Abstract
This study investigates the paleodepositional conditions of the No. 6 Seam of the Madzaringwe Formation in Makhado and Voorburg south area of the Soutpansberg Coalfield (Limpopo Province, South Africa) utilizing organic petrography and inorganic geochemical proxies. The coals are predominantly high-volatile bituminous B-A rank with high ash yields (avg. 36.1 wt%), characterized by high-vitrinite (~ 41.5 vol%), moderate-to-high inertinite (9.8 vol%–33.7 vol%) and low liptinite (~ 2.3 vol%). The distribution of inertinite varies among different coal horizons (from bottom-lower to middle-upper), suggesting differential oxidation conditions and/or paleofire occurrence. Vitrinite-to-inertinite (V/I) ratio, tissue preservation–gelification index (TPI–GI), and groundwater–vegetation index (GWI–VI) plots, indicate that the peat-forming forest-swamp vegetation accumulated under mesotrophic-to-rheotrophic hydrological conditions. The presence of structured macerals (i.e., telinite, collotelinite, fusinite, and semifusinite) suggests well-preserved plant tissues, whereas framboidal pyrite and sulphur content (0.24 wt%–2.16 wt%) point to brackish-water influence at the peat stage. The coals contain quartz, kaolinite, siderite, muscovite, dolomite, calcite, and pyrite minerals, most of which were likely sourced from felsic igneous rocks. The Al/(Al+Fe+Mn) and (Fe+Mn)/Ti ratios for the studied samples range between 0.24–0.97 and 0.57–70.10, respectively. The ratios, Al–Fe–Mn plot, and presence of massive botryoidal-type pyrite imply some influence of meteoric waters or fluids from hydrothermal activity post-deposition. Moreover, the chemical index of alteration (CIA: 98.25–99.67), chemical index of weathering (CIW: 92.04–97.66), and A–CN–K ternary diagram suggest inorganic matter suffered strong chemical weathering, indicating warm paleoclimatic conditions during the coal formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
South Africa has extensive coal deposits, constituting approximately 3.5% of the world's total coal resources, as outlined in the National Coal Strategy for South Africa in 2018. These coal deposits are hosted within five Permian age sedimentary basins, namely, the Main Karoo (MKB), Springbok Flats, Tuli, Waterberg, and Limpopo Basins (Cairncross 2001; Catuneanu et al. 2005). Among these basins, the MKB holds the majority of the coal deposits and mining activities. The primary mining regions are within Witbank, Highveld, and to a lesser extent, the Ermelo Coalfield (Hancox and Götz 2014). Over the past two decades, extensive research has been conducted in the Witbank and Highveld Coalfields to unravel the depositional environments of the coal-bearing strata. These studies used a combination of maceral composition, mineral geochemistry, sedimentology, palynology, and other interdisciplinary approaches, as evidenced by the works of Falcon (1986, 1989), Cadle and airncross (1993), Pinetown et al. (2007), Gӧtz and Ruckwied (2014), Matjie et al. (2015), Wheeler and Gӧtz (2017), Moroeng et al. (2018, 2019), Krzeszowska (2019), and Denge and Baiyegunhi (2021). In a recent study, Mahooana et al. (2022) conducted an investigation into the A and B Seams of the Ermelo Coalfield using organic petrology and mineralogy, with the aim of elucidating the depositional conditions that prevailed during the formation of these coal seams.
The majority of South Africa's Permian coal deposits were formed through fluvial and deltaic processes, with the organic matter mainly originating from conifers, gangamopteris, and glossopteris flora (Falcon 1986; Cairncross et al. 1990; Cairncross 2001; Bamford 2004; Catuneanu et al. 2005; Jasper et al. 2013; Gӧtz and Ruckwied 2014; Wagner et al. 2019). These coals are typically characterized by high inertinite and mineral matter contents, with less vitrinite and minor liptinite (Steyn and Smith 1977; Falcon 1986, 1989; Hagelskamp and Snyman 1988; Glasspool 2003a, b; Wagner et al. 2018).
