval differences were −5.3 days (−5.9 to −4.7) for FG-LAI, 0.3 days (0.1 to 0.6) for R-LAI and 0.8 days (−0.2 to 1.7) for P-LAI (Fig. 2B). Among the FG-LAIs, the greatest difference was observed for fluphenazine (−11.8 days), followed by haloperidol (−4.9 days), flupentixol (−4.6 days) and zuclopenthixol (1.6 days).
3.6 Persistence
Kaplan-Meier curves were similar for all algorithms, and log-rank and Wilcoxon tests showed no statistically significant differences (Fig. 3, ESM Fig. 2, ESM Table 1). PPC results also showed that persistence estimates from DPIN dispensations were similar to those from the medical record (Fig. 4, ESM Fig. 3). The algorithms accurately estimated trends in persistence across all strata investigated (ESM Figs. 4–6). However, DPIN-derived estimates of PPC overestimated PPC for FG-LAI and P-LAI and underestimated PPC for R-LAI (ESM Figs. 7–9).
4 Discussion
This study establishes the validity of DPIN dispensations as a proxy for long-acting injectable antipsychotic administration, with a PPV of 80% with a 0-day grace window, and >90% with a 7-day window. When stratified by LAIA type, PPV for R-LAI and P-LAI was 100% at 0 days. Among true positive doses, quantity dispensed and days supplied fields slightly underestimated dose and interval for FG-LAIs but provided valid estimates for SG-LAIs. However, this study also found that a large proportion of administered doses could not be linked with a dispensation, and the absence of dispensation in DPIN does not rule out LAIA administration. Possible explanations for this finding include the use of samples or dispensing from an inpatient pharmacy. DPIN-derived estimates of persistence over 1 year were similar to medical record-derived estimates using Kaplan-Meier survival analysis.
A previous validation study comparing Medicaid claims data with medical records showed the days supplied variable in claims data significantly underestimated days supplied compared with the medical record, and only 13.8% of LAIA dispensations had days supplied values that exactly matched the medical record [13]. This study was published in 1999, before the development of SG-LAIs. While the FG-LAI stratum from the present study showed smaller differences in days supplied than reported by Shireman et al. (5.3 days vs 62.6 days), both studies showed underestimation of days supplied in claims data. In the current study, days supplied in DPIN data was much more reliable among the SG-LAI dispensations, which are supplied in single-use vials or pre-filled syringes. Subsequently, prescription claims data may be able to classify SG-LAI exposure with greater certainty than for oral medications. The prolonged exposure to therapeutic blood levels after an LAIA injection eliminates the need to assess or make assumptions about adherence between injections. Even if an injection is delayed, therapeutic drug levels will subside gradually over time; patients who have achieved steady state may sustain therapeutic levels for weeks or even months after their last dose. As a result, the choice of grace window between dispensations had minimal impact on persistence estimates.
DPIN data showed PPV consistently >80% compared against the medical record reference standard, regardless of the specific agent used or selection of grace window between dispensing and administration. We have identified algorithms with a high specificity, which can be applied in future studies of LAIA safety and effectiveness. However, the algorithms that maximized specificity had many false negative doses, and a cohort formed from this data source will not capture all LAIA users. False negatives were particularly prevalent in the FG-LAI stratum. Excluding doses administered from multidose vials reduced the proportion of doses classified as DPIN negative. This led to only a modest reduction in false negatives and slight improvement in true negatives in the shorter grace window algorithms (≤ 30 days). This resulted in reduced specificity but improved sensitivity and NPV, and a positive or neutral effect on PPV; overall the impact was small.
All algorithms performed poorly based on kappa values. This may be explained by the small number of negative doses observed in medical record data, particularly for FG-LAI and R-LAI, resulting in some time windows having no doses classified as false negative or true negative. The kappa statistic may have limited value when the prevalence of one classification category approaches zero, regardless of model specificity and sensitivity [27, 28]. Nevertheless, we have reported kappa values as the low prevalence of negatively classified doses was unexpected, and we planned to calculate kappa values at the design of this study. Post-hoc algorithm assessment using additional performance measures can be found in ESM Table 1.
Generalizability of our findings outside of the validation sample population may be limited. While demographic characteristics of the validation sample were similar to those of the general population of LAIA users, there were some clinical differences. Fewer individuals in the validation sample had comorbid mood or anxiety disorders; concomitant prescriptions for oral antipsychotics, mood stabilizers, antidepressants and sedative-hypnotics were also less prevalent. This may indicate the validation sample patients had more stable disease, or may reflect differences in diagnosis and treatment patterns for patients enrolled in a specialized treatment program versus usual care. Still, the clinic from which subjects were randomly selected for the validation sample comprised almost 25% of all LAIA users in the province of Manitoba in the study period, and LAIA users are closely monitored by care providers regardless of care setting. These findings may be relevant in other populations with comparable demographic and clinical profiles in similar care settings.
