Abstract
The extraction of valuables from waste has gained momentum. Thermal influence alters both the organic and inorganic components of coal. Insufficient knowledge on the association of rare earth elements (REEs) with the parent matrix of thermally altered high-ash coals (63% ash) limits the potential for such coals being utilized for isolation of valuables. In this study, we analyzed the distribution and occurrence modes of REEs within a magmatically altered high-ash coal via nine-step sequential extraction, combining Tessier and BCR methods. The total concentration of REEs in the coal sample, on whole coal basis, was found to be 820 ppm, which is significantly higher than the world average. Major mineral oxides were deduced to be those of Si, Fe, Al, Ca, Mg, and Ti. Sequential extraction confirmed that about 66% of HREE and 25% of LREE were included in the residual fraction. LREEs were concluded to be primarily in ionic form, whereas HREEs were speculated to be associated with the TiO2 phase. XRD analyses showed that thermal alteration affected the dolomite phase specifically, which selectively got removed where carbonate-bound elements were assessed. Petrographic analysis supported the magmatic influence and demonstrated the presence of mosaic structures and pores containing unfused vitrinite, with a reflectance value of 3.6. To summarize, the present study pertaining to delineation of association of valuables in high-ash heat-altered coals from an Eastern coalfield in India can potentially open up new avenues for utilizing such coals, which are otherwise considered waste.
Article Highlights
-
A nine-step sequential chemical leaching process employing eco-friendly lixiviants was implemented to delineate the mineralogical associations in high-ash heat altered coal of Indian origin.
-
Dolomite was completely eradicated at the complexation stage implying alteration of this mineral phase due to magmatic influence.
-
LREEs were concluded to be present primarily in the ionic form while HREEs were strongly associated with anatase phase.
-
Petrographic analysis revealed presence of mosaic structures with porous morphology containing un-fused vitrinite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Globally, the demand for rare earth elements has increased manifold in recent years in line with their augmented usage in high-end technologies and economic areas. The potential risk of disrupted supply of these valuables from China has spurred growing interests in countries worldwide to explore their indigenous resources. However, identification of coal blocks enriched with REEs, knowledge of how they are associated with the coaly matrix, their geochemical origin, and depositional conditions are crucial in order to develop efficient extraction strategies.
Magmatic intrusion into coal seams is a well-known phenomenon, and various studies have been done on the changes observed in mineralogy and geochemistry for such heat-altered coals (Dai and Ren 2007). Such intrusions not only affect the organic matter (Sarana and Kar 2011) but also the non carbonaceous constituents of the coal. Hence, the association pattern of various elements present in the coal matrix is expected to be altered, thereby, affecting the leaching of these elements. A similar challenging situation is usually experienced while optimizing the leaching parameters of REEs from coal ash (both bottom and fly ash) obtained from thermal power plants where the valuables are speculated to get encapsulated in the matrix during the combustion process (Fu et al. 2022). Consequently, suitable pre-treatment is often considered an absolute necessity to improve upon the leaching potential of REEs (Zhang and Honaker 2020). Numerous studies were previously performed on understanding the rank behavior of thermally altered coals of Indian origin (Chandra and Chakrabarti 1989; Pareek 1988; Prakash and Sarate, 1993; Mishra and Cook 1992; Singh et al. 2013), delineation of the genesis of natural cokes (Singh et al. 2007; 2008) and the impact of heat alteration on the reactivity of coal macerals (Choudhary et al. 2008). Minerological enrichment from the dike into the altered coal was shown to be higher for elements such as As, Ca, Cu, Mg, Mn, Sr, Th, and rare earths (Goodarzi and Cameron 1990).
The distribution of REEs along with their modes of occurrence has been thoroughly investigated for unaltered thermal coals aiming at improving the efficiency of extraction of valuables (Fu 2022; Banerjee et al. 2021, 2022; Das et al. 2023; Pan et al. 2019). Sequential leaching has been widely acknowledged as an important tool to delineate the elemental distribution in coal (Mittermüller et al. 2016; Pan et al. 2021). However, igneous intrusions are known to cause pronounced changes in the vitrinite reflectance, mineralogical profile (both associations and distribution), and geochemistry of the coal seams. It would be interesting to understand its impact on the association of REEs, in particular, with the major oxides in the coal matrix which have not been examined. In the Indian scenario, such investigations are even more crucial since about 2% of the coals are thermally metamorphosed and rendered useless (Singh et al. 2007). A preliminary knowledge of the geological enrichment of REEs in coal was considered necessary prior to a detailed study.