The Soutpansberg Coalfield, located in the Limpopo Province to the north of the country, has recently become a focal point for exploration because it has the country's largest deposits of hard-coking coal, with the potential to grow into a significant coal mining hub (Sparrow 2012; Sebola et al. 2022). The coals of the Soutpansberg in the Madzaringwe Formation are early Permian in age (Cadle and Cairncross 1993; Cairncross 2001; Sparrow 2012). In contrast to the coals of the Vryheid Formation of the MKB, there are limited studies on the peat-forming conditions, vegetation type, and post-depositional processes of the Madzaringwe Formation (Hancox and Gotz 2014). According to Mphaphuli (2017), the coals of the Madzaringwe Formation are high in vitrinite, whereas inertinite and liptinite are lower. This contrasts with the coals found in the MKB, which often have a high inertinite content (Falcon 1986, 1989; Hagelskamp and Snyman 1988; Wagner et al. 2018; Moroeng et al. 2018; Mahooana et al. 2022). These differences in maceral composition suggest variations in the paleoenvironmental conditions of the paleomires in the two basins. Organo-petrographic and mineralogical studies provide insights into the conditions under which peat accumulated (e.g., Stach et al. 1982; Teichmüller 1989; Diessel 1992; Taylor et al. 1998; Ward 2016; Dai et al. 2020).
The present study reports detailed maceral and mineral compositions for the No. 6 Seam of the Madzaringwe Formation of the Soutpansberg Coalfield, South Africa. The study aims to decipher the organic and inorganic matter provenance, paleodepositional conditions, and paleoclimatic conditions prevailing during the peat formation and/or accumulation in the Soutpansberg Coalfield based on petrographic and geochemical indices [i.e., chemical index of alteration (CIA) and chemical index of weathering (CIW)] (following Nesbitt and Young 1982; Diessel 1986; Mukhopadhyay 1986; Harnois 1988; Kalkreuth and Leckie 1989; Calder et al. 1991; Marchioni and Kalkreuth 1991; Biswas et al. 2021). This information is useful for acquiring insights into the possible occurrence of critical rare earth elements in this coal.
2 Geological setting
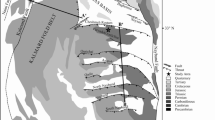
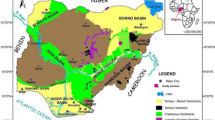
The Soutpansberg Coalfield, situated within South Africa's Limpopo Province between latitudes 22° S to 23° S and longitudes 28° E to 32° E, is a geological rift basin of Karoo age formed within an intracratonic region (Brandl 1981; Malaza 2013; depicted in Fig. 1). The shape and structural configuration of this basin have been significantly influenced by major fault systems such as the Tshipise, Klein Tshipise, and Bosbokpoort Faults (Brandl 1981; Hancox and Gotz 2014). Subsequent to the Karoo period, these fault systems were reactivated, persistently shaping the landscape by generating half-grabens, and thus creating a complex structural setting (Brandl 1981; Telfer and Njowa 2012). The Soutpansberg Coalfield is noteworthy for its preservation of the complete strata of the Karoo Supergroup, as documented by Hancox and Gotz (2014). The economically important interbedded coal occurs in the middle Ecca or Madzaringwe Formations (Malaza 2013) (Fig. 2).
Geological map of the Makhado and Voorburg south coal area (marked with a red square) of the Soutpansberg Coalfield, South Africa (after Hancox and Gotz 2014)
Generalized stratigraphy of the Soutpansberg Coalfield (after Sparrow 2012)
Based on research conducted at CoAL's Makhado and Voorburg Project, the No. 6 seam has been divided into six potentially mineable coal horizons, which are denoted as bottom lower (BL), bottom middle (BM), bottom upper (BU), middle lower (ML), middle upper (MU), and upper seam (US) (Telfer and Njowa 2012; Malaza 2013). Brown (2013) reported that this project has the potential to yield approximately 2.3 million tons of coking coal and around 3.2 million tons of thermal coal annually, with the potential to process roughly 12.6 million tons of run-of-mine coal per year, spanning an estimated mine lifespan of 16 years. The No. 6 coal seam in the Makhado region has a dip of about 12°, while it has a gentler incline of approximately 5° in the Voorburg area (Hancox and Gotz 2014). This coal typically possesses bright and brittle characteristics, with a high vitrinite content (up to 90%), classified as hard-coking coal (Malaza 2013; Sebola et al. 2022). However, in certain regions, coal with a higher inertinite content has also been observed (Mphaphuli 2017).