5 Conclusions
Prescription claims data are a valuable tool for clinicians conducting medication histories, and an important data source for drug safety and effectiveness studies. We have shown prescription claims data are a valid source of data on positive LAIA exposures, particularly for SG-LAI. Quantity dispensed and days supplied from dispensation data provide approximate estimates of dose and dosing interval for LAIA medications. Persistence estimates using claims data were not significantly different from the medical record.
References
Leucht C, Heres S, Kane JM, Kissling W, Davis JM, Leucht S. Oral versus depot antipsychotic drugs for schizophrenia—a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011. https://doi.org/10.1016/j.schres.2010.11.020.
Weiden PJ, Roma RS, Velligan DI, Alphs L, DiChiara M, Davidson B. The challenge of offering long-acting antipsychotic therapies: a preliminary discourse analysis of psychiatrist recommendations for injectable therapy to patients with schizophrenia. J Clin Psychiatry. 2015. https://doi.org/10.4088/JCP.13m08946.
Acosta FJ, Ramallo-Fariña Y, Siris SG. Should full adherence be a necessary goal in schizophrenia? Full versus non-full adherence to antipsychotic treatment. Compr Psychiatry. 2014. https://doi.org/10.1016/j.comppsych.2013.09.005.
Rezansoff SN, Moniruzzaman A, Fazel S, McCandless L, Somers JM. Adherence to antipsychotic medication and criminal recidivism in a Canadian provincial offender population. Schizophr Bull. 2017. https://doi.org/10.1093/schbul/sbx084.
Olivares JM, Sermon J, Hemels M, Schreiner A. Definitions and drivers of relapse in patients with schizophrenia: a systematic literature review. Ann Gen Psychiatry. 2013. https://doi.org/10.1186/1744-859X-12-32.
Brissos S, Veguilla MR, Taylor D, Balanzá-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014. https://doi.org/10.1177/2045125314540297.
Tiihonen J, Mittendorfer-Rutz E, Majak M, Mehtälä J, Hoti F, Jedenius E, Enkusson D, Leval A, Sermon J, Tanskanen A, Haipale H. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiat. 2017. https://doi.org/10.1001/jamapsychiatry.2017.1322.
Taipale H, Mittendorfer-Rutz E, Alexanderson K, Majak M, Mehtälä J, Hoti F, Jedenius E, Enkusson D, Leval A, Sermon J, Tanskanen A, Tiihonen J. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018. https://doi.org/10.1016/j.schres.2017.12.010.
Stip E, Lachaine J. Real-world effectiveness of long-acting antipsychotic treatments in a nationwide cohort of 3957 patients with schizophrenia, schizoaffective disorder and other diagnoses in Quebec. Ther Adv Psychopharmacol. 2018. https://doi.org/10.1177/2045125318782694.
Kishi T, Matsunaga S, Iwata N. Mortality risk associated with long-acting injectable antipsychotics: a systematic review and meta-analyses of randomized controlled trials. Schizophr Bull. 2016. https://doi.org/10.1093/schbul/sbw043.
Burden AM, Paterson JM, Gruneir A, Cadarette SM. Adherence to osteoporosis pharmacotherapy is underestimated using days supply values in electronic pharmacy claims data. Pharmacoepidemiol Drug Saf. 2015. https://doi.org/10.1002/pds.
Campagna EJ, Muser E, Newcomer JW, Parks J, Morrato EH. Methodological considerations in estimating drug adherence for a long-acting injectable medication. J Manag Care Pharm. 2014. https://doi.org/10.18553/jmcp.2014.20.7.756.
Shireman T, Svarstad B, Sweeney J. Validity of claims data for long-acting injectable medications. J Pharmacoepidemiol. 1999;7:41–55.
Kozma CM, Durham M, Durkin M, Dickson M, Howe A. Validity of administrative claims data for calculating adherence measures for long-acting injectable (LAI) antipsychotic therapies. Value Heal. 2012. https://doi.org/10.1016/j.jval.2012.03.529.
Kozyrskyj AL, Mustard CA. Validation of an electronic, population-based prescription database. Ann Pharmacother. 1998. https://doi.org/10.1345/aph.18117.
Manitoba Health and Seniors Care. About the Manitoba Pharmacare Program [Internet]. https://www.gov.mb.ca/health/pharmacare/. Accessed 20 Aug 2021.
WHOCC - ATC/DDD Index [Internet]. https://www.whocc.no/atc_ddd_index/. Accessed 31 Mar 2021.
Nosè M, Tansella M, Thornicroft G, Schene A, Becker T, Veronese A, Leese M, Koeter M, Angermeyer M, Barbui C. Is the defined daily dose system a reliable tool for standardizing antipsychotic dosages? Int Clin Psychopharmacol. 2008. https://doi.org/10.1097/YIC.0b013e328303ac75.