Eastern Coalfield is a store-house for good-quality coal, catering to both the steel sector and thermal power plants. The geological aspects of this geographical location have been extensively studied, along with the mineralogical distribution (Chatterjee and Pal 2010; Das and Chakrapani 2011; Saha et al. 2022). A preliminary analysis of coal samples from Eastern coalfield gave promising results with respect to REE concentrations (unpublished results). Nevertheless, a considerable portion of the coal in this basin is affected by igneous intrusions (Sarana and Kar 2011), which not only limits their subsequent utilization but also indicates a possible alteration in the inherent chemistry of mineralogical association. Evaluation of such alterations is crucial to devising the best possible leaching strategy for valuables, both trace elements and REEs, from magmatically affected coals that are otherwise rendered useless.
The primary objective of the present study was to conduct a systematic analysis of the content of REE and delineate the crucial associations with the inorganic plexus of a high-ash coal acquired from Eastern Coalfield, India. A novel nine-step sequential chemical extraction using a combination of BCR and Tessier methods (Park et al. 2021) was performed incorporating eco-friendly reagents and acids. Thorough mineralogical analysis (using XRF, ICP-OES and ICP-MS) in combination with petrography, zeta potential, X-ray diffraction (XRD), and scanning electron microscopy (SEM–EDX) was performed to gain a complete understanding of how the REEs are distributed and associated in the magmatically altered coal from the chosen area.
2 Scope of the paper/background literature
In prior literature, sequential leaching has been adopted as the preferred way to understand the pattern of occurrence of important elements in multiple sources, such as natural kaolinite, gold-bearing mineral, soil, and tailings, coal fly ash, and many more. Some common multi-step sequential leaching studied to understand the association of REE in various sources is tabulated in Table 1.
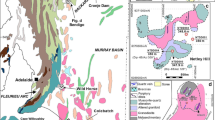
The chosen heat altered coal was collected from Eastern Coalfield, India, which has total coal reserves of more than 50 billion tonnes and geographically spreaded across Indian states of West Bengal and Jharkhand. It is known to consist of heat altered coals originating from multiple types of igneous intrusions, such as mica peridotite dykes and sills and dolerite dykes (Paul 2005). A geological map of the chosen study area is shown in Fig. 1.
3 Sample collection and preparation
Coal sample was collected from the chosen coal block located in Eastern Coalfield, India (Fig. 1). The coal seam was located at a depth of 885 cm and the thickness of the seam was about 2 m (885–887 m). In the present study, a total of 20 kg of coal sample was procured in 50 mm size range which was homogenized using quarter-coning first. Subsequently, it was subjected to grinding using both primary and secondary crushers to two types of sample size range, a) 3 mm particle size range (which was used for petrographic analysis) and b) 200 mesh particle size (which was utilized towards sequential extraction studies).
4 Methods and materials
Heat altered coal of Indian origin was chosen for the present study. pH measurements were done with pH meter CL 54 + . Analytical grade nitric acid (65%) which was used throughout the experiments was obtained from Rankem, India. All other chemicals (analytical grade) along with their respective makes were as follows: methanesulphonic acid and sodium hydroxide (Merck), lithium metaborate and tetraborate (Loba Chemie), ammonium sulphate (Fisher Scientific), tartaric acid (Loba Chemie), hydroxylamine hydrochloride (Loba Chemie), ammonium oxalate (Loba Chemie), oxalic acid (Sisco Research Laboratories), ascorbic acid (Sisco Research Laboratories), 30% hydrogen peroxide (Merck) and ammonium acetate (Merck). Milli Q water (using Millipore, 18.2 Ω) was used to produce deionized (DI) water, which was used for all the leaching and extraction experiments.
An X-ray fluorescence (XRF) spectrophotometer (S8 Tiger, Bruker) was used for mineralogical profiling using the press pellet method. An X-ray diffractometer (X’pert PRO, PANalytical) was used to determine the primary mineral phases present in the coal ash samples. The experiments were performed over a 2θ range of 5°- 90° using Ni-filtered Cu Kα radiation. The identification of phases was performed using High Score Plus software on the basis of the JCPDS database. Morphological and qualitative mineralogical analysis of the heat altered coal sample was accomplished using scanning electron microscopy (SEM) (FEI NOVA NANOSEM 430 at 15 kV) equipped with EDX. REE quantification was accomplished using inductively coupled plasma–mass spectrometer (ICP-MS) (Agilent 7800). Major and minor trace elements were analyzed using an inductively coupled plasma-optical emission spectrophotometer (ICP-OES) (Thermo Scientific, model i CAP 7000 series). Petrographic analysis was performed on polished blocks (prepared using a − 1 mm coal sample size) under both reflected white and fluorescent lights, with 50 × and 20 × oil- immersion objectives employing a Leica DM4500P microscope. Zeta potential measurements were carried out at ambient temperatures (25 °C) using a Zetasizer, Nano-ZS (Malvern, Model ZEN 3600).