3 Materials and methods
Coal samples from the Madzaringwe Formation (Middle Ecca) were collected from four boreholes (viz. T190BS03, T07BS, C699001, and R637004) drilled in the Makhado and Voorburg south areas of the Soutpansberg Coalfield, South Africa. The samples were obtained from the bottom-lower (BL), bottom-upper (BU), middle-lower (ML), and middle-upper (MU) parts of the No. 6 coal (Fig. 2). For this study, representative samples from each horizon were selected (total of 11 samples), crushed, and milled for further analyses.
Two splits of each sample were obtained, one part was used for organic petrography and another half was kept for X-ray fluorescence (XRF) and X-ray diffraction (XRD) analyses. The coal particles (< 1 mm) were mounted in epoxy resin, ground, and polished to a final 0.5 μm polish, to achieve a scratch-free surface following the South African National Standard method (SANS/ISO 7404-2 2015). The polished blocks were analyzed with a reflected light Zeiss Axio Imager M2m microscope coupled to Hilgers Diskus Fossil software for point-counting. A minimum of 500 distinct points were recorded on each polished block to determine maceral and mineral assemblages (SANS/ISO 7404-3 2016). The macerals were classified following the International Committee for Coal and Organic Petrology, ICCP System 1994 (ICCP 1998, 2001; Pickel et al. 2017). The pellets were also used to measure mean random vitrinite reflectance (%RoV), following SANS/ISO 7404-5 (2016). The reflectance was measured on homogenous and impurity-free vitrinite grains (collotelinite); about one hundred reflectance measurements were taken per sample. Before beginning with the reflectance analysis, the Yttrium–Aluminum-Garnet (YAG with a reflectance of 0.90%, supplied by Klein & Becker GmbH & Co., Germany) standard was used for calibration.
Further, representative samples were milled to − 212 μm and split utilizing a rotary splitter for proximate and total sulphur (TS) analysis, as well as gross calorific value (GCV). The proximate analysis was carried out at the School of Chemical and Metallurgical Engineering, WITS (South Africa), using a Perkin-Elmer Thermo-Gravimetric analyzer (TGA). The total moisture, ash yield, volatile matter yield, and fixed carbon were determined by the American Society for Testing and Materials (ASTM) standard method D7582 (ASTM 2011). The GCV was determined using a Dry Cal bomb calorimeter in accordance with SANS/ISO 1928 (2009) and the TS content using a Leco SC632 analyzer following SANS/ISO 334 (2020).
The major element oxides in the samples were determined using wavelength-dispersive X-ray fluorescence (XRF) spectrometer and mineralogy through X-ray diffraction (XRD) analyses. Dry powdered samples of − 212 µm size were sent to the XRD Analytical and Consulting in Pretoria, South Africa, for both analyses. The samples were prepared for XRD analysis using the back-loading preparation method. The diffractometer used Co-Kα radiation filtered through iron, and the resulting data were analyzed using X’Pert Highscore plus software and PAN-ICSD for phase identification. The Rietveld method was used to estimate the relative phase quantities (wt%) in the studied coal. The XRF analysis was performed with the ARL PERFOM’X series XRF spectrometer. Initially, powdered ( − 212 µm) samples were ignited at 815 °C for 4 h to remove organic composites and water molecules from the samples. The resulting material was transformed into a fused bead through fusion with lithium tetraborate (Li2B4O7) (following method by Bennett and Oliver 1992). The prepared fused beads were placed in the spectrometer using a sample holder for major oxide characteristics.
Scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM – EDX) investigations were carried out using a TESCAN Vega 3 XMU scanning electron microscope (SEM) at the University of Johannesburg, South Africa, to understand the modes of occurrence for specific mineral phases. A backscattered electron detector at 20 kV was employed for imaging the samples. For obtaining clear and focused images the working distance of ~ 15 mm between the sample and the detector was maintained. Aztec software was used with the EDX system to acquire spectrum peaks of different minerals in the samples.