Grymonpre R, Cheang M, Fraser M, Metge C, Sitar DS. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006. https://doi.org/10.1097/01.mlr.0000207817.32496.cb.
Manitoba Health, Seniors and Active Living. Manitoba Health, Healthy Living and Seniors Population Report June 1, 2016 [Internet]. 2016 [cited 2021 Mar 31]. Available from: https://www.gov.mb.ca/health/population/2016/pr2016.pdf
Janzen D, Bolton J, Kuo I, Leong C, Alessi-Severini S. Trends in the use of long-acting injectable antipsychotics in the province of Manitoba, Canada. J Clin Psychopharmacol. 2020. https://doi.org/10.1097/JCP.0000000000001148.
Leong C, Sareen J, Leslie WD, Enns MW, Bolton J, Alessi-Severini S, Katz LY, Logsetty S, Snider C, Berry J, Prior HJ, Chateau D. Validity of the days supply field in pharmacy administrative claims data for the identification of blister packaging of medications. Pharmacoepidemiol Drug Saf. 2017. https://doi.org/10.1002/pds.4288.
Janssen Inc. Risperdal Consta [product monograph]. 2020.
Janssen Inc. Invega Sustenna [product monograph]. 2020.
Metge C, Chateau D, Prior HJ, Soodeen R, De Coster C, Barré L. Composite measures/indices of health and health system performance. Winnipeg, MB: Manitoba Centre for Health Policy, August 2009.
Rasmussen L, Pratt N, Hansen MR, Hallas J, Pottegård A. Using the “proportion of patients covered” and the Kaplan-Meier survival analysis to describe treatment persistence. Pharmacoepidemiol Drug Saf. 2018. https://doi.org/10.1002/pds.4582.
Maclure M, Willett WC. Misinterpretation and misuse of the kappa statistic. Am J Epidemiol. 1987. https://doi.org/10.1093/oxfordjournals.aje.a115065.
Donker DK, Hasman A, Van Geijn HP. Interpretation of low kappa values. Int J Biomed Comput. 1993;1993(33):55–64.
Acknowledgement
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Manitoba Population Research Data Repository under project #2020-040 and 2017-016 (HIPC#2019/2020-19 and 2016/2017-64). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred. Data used in this study are from the Manitoba Population Research Data Repository housed at the Manitoba Centre for Health Policy, University of Manitoba and were derived from data provided by Manitoba Health and Winnipeg Regional Health Authority. The authors acknowledge Dr Sherif Eltonsy and Alekhya Lavu for their review of this manuscript, as well as Dr Joseph Delaney for his assistance in interpreting the results. We also acknowledge the WRHA Pharmacy Program, and in particular Bob Bulloch, Jarrid McKitrick and Wendy Simoens for their assistance with identifying eligible study subjects, and HSC Health Information Services staff, Rachael Porter, Chrissy Hasse and Lorna Sasley for their assistance with the medical records review. We acknowledge Judy Dyrland, Jason Berry, Saila Parveen, Charles Burchill and Dave Towns for their assistance in obtaining the necessary data access approvals and de-identifying the medical record data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was supported by the Evelyn Shapiro Award for Health Services Research to DJ, the Leslie F. Buggey Professorship in Pharmacy to SAS, and the University of Manitoba.
Conflicts of interest/Competing interests
DJ, RR, JB, CL, IK and SAS all declare no conflicts of interest.
Availability of data and material
Data used in this article was derived from administrative health and social data as a secondary use. The data was provided under specific data sharing agreements only for approved use at Manitoba Centre for Health Policy (MCHP). The original source data is not owned by the researchers or MCHP and as such cannot be provided to a public repository. The original data source and approval for use has been noted in the acknowledgments of the article. Where necessary, source data specific to this article or project may be reviewed at MCHP with the consent of the original data providers, along with the required privacy and ethical review bodies.
Code availability
SAS code is available on request.
Author contributions
Design and conceptualization of the study: DJ, JB, CL, IK and SAS. Data extraction: DJ, RR. Analysis and data interpretation: DJ, JB, CL, IK and SAS. Drafting and revising the manuscript: DJ, RR, JB, CL, IK and SAS. All authors give final approval of the manuscript.
Ethics approval
This study was approved by the Health Research Ethics Board of the University of Manitoba under study numbers HS22875 (H2019:210) and HS20380 (H2016:468).
Consent to participate
Participant consent was waived due to the retrospective nature of the study.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Janzen, D., Ramkissoon, R., Bolton, J.M. et al. Long-Acting Injectable Antipsychotics in a Prescription Claims Data Source: A Validation Study. Drugs - Real World Outcomes 9, 517–527 (2022). https://doi.org/10.1007/s40801-022-00297-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-022-00297-4