4.1 Estimation of total REE content in coal sample
The total REE content in coal samples was evaluated using borate fusion method (Banerjee et al. 2021) after determining the loss on ignition (LOI). In nutshell, 0.1 g of coal ash was fused with a mixture of lithium tetraborate and lithium metaborate (1:1.3) at 950 °C for 1 h in a muffle furnace. The resultant sample was cooled down in a stepwise manner followed its dissolution in 100 ml of 10% nitric acid. The quantification of REE in the solution was performed using ICP-MS instrument.
4.2 Procedure for sequential leaching of coal
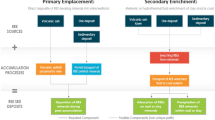
Based on prior literature, sequential chemical extraction has been implicated as one of the most preferred methodologies in order to ascertain the association pattern of REEs in host material (Huggins 2002; Zhang and Honaker 2020). Typically, the process entails sequential reactions with a series of reagents under different experimental conditions, where a result from each step indicates the occurrence for an element. In order to investigate how the modes of occurrence of REEs get altered when magmatic intrusion happens, sequential chemical extraction tests were performed on the chosen coal sample. To start with, 15 g of the coal sample was taken in a 100 mL centrifuge tube. The centrifuge tube containing the coal sample and reagent was shaken in an orbital shaker for each step. An overall scheme for the sequential chemical extraction adopted for this study is presented in Fig. 2. After each step, the resultant coal slurry was filtered using Whatman 42 filter paper. The clear filtrate thus collected was analyzed using ICP-MS. The remaining filter cake was thoroughly rinsed using MilliQ water and dried at 110 °C for 12 h prior to proceeding for the next step in the sequence. The resultant product was weighed and taken forward for the next stage. In the last extraction step, the solid acquired was weighed and completely digested using lithium borate fusion method (Banerjee et al. 2021). In the final step, the digestion solution was collected to determine the concentration of REEs using ICP-MS.
5 Results and discussion
Isolation of valuables such as REEs from magmatically altered coal has dual advantage of utilization of waste as well as exploration of the leaching potential from a novel unexplored type of coal. The leaching potential, in turn, is highly dependent on the association of the elements with the coal matrix as a whole, comprising of organic and inorganic components. Therefore, in the present study a detailed sequential leaching process was explored comprising of water soluble, ion exchangeable, carbonate bound, mild moderate and strongly reducible, oxidizable and mineral acid soluble. The product obtained in each stage was characterized using analytical tools to delineate the mineralogical associations.
5.1 Characterization of coal and coal ash
The results obtained from preliminary analysis of the coal sample are shown in Table 2, which demonstrates that it possesses a high content of mineral matter (63% ash) and a low sulphur content (0.18 wt%). Owing to heat alteration, the calorific content of the chosen coal was considerably low (2967 kcal/kg). The comprehensive mineralogical analysis of the major oxides in the coal ash using XRF is tabulated in Table 3. The individual concentrations of the major oxides in the coal ash were as follows: SiO2 (61 wt%), Al2O3 (14 wt%), Fe2O3 (6 wt%), MgO (6 wt%), TiO2 (4 wt%), and CaO (4 wt%). The SEM-EDAX results for the chosen heat affected raw coal is shown in Fig. 3, which further substantiates the presence of elevated concentration of Ca and Mg, along with the Si, Al, and Fe, which are typically present in abundance in coal. Using ICP-MS, the total REE concentration of the coal sample (on whole coal basis) was determined to be 820 ppm (Table 4) which is significantly higher than the world average on whole coal basis (115–130 ppm) which justified the importance of the chosen coal block for exploration based study. Individually, the total concentration of LREE was 750 ppm, whereas only 69 ppm of HREE was present. Among the LREEs, the highest enrichment was that of Ce (330 ppm) followed by Nd (163 ppm) and La (144 ppm). Among the HREEs whose concentration was in the detectable range, the highest concentration was found to be for Y (46 ppm) followed by Dy (10 ppm). In nutshell, on the basis of mineralogical profiling, the chosen coal block of Eastern Coalfield, India was concluded to be highly enriched in valuables.
5.2 Petrography
Petrographic analysis, including maceral quantification and vitrinite reflectance measurements of the coal sample, was performed in order to understand the alterations in inherent chemistry caused by the magmatic alteration. The results obtained for the quantification of macerals and sub macerals for the chosen coal are shown in Table 5 and the photomicrographs of the sample is shown in Fig. 4.