4 Results
4.1 Standard coal characteristics
The data presented in this section includes results from proximate, GCV, and TS analyses (Table 1). The studied Soutpansberg coals are characterized by relatively high ash (18.00 wt%–58.11 wt%), medium to high volatile matter yields (15.18 wt%–27.55 wt%), and low to medium TS contents (0.24 wt%–2.16 wt%), on a dry-ash basis. The moisture content varies between 0.52 wt% and 1.01 wt% (avg. 0.70 wt%). The fixed carbon (FC) ranges from 25.22 wt% to 56.38 wt%. There is a decrease in the trend of ash yields from BL to MU, whereas the fixed carbon contents increase. The GCV values range from 11.51 to 30.20 MJ/kg. On average, the Makhado samples are comparable to the Voorburg south samples in terms of ash yields and GCV values, though the former samples generally report higher ash (avg. 37.13 wt%) contents and lower GCV values (avg. 18.67 MJ/kg). The studied samples have TS contents varying from 0.24 wt% to 2.16 wt%, with an average of 0.94 wt%. The MU coal seams report relatively higher TS than the other seams in both the Makhado and Voorburg south areas (Table 1).
4.2 Organic petrography
The mean random vitrinite reflectance values (%RoV) values, indicative of coal rank, are presented in Table 2. The %RoV values for the studied coals range between 0.70% and 0.95% (avg. 0.83%), classifying the coals as high volatile bituminous B-A in rank. The distribution of macerals and microscopically observable mineral matter in the studied samples are listed in Table 2; representative photomicrographs are given in Fig. 3. Vitrinite is most abundant in these samples (except coal samples from BU and BL seams of borehole number C699001 in Makhado area), varying from 29.1 vol% to 67.0 vol% (avg. 46.3 vol%), and mainly comprised of collotelinite (avg. 22.3 vol.%; Fig. 3a) and collodetrinite (avg. 14.6 vol.%; Fig. 3c), corpogelinite (avg. 5.2 vol%; Fig. 3b), vitrodetrinite (avg. 2.9 vol%), with minor telinite (avg. 0.7 vol%; Fig. 3a), and gelinite (avg. 0.6 vol%). The inertinite group is represented mainly by inertodetrinite (avg. 7.9 vol%), fusinite (avg. 6.9 vol%; Fig. 3d), semifusinite (avg. 3.9 vol%; Fig. 3e), secretinite (avg. 1.4 vol%), micrinite (avg. 0.5 vol%), and minor macrinite. Liptinite macerals constitute a very minor proportion of the organic composition of the Soutpansberg coals (avg. 2.3 vol%), mainly represented by sporinite, cutinite, and liptodetrinite (Figs. 3g, h). Interestingly, the BU and BL coals contain relatively higher inertinite (avg. 25.2 vol%) than MU and ML coals (avg. 12.8 vol%), mostly represented by inertodetrinite (avg. 10.1 vol%), fusinite (avg. 7.6 vol%), and semifusinite (avg. 5.1 vol%) (Table 2).
Petrographically observed mineral matter in the coals ranges between 17.6 and 55.5 vol% (av. 35.5 vol%; Table 2) and mainly comprises silicate (i.e., clay and quartz), carbonate, and sulphide minerals. The sulphide mineral identified during petrography is likely pyrite, and siderite and calcite are the common carbonates. Interestingly, in sample 15136 (from Voorburg south area), the sulphide content (pyrite) is high (up to 10.4 vol%), possibly indicating variations in depositional conditions within the paleomire.
Different types of pyrite were also identified during the petrographic analysis including infilling cell or pore structures, disseminated euhedral, massive, infilling cracks and replacing other minerals, and framboidal pyrite sometimes exhibiting irregular morphologies (Fig. 4). Overgrowth of framboidal pyrite showing concentric rings was noted in samples 15136 and 15143 from the Voorburg south and Makhado areas, respectively (Fig. 4f). The concentration of carbonate minerals seems higher in sample 15136 from Voorburg south (i.e., 9.6 vol%, Table 2) and in samples 15140 and 15142 from the Makhado areas, at 6.0 vol% and 8.0 vol.%, respectively.