Photomicrographs of thermally altered coal samples collected from Eastern coalfield, India, obtained using an advanced optical microscope: a Highly porous structure formed due to heat effect b Natural coke showing flow structure with un-fused vitrinite at their edges c Heat affected on vitrinite band at the very initial stage as high porosity due to devolatilization d Un-fused vitrinite sandwiched between natural coke e–f Un-fused vitrinites showing mosaic structure cavities filled with mineral matter g Mosaic structure with un-fused vitrinite patches h–j Demonstrating the abundance of pyrite in the chosen heat altered coal
Mineral suites identified by microscopic examination, particularly quartz, carbonate, and clay possibly associated with dykes or igneous intrusions, suggested that the coal under investigation was heat altered (Das et al. 2023). A higher vitrinite reflectance of 3.60 was attributed to the magmatic influence in coal. However, the sample did not metamorphose or get converted to coke completely because it demonstrated the presence of unfused vitrinite (Fig. 4b). The variations in the texture of the macerals and minerals showed initial heating for the coaly mass. It was porous, with pores having irregular shapes indicative of the removal of volatiles during metamorphism. The formation of mosaic structures was evidence of the intermediate plastic stage during heat alteration. The texture of the coal sample had a close resemblance to that of a brecciated semi-coke, as reflected by its reduced porosity, and the vitrinite was less anisotropic as well. Further, the presence of pyrite indicated that thermal alteration must not be caused due to significantly higher temperatures (> 600 °C); otherwise, the pyrite would have decomposed. The presence of pyrite in the chosen area is demonstrated in Figs. 4h–j.
REEs in coal were speculated to be introduced through widespread volcanic ash fall, igneous intrusions, and fluvial-detrital transport. Mineral and maceral structures identified by microscopic studies further supported that the primary source of REEs in the chosen heat altered coal samples was due to the igneous intrusions in the basin.
5.3 Sequential leaching
The speciation and fractionation of REE and the other major elements in the heat altered carbonaceous shale was chemically determined using the 9-step sequential leaching process (Rauret 1999). The optimization of the number of steps, solid to liquid ratio, reagents used, temperature, and duration depends upon the speciation element and the matrix under consideration. The 9-step sequential leaching process adopted here encapsulates all the possible phases to be considered in understanding the association of elements in the matrix. The solid to liquid ratio was maintained at 1:20 throughout the leaching experiments to maintain consistency. On another note, both inorganic (HNO3) and organic acids (MSA) were investigated in the key leaching steps in order to compare their respective leaching potentials, since their mechanism of extraction are significantly different.
5.4 Water-soluble and ion-exchangeable fractions
The first two steps in the sequential leaching scheme were meant to identify the water-soluble and ion-exchangeable elements that were loosely attached to the coal matrix or are typically present in cation-exchangeable form. The elemental analysis of the filtrate demonstrated significantly lower concentrations of REEs (< 1%) (Fig. 5) being leached out, thus proving that the REEs were embedded or tightly bound to the coal matrix and were not present in an ion-exchangeable form.
Results obtained for all the individual steps in sequential leaching of heat altered coal for LREE; HREE; Si; Fe; Al; Ca; Mg and Ti. The steps were as follows: S1 and S2 represent water soluble and ion-exchangeable fractions, S3 represent complex/carbonate bound fractions, S4–S6 represents the reducible fractions, S7 represents the oxidizable fractions, S8 was for solubility in strong acids and lastly S9 was for the residual fusion step
5.5 Complex/carbonate bound elements
The next stage of leaching (Step 3) includes reagents which can potentially leach out elements which possess higher complexing ability or are naturally present as carbonate bound. In order to ascertain the complexing potential, tartaric acid was chosen as the suitable reagent due to its high denticity (tetradented) and complex formation ability (Manning 1963; Banerjee et al. 2021, 2023). When the coal sample was leached using 0.3 M tartaric acid at room temperature, 20% of the LREEs were leached out along with 7% of the HREEs (Fig. 5). This demonstrated that the magmatic alteration might have occurred at a lower temperature (< 700 °C) and hence didn’t lead to the formation of a complete melt of the aluminosilicate matrix, which typically encapsulates REEs. Instead, the REEs were concluded to be complexly bound in the coal matrix.
Out of all the major elements that were examined, the leaching percentages were as follows: Ca (55%), Mg (28%), Fe (17%), Al (4%) and Si (1%) (Fig. 5). In the present coal block, on the basis of XRD analysis, Ca, and Mg were concluded to be primarily present as dolomite minerals (Fig. 7d). In accordance with prior literature, dolomite disintegrates upon attainment of 600 °C, leading to the formation of decomposition products such as Mg-calcite (MgCaCO3) along with the major oxides (MgO and CaO) (Zhang et al. 2022). Petrographic analysis of this coal sample revealed mineralogical evidence of the thermal effect being caused by lower temperatures (approx. 500–600 °C) (Fig. 4). Thus, under the same thermal effect, dolomite was partially or completely decomposed to MgO and CaO, which got leached out in higher concentrations in the presence of acid (55% of Ca and 28% of Mg) (González et al. 2021). The disappearance of dolomite was supported by the XRD results (Fig. 7d). Apart from the concentration that got leached out, the rest of the quantity is speculated to be associated with the refractory minerals (Vassilev et al. 1995).
Iron in coal is primarily present as pyrite (Fe2S2) in coal. In presence of acid, pyrite typically gets fragmented into sulphate and iron oxyhydroxides (Ma et al. 2022). Mineral acids (~ 0.5–1 M) are usually employed to study the chemical reactions of pyrite. Interestingly, in the present investigation, leaching with lower concentrations of organic acid (0.3 M tartaric acid, pH 2.2) resulted in extracting out 17% of the total iron into the leach filtrate. The ease of complexation of Fe with a mild organic acid could be indicative of presence of complex bound Fe3+ ions (non-embedded) in the coal matrix.
5.6 Reducible
The reducible fractionation was studied elaborately in 3 steps: Step-4 (mildly reducible conditions) where 0.1 M hydroxylamine hydrochloride was used; Step-5 (moderately reducible) where 0.2 M ammonium oxalate and 0.2 M oxalic acid were used and lastly, the strongly reducible step (Step-6) where ascorbic acid (0.1 M) was additionally used along with the reagents from previous step. Each step has individual significance in the identification of a particular phase (Bauer 2022). The mildly reducible stage was aimed at the identification of amorphous Mn or other similar divalent oxides whereas the moderately reducible condition was performed to identify crystalline Mn oxides or amorphous Fe oxides (Bauer 2022). The strongly reducible step typically identifies the crystalline Fe oxides. A detailed investigation of the alteration in the mineral phases from the XRD analysis was thought to be the ideal way to justify the alteration in mineral phases in a stepwise manner. The combined phase alteration results of the two steps, mild and moderately reducing is shown in Fig. 7e. When compared to the mineral phases present in the raw sample (Fig. 7 a), no significant difference could be observed after treating the coal sample with reducing reagents. However, when strong reducing conditions were employed using an additional dose of ascorbic acid (0.1 M) (Lin et al. 2018), crystalline Fe2O3, which in this case is the magnetite phase, got completely removed (Fig. 7f). Nevertheless, no other mineral phase seemed to have gotten affected. Of note, there was no leaching of REEs, neither LREE nor HREE, under the reducing conditions which is indicative of absence of their association with either amorphous or crystalline oxides present in coal.
5.7 Oxidizable
The next step (Step 7) was aimed at the isolation of oxidizable or pyritic fractions or organic compound states of the elements following the BCR method (Park et al. 2021). Mild oxidizing agent (30% H2O2) was employed for the same in a two-step process (at 25 °C followed by 85 °C, 1 h each), and the results obtained are manifested in Fig. 7g. Coal primarily consists of humic acid framework and mineral matter. Oxidative degradation of humic acids and lignite from Mír mine (South Moravian region, Czech Republic) using H2O2 was demonstrated to result in both oxidation and cleavage of aromatic units from the parent structure (Doskočil et al. 2014). Under the experimental conditions, only 8% LREE and 3% HREE were leached out, indicating their association to be primarily with the inorganic matter in coal. Among the rest of the major elements, 10% Ca was leached out, followed by 3% Mg and 2% Fe. In our knowledge, the only organic association between Ca and other similar elements that has been reported previously was with the carboxyl group (Huggins et al. 2009). Therefore, it could be concluded that with the cleavage of organic units, dissociation of Ca and possibly Mg occurred in relatively higher concentrations, which got leached out during the oxidation stage. None of the mineral phases (vermiculite, muscovite, quartz and anatase) got affected during the oxidation step (Fig. 7g).
5.8 Acid soluble
REEs are most commonly leached out from their parent ores employing strong mineral acids (Ji et al. 2022) The implications of strong acid on the present pre-treated matrix were ascertained in Step 8 which was performed mainly to evaluate the association of elements (loosely bound/embedded) in the aluminosilicate matrix. Instead of going the conventional way of using strong mineral acids, an eco-friendly approach was adopted for this step. Strong organic acid (methanesulphonic acid, 2 M, reflux) was chosen for this step owing to its compatible potential in leaching of REEs with that of mineral acid as demonstrated previously (Banerjee et al. 2022). The chosen organic acid was able to leach out both REEs and other transition and alkaline earth metals with equal potency; the concentrations were as follows: LREE (47%), HREE (25%), Si (4%), Al (31%), Fe (36%), and Ca (17%) (Fig. 5). Simultaneously, leaching was also performed using an equimolar concentration of HNO3, and similar concentration of REEs and major elements were extracted.The XRD analysis demonstrated no visible change in the mineral phases of refractory elements (Fig. 7h). Given that the maximum leaching was observed at the acid dissolution stage, it was reasonable to conclude that REEs and other major elements are very strongly bound in the present situation, resembling the crystalline structure-bound forms.