Photomicrographs of different forms of pyrite under petrographic study under reflected white light, a Infilling cell and pore structures; bSubhedral to euhedral pyrite crystals; c Massive pyrite; d: Pyrite infilling cracks in vitrinite, enclosed in a siderite grain; e Syngenetic framboidal pyrite; and f Framboidal pyrite with concentric rings formations. Explanation: py = pyrite, cdt = collodetrinite, ct = collotelinite, and sid = siderite
4.3 Petrographic indicators based on macerals
Diessel (1986) introduced the tissue preservation index (TPI) and gelification index (GI) to understand the paleoenvironmental conditions for Permian coals. There are contradictory views on deciphering paleoenvironment using these models (DiMichele and Phillips 1994; Scott 2002a, b; Moore and Shearer 2003); however, there are several studies that use coal facies indices to reconstruct the paleoenvironmental conditions for Permian coals from around the world (e.g., Silva and Kalkreuth 2005; Silva et al. 2008; Mendhe et al. 2018; Samad et al. 2020; Wagner et al. 2019, Dai et al. 2020). The equations by Diessel (1986) are used to compute the TPI and GI values in this investigation. The TPI is the ratio of tissue-derived structured macerals to unstructured macerals; it reflects the amount of wood that has contributed to the peat, whereas GI quantifies the relative dryness or wetness in the peat-forming environment (Diessel 1992). TPI values greater than 1 imply good plant tissue preservation, whereas high GI values more than 1 suggest relatively wetness during peat accumulation (Diessel 1992). For the studied coals, TPI varies from 0.8 to 2.0 (Table 2). Most of the studied samples have TPI values above 1, except sample 15145 (Table 2). TPI shows positive relationships with telinite, collodetrinite, fusinite and semifusinite (r2 = 0.51, 0.90, 0.72, and 0.34), indicate well preserved structured macerals. The GI ranges widely from 0.5 to 8.8 (avg. 3.1); sample 15136 from the Voorburg south area has a very high GI value of 8.8 (Table 2).
The vegetation index (VI) and groundwater index (GWI) were first used by Calder et al. (1991). VI values more than 1 represent coal derived from woody plants, whereas VI values less than1 indicate the contribution of herbaceous and/or aquatic plants to peat (Thompson et al. 1985; Jiu et al. 2021). The GWI reflects the amount of precipitation during peat formation as well as the groundwater level. According to Silva et al. (2008) and Amijaya and Littke (2005), mires often develop as a result of repeated changes in hydrological conditions between rheotrophic, mesotrophic, and ombrotrophic states. A GWI value less than 0.5 suggests ombrotrophic conditions, whereas a value greater than 1 indicates rheotrophic conditions, and GWI values between 0.5 and 1 define mesotrophic conditions. The VI and GWI values for the studied coals range between 0.7 and 2.0, and 0.4 and 10.0, respectively (Table 2). The VI value has some positive relation with telinite, collodetrinite, fusinite, semifusinite (r 2 = 0.57, 0.89, 0.73, 0.34) and negative relation with liptinite (r2 = 0.57). This is indicative of input of forest vegetation in the formation of this coal and fluctuating water tables in the paleomires.
4.4 Major oxides composition
The oxide compositions are important for assessing sediment source regions, their degree of chemical weathering, paleodepositional environment, and paleoclimatic conditions (Nesbitt and Young 1982, 1984; Bhatia 1983; Dickinson et al. 1983; Roser and Korsch 1988; Condie 1993; McLennan et al. 1993; Nesbitt et al. 1996; Hayashi et al. 1997; Biswas et al. 2021). For this study, the results indicate that SiO2 is the most abundant oxide in the coals, varying between 43.04 wt% and 72.40 wt% (Table 3), Al2O3 between 12.82 wt% and 32.11 wt%, and an average 6.96 wt% for Fe2O3. Sample 15136 from the Voorburg south area, also reporting high pyrite and TS contents (Tables 1 and 2), has the highest Fe2O3 of ~ 30.09 wt% (Table 3). In addition to the three main oxides, the samples also have minor contents of CaO (avg. 1.73 wt%) and TiO2 (avg. 1.01 wt%). The remaining oxides, i.e., K2O, MgO, MnO, Na2O, P2O5, BaO, etc., are generally present in quantities less than ≤ 1 wt%, typical for South African coals (Pinheiro 1999).
Ratios for selected oxides, i.e., K2O/Al2O3, MgO/Al2O3, K2O/Na2O, and Al2O3/TiO2, are given in Table 3; these are useful for determining paleoclimate and paleoweathering conditions. To determine the organic affinity of the oxides (Table 4), Pearson correlation coefficients have been calculated for the studied samples (Table 5). The chemical index of alteration (CIA) and chemical index of weathering (CIW) are useful in determining paleoclimatic conditions (Nesbitt and Young 1982; Harnois 1988). The CIA is computed as follows: Al2O3 × 100/(Al2O3 + CaO* + Na2O + K2O) (Nesbitt and Young 1982), and the CIW as: Al2O3 × 100/(Al2O3 + CaO* + Na2O) (Harnois 1988); CaO* represents the amount of CaO that is incorporated into the silicate fraction of rocks and is calculated as per Bock et al. (1998). The studied samples have CIA and CIW index values ranging from 92.04 to 97.66 (avg. 95.40) and 98.25 to 99.67 (avg. 99.26) (Table 3), indicating a high degree of weathering.
4.5 Mineral composition
The XRD patterns for the studied coals revealed that the minerals included in the coal samples are kaolinite (10.1%–38.6%), quartz (8.9%–28.2%), muscovite (1.2%–5.7%), siderite (0.1%–5.2%), dolomite (0.2%–1.5%), pyrite (0.1%–0.6%), and calcite (0.1%) (Table 4 and Fig. 5), in agreement with the XRF results (Table 3). The relative proportions of quartz and muscovite are similar in the studied coal. In addition, there is a relative increase in the amount of kaolinite from MU to BL of the coal seam. Siderite and pyrite seem to be higher in MU than other parts of the coal seam, whereas calcite and dolomite are more common in the bottom part of the seam. Interestingly, siderite appears to be very high in the MU section of the seam in the Voorburg south area.
Similar to organic petrography, the SEM–EDX analysis shows the presence of pyrite, siderite, and quartz in Soutpansberg coals. Pyrite occurs in a wide variety of forms, including micron-size framboids to more massive, and coarse, vein-filling forms (Fig. 6a–d). In sample 15136, pyrite was observed enclosed in a siderite grain (Fig. 4d), indicative of siderite replacement (Ward 2016; Kusebauch et al. 2018). In addition, framboidal pyrite is commonly observed occurring within vitrinite (Figs. 4e and 6b); in some MU coal samples, these pyrites are found with concentric ring overgrowth and massive pyrite of botryoidal-type (Fig. 6d). Due to its morphology, the framboidal pyrite may be syngenetic in origin (Ward 2016); however, the overgrowth of botryoidal pyrite (Fig. 6d) may be epigenetic, developed after the framboidal pyrite had already formed (Widodo et al. 2010; Chou 2012; Ward 2016; Kusebauch et al. 2018).
5 Discussion
5.1 Paleodepositional conditions and paleovegetation: based on maceral assemblages
The Soutpansberg coals are generally rich in vitrinite, with varying inertinite contents, and very low liptinite indicating that the peat was deposited under generally waterlogged-forest-swamp conditions (Flores 2002; Petersen et al. 2009). Likewise, the low inertinite content implies oxygen-deficient depositional conditions (Hower et al. 2010, 2011; O'Keefe and Hower 2011). Vitrinite (V) forms in wet and moderately anoxic conditions, whereas inertinite (I) results from relatively dry and oxidizing conditions (Stach et al. 1975; Taylor et al. 1998; Dai et al. 2020). Thus, the V/I ratio provides information regarding changes in the oxidation–reduction environment in the paleaomire (Sen and Banerjee 2015). According to Teichmüller and Teichmüller (1982), a V/I ratio greater than 1 indicates a wet forested depositional environment, whereas a ratio greater than 4 denotes a highly water-covered environment (also see Xu and Fang 2005); a value less than 0.25 indicates a dry/oxidizing environment (Sen and Banerjee 2015; Xu and Fang 2005). The studied Soutpansberg coal samples have V/I values between 0.5 and 6.2 (Table 2). The MU and ML coals are observed to have high values greater than 4 owing to their very high vitrinite (56.1 vol%) and low inertinite (12.8 vol%) contents. This indicates that peat mostly accumulated under reducing conditions with high water cover (Stach et al. 1975; Teichmüller and Teichmüller 1982). The occurrence of framboidal pyrite in the vitrinite also supports this interpretation (Figs. 4e and f). Most of the other studied coals have an average V/I ratio of less than 4 suggesting that peat accumulation took place in a telmatic, forested paleomire under reducing conditions due to the high-water cover. The lowest V/I value is for the BU coal samples from borehole number C699001 (sample 15145), indicative of relatively dry conditions hence the abundance of inertinite (~ 33.7 vol%). The occurrence of high amounts of inertodetrinite, semifusinite, and fusinite is indicative of the occurrence of mild to severe oxidation, likely by paleofires (including wildfires) occurring in and/or around the paleomire (Glasspool 2003a, b; Hower et al. 2011). Significant inertodetrinite enrichment may be due to the remobilization of charcoal from peripheral areas into the paleomire as a result of flooding (Moroeng et al. 2018; Mahooana et al. 2022).