5.9 Residual
The last step (Step 9), or residual analysis step, was investigated by the lithium metaborate-tetraborate fusion method. The coal sample, which was obtained after 8 steps, was thoroughly dried and fused with a mixture of lithium tetraborate and lithium metaborate (1:1.3) at 950 °C (Banerjee et al. 2022). It was performed to identify the fractions that were not present in their extractable form. As shown in Fig. 5, the leaching percentages of the following were reasonably high; they were HREE (66%), Si (90%), Al (57%) and Fe (26%), which thereby suggests that the HREEs were strongly bound to the aluminosiliate matrix which also consisted of encapsulated Fe. Interestingly, 95% of the total Ti was extracted only at the fusion stage (Fig. 5). According to prior literature, anatase (TiO2), which is present in the coal sample (Fig. 7a) is a commonly known REE bearing mineral. Considering the co-leaching of HREE and Ti at the very last fusion step, it was convincingly concluded that for this particular heat altered coal, instead of the organic matrix, HREEs were primarily associated with the anatase phase.
6 Zeta potential
Zeta potential is a well-known parameter to measure the potential developed at the surface of coal particles, where it is used as an indicator of charge originating from the surface functionality (Aktas 2000). Although, the effect of surfactants have been widely studied with respect to alteration in surface charge using zeta potential measurements (Chakladar et al. 2022), such alterations due to thermal effects have not been examined previously. Magmatic intrusion typically leads to partial loss of volatile matter, and therefore it was speculated to have obvious effect on surface charge. This phenomenon is expected to cause enrichment of mineral matter in the coaly mass, thus making it hydrophilic in nature. The results obtained for both coal and coal ash in the range of pH 2–11 are shown in Fig. 6. The negative zeta potential suggested the development of a surface negative charge on the coal particles owing to magmatic alteration. The iso-electric point for the coal and coal ash was at pH 9.5 and pH 11, respectively.
X-ray diffraction analysis was performed primarily for two reasons: (1) to examine the elemental association in different mineralogical phases in the chosen coal sample; and (2) to identify the phase transformations that may have occurred during the individual steps of chemical treatment. The primary mineral phases in the raw coal sample was found to be vermiculite ((Mg,Fe)3[(Al,Si)4O10]) (10%), muscovite ((KF)2(Al2O3)3(SiO2)6) (15%), sanidine (K(AlSi3O8) (10%), magnetite (Fe2O3) (12%), quartz (SiO2) (30%), anatase (TiO2) (8%) and dolomite (CaMg(CO3)2) (15%) (Fig. 7a). There was no observable change after the first two steps as evident from Figs. 7b and c. However, in step 3 which was performed to ascertain the complex/carbonate bound fractions, there was a significant change in the composition of mineralogical phases where dolomite mineral got completely removed (Fig. 7d). This was indicative of the liberation of CaO and MgO under the magmatic influence. The next noticeable change was in step-6 (Fig. 7f), wherein the coal sample was treated with a strongly reducing agent aimed at the identification of crystalline iron oxide phases. The magnetite phase got completely leached out at this step in the presence of 0.1 M ascorbic acid. In step-8, where the solubility of fractions in strong acid was examined, only a slight reduction in the concentration of vermiculite was observed, leaving the rest of the mineral phases intact. To sum it up, the results obtained from XRD analysis supported the speculated changes based on chemical treatment in each step of the stepwise extraction, where the removal of dolomite under the influence of 0.3 M tartaric acid in step 3 was the most significant observation for the chosen heat-altered coal.
7 Conclusions
In this study, the mineralogical distribution of a high ash heat-altered coal sample of Indian origin was examined in detail. A nine-step sequential chemical leaching process employing eco-friendly lixiviants was implemented, which included the identification of water-soluble and ion-exchangeable fractions, complex/carbonate-bound fractions, reducible and oxidizable fractions, and lastly, acid soluble fractions. The key observations and conclusions were as follows:
-
(1)
XRD analysis revealed the presence of vermiculite, dolomite, anatase, magnetite, quartz, sanidine and muscovite as the major mineral phases in the heat altered coal.
-
(2)
Dolomite was completely eradicated at the complexation stage implying partial dissociation of the mineral phase under thermal influence into Mg and Ca oxides.
-
(3)
Upon leaching with strongly reducible reagents, selective removal of magnetite phase was observed, leaving the remaining mineral phases intact.
-
(4)
LREEs got primarily leached out (47%) at the stage where acid-soluble fractions were ascertained, suggesting their presence in primarily ionic form.
-
(5)
The co-leaching of Ti (95%) and HREEs (66%) at the last borate fusion step strongly supported the possible association of HREEs with anatase instead of the organic matrix of coal.
-
(6)
Petrographic analysis revealed the presence of highly porous structures caused by thermal alteration, along with presence of mosaic structures with unfused vitrinite in them. The presence of pyrite convincingly proved that the magmatic alteration in the chosen area of the Eastern coalfield occurred at a lower temperature (< 700 °C).