Maceral indices plots such as TPI versus GI, and VI versus GWI are used to understand the vegetation, preservation of plant tissues, and paleohydrological conditions (Diessel 1986; Calder et al. 1991; Biswas et al. 2020). The GI values for the Soutpansberg coals range from 0.5 to 8.8, which indicates generally wet conditions (Table 2). These environmental conditions are not suited to the gelification of precursor plant tissues (Diessel 1986); hence the studied coals have reduced amounts of gelovitrinite (i.e., gelinite, and corpogelinite). The TPI value varies between 0.8 and 2.0 (Table 2), reflecting well-preserved tissues and significant input of woody plants into the paleomire of the Soutpansberg coals (Singh and Singh 1996; Flores 2002). The presence of structured macerals including telinite, collotelinite, fusinite, and semifusinite further support this observation (Fig. 3). Most of the samples plot in a zone where both GI and TPI are greater than 1, except for the BU seam (sample 15145) (Fig. 7). This suggests that the studied coals formed under a wet-forest swamp depositional setting. The high ash yields (avg. 36.08 wt.%) along with the high TPI and GI values further support the wet-forest conditions in the paleomire (Diessel 1992; Sen et al. 2016). The BU coal (sample no. 15145) has low TPI value, less than 1, which indicates low tree density and that the peat may have accumulated during periods of relatively lower water table within the paleomire (Fig. 7). The variation in TPI values may be caused due to the increased rate of basin subsidence (Diessel 1992).
GI-TPI plot shows the peat depositional environment for the studied coal of Makhado and Voorburg coal area of the Soutpansberg Coalfield, South Africa (after Diessel 1986)
Similarly, VI and GWI show the influence of hydrological (groundwater) conditions and vegetation (trees, herbs/shrubs, etc.) on the formation of peat (Calder et al. 1991). The VI and GWI values for the studied coal range between 0.7 and 2.0 (avg. 1.1), and 0.4 and 10.0 (avg. 2.2), respectively (Table 2). VI values greater than 1 reflect forested vegetation, whereas values less than 1 suggest herbaceous or aquatic plants (Thompson et al. 1985; Jiu et al. 2021). The VI values for the studied coals indicate significant input of forest vegetation into the paleomire of the Makhado and Voorburg south area of the Soutpansberg Coalfield. The plot of GWI and VI indicates that the peat-forming vegetation was mostly deposited under mesotrophic-to-rheotrophic hydrological conditions (Fig. 8). The ternary plot based on maceral assemblages (Fig. 9) also indicates that peat accumulation took place under mildly oxic to anoxic conditions, likely due to fluctuating water level in the paleomire (Mukhopadhyay 1986). The GWI values of the studied coal also support this observation. In addition, there is some agreement between the peat-forming conditions inferred based on Figs. 8 and 9 and it is inferred that these Soutpansberg coals were likely formed from mixed vegetation, mainly composed of forest plants with the input of herbaceous plant species such as reed, marsh, helophytes, etc. (following Mukhopadhyay 1986).
GWI versus VI plot shows relative changes in hydrological conditions for the studied samples (after Calder et al. 1991)
Ternary diagram showing peat-forming environments (after Mukhopadhyay 1986)
5.2 Paleoenvironment and Paleoweathering conditions: based on major-oxide geochemistry
The paleoweathering conditions during the sedimentation can be evaluated from the major oxide composition. The intensity of chemical weathering depends on the inorganic matter source and paleoclimatic conditions (Nesbitt and Young 1982, 1984; Bhatia 1983; Dickinson et al. 1983; Roser and Korsch 1988). Elevated contents of SiO2, Al2O3, and TiO2 indicate detrital