-
(7)
The enrichment of major elements and REEs is believed to have originated from the dykes in the altered coal.
The results of this detailed examination further enhanced the possibility of a sustainable utilization of magmatically altered coal of Indian origin with respect to isolation of valuables.
Availability of data and material
All the data generated during the course of the present study are included in this published article.
Abbreviations
- XRF:
-
X-ray fluorescence
- ICP-MS:
-
Inductively coupled plasma-mass spectrometry
- XRD:
-
X-ray diffraction
- ICP-OES:
-
Inductively coupled plasma optical emission spectroscopy
- VM:
-
Volatile matter
- GCV:
-
Gross calorific value
- FC:
-
Fixed carbon
- S1–S7:
-
Step 1–step 7
- BCR:
-
European Community Bureau of Reference
References
Aktas Z (2000) Effect of non-ionic reagent adsorption on zeta potential of fine coal particles. Turk J Chem 24(2):117–130
Aubert D, Probst A, Stille P (2004) Distribution and origin of major and trace elements (particularly REE, U and Th) into labile and residual phases in an acid soil profile (Vosges Mountains, France). Appl Geochem 19(6):899–916
Banerjee R, Mohanty A, Chakravarty S, Chakladar S, Biswas P (2021) A single-step process to leach out rare earth elements from coal ash using organic carboxylic acids. Hydrometallurgy 201:105575
Banerjee R, Chakladar S, Mohanty A, Chattopadhyay SK, Chakravarty S (2022) Leaching characteristics of rare earth elements from coal ash using organosulphonic acids. Miner Eng 185:107664
Banerjee R, Chakladar S, Chattopadhyay SK, Chakravarty S (2023) Leaching of rare earth elements from coal ash using low molecular weight organocarboxylic acids: complexation overview and kinetic evaluation. Int J Chem Kinet 55:606
Bauer S, Yang J, Stuckman M, Verba C (2022) Rare earth element (REE) and critical mineral fractions of central appalachian coal-related strata determined by 7-step sequential extraction. Minerals 12(11):1350
Chakladar S, Vishwakarma D, Patar PK, Mohanty A, Chakravarty S, Patra C (2022) Effect of surfactant, ionic strength, mineralogical composition, and surface morphology on zeta potential of bituminous coals of Indian origin. Int J Coal Prep Util 43:538
Chandra D, Chakrabarti NC (1989) Coalification trends in Indian coals. Int J Coal Geol 13(1–4):413–435
Chatterjee R, Pal PK (2010) Estimation of stress magnitude and physical properties for coal seam of Rangamati area, Raniganj coalfield, India. Int J Coal Geol 81(1):25–36
Choudhury N, Mohanty D, Boral P, Kumar S, Hazra SK (2008) Microscopic evaluation of coal and coke for metallurgical usage. Curr Sci 94:74–81
Dai S, Ren D (2007) Effects of magmatic intrusion on mineralogy and geochemistry of coals from the Fengfeng—Handan Coalfield, Hebei, China. Energy Fuels 21(3):1663–1673
Das SK, Chakrapani GJ (2011) Assessment of trace metal toxicity in soils of Raniganj Coalfield, India. Environ Monit Assess 177:63–71
Das A, Kumar R, Patel SS, Saha C, Das A, Mandal H, Patel RS (2023) Geochemical variations of major, trace, rare earth elements in some Gondwana and Eocene coals of India with a comparison of their germanium, lithium, and mercury content. Geochemistry 83(2):125960
Doskočil L, Grasset L, Válková D, Pekař M (2014) Hydrogen peroxide oxidation of humic acids and lignite. Fuel 134:406–413
Fu B, Hower JC, Zhang W, Luo G, Hu H, Yao H (2022) A review of rare earth elements and yttrium in coal ash: Content, modes of occurrences, combustion behavior, and extraction methods. Prog Energy Combust Sci 88:100954
González Y, Navarra A, Jeldres RI, Toro N (2021) Hydrometallurgical processing of magnesium minerals–a review. Hydrometallurgy 201:105573
Goodarzi F, Cameron AR (1990) Organic petrology and elemental distribution in thermally altered coals from Telkwa, British Columbia. Energy Sour 12(3):315–343
Huggins FE, Seidu LBA, Shah N, Huffman GP, Honaker RQ, Kyger JR, Seehra MS (2009) Elemental modes of occurrence in an Illinois# 6 coal and fractions prepared by physical separation techniques at a coal preparation plant. Int J Coal Geol 78(1):65–76
Ji B, Li Q, Honaker R, Zhang W (2022) Acid leaching recovery and occurrence modes of rare earth elements (REEs) from natural kaolinites. Miner Eng 175:107278
Lin R, Stuckman M, Howard BH, Bank TL, Roth EA, Macala MK, Granite EJ (2018) Application of sequential extraction and hydrothermal treatment for characterization and enrichment of rare earth elements from coal fly ash. Fuel 232:124–133
Ma M, Wang W, Zhang K (2022) Occurrence characteristics of fine-grained pyrite in coal and its scaling effect on flotation desulfurization. ACS Omega 7(46):42467–42481
Manning PG (1963) Tartrate complexes of the rare-earth elements: I. the d-, dl-, and meso-tartrate complexes OF Tb and Eu. Can J Chem 41(10):2557–2565
Mishra HK, Cook AC (1992) Petrology and thermal maturity of coals in the Jharia Basin: implications for oil and gas origins. Int J Coal Geol 20(3–4):277–313
Mittermüller M, Saatz J, Daus B (2016) A sequential extraction procedure to evaluate the mobilization behavior of rare earth elements in soils and tailings materials. Chemosphere 147:155–162
Pan J, Zhou C, Tang M, Cao S, Liu C, Zhang N, Ji W (2019) Study on the modes of occurrence of rare earth elements in coal fly ash by statistics and a sequential chemical extraction procedure. Fuel 237:555–565
Pan J, Hassas BV, Rezaee M, Zhou C, Pisupati SV (2021) Recovery of rare earth elements from coal fly ash through sequential chemical roasting, water leaching, and acid leaching processes. J Clean Prod 284:124725
Pareek HS (1988) Petrographic characteristics of the solid fuels of India with particular reference to the coking coals. Int J Coal Geol 10(3):285–306
Park S, Kim M, Lim Y, Yu J, Chen S, Woo SW, Kim HS (2021) Characterization of rare earth elements present in coal ash by sequential extraction. J Hazard Mater 402:123760
Paul DK (2005) Petrology and geochemistry of the Salma dike, Raniganj coalfield (Lower Gondwana), eastern India: linkage with Rajmahal or Deccan volcanic activity? J Asian Earth Sci 25(6):903–913
Prakash A, Sarate OS (1993) Nature, composition and rank of Lower Gondwana from Pathakhera Coalfield, Satpura Graben. Geophytology 23:115–130
Rauret G, López-Sánchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller P (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1(1):57–61
Saha D, Keshri JP, Saha NC (2022) Comprehensive study on Raniganj coalfield area, India: a review. Ecol Environ Conserv 28:S387–S398
Sarana S, Kar R (2011) Effect of igneous intrusive on coal microconstituents: study from an Indian Gondwana coalfield. Int J Coal Geol 85(1):161–167
Singh AK, Singh MP, Sharma M, Srivastava SK (2007) Microstructures and microtextures of natural cokes: a case study of heat-affected coking coals from the Jharia coalfield, India. Int J Coal Geol 71(2–3):153–175
Singh AK, Sharma M, Singh MP (2008) Genesis of natural cokes: some Indian examples. Int J Coal Geol 75(1):40–48
Singh AK, Sharma M, Singh MP (2013) SEM and reflected light petrography: a case study on natural cokes from seam XIV, Jharia coalfield, India. Fuel 112:502–512
Tohar SZ, Yunus MM (2020) Mineralogy and BCR sequential leaching of ion-adsorption type REE: a novelty study at Johor, Malaysia. Phys Chem the Earth Parts a/b/c 120:102947
Vassilev SV, Kitano K, Takeda S, Tsurue T (1995) Influence of mineral and chemical composition of coal ashes on their fusibility. Fuel Process Technol 45(1):27–51
Zhang W, Honaker R (2020) Characterization and recovery of rare earth elements and other critical metals (Co, Cr, Li, Mn, Sr, and V) from the calcination products of a coal refuse sample. Fuel 267:117236
Zhang Y, Ta X, Qin S (2022) Effect of heat treatment on physico-mechanical behaviour of a natural building stone: Laizhou dolomite marble. J Build Eng 47:103885
Acknowledgements
The authors of the paper are thankful to the Director, National Metallurgical Laboratory, for his cooperation and permission to publish this work. Special thanks to CMPDIL (Coal India Limited) for to provide the coal samples.
Funding
The present study was not funded by any funding agency.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Project planning and execution and entire experimental work: Riya Banerjee. Writing—Manuscript preparation, review and editing: Saswati Chakladar; Petrographic analysis and write up: Alok Kumar. Funding acquisition: SanchitaChakravarty; Supervision: SanchitaChakravarty and Shyamal Chattopadhyay. All the five authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no conflict of interest among themselves.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banerjee, R., Chakladar, S., Kumar, A. et al. Petrology and association of rare earth elements in magmatically altered high-ash coal of Indian origin. Int J Coal Sci Technol 11, 52 (2024). https://doi.org/10.1007/s40789-024-00709-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-024-00709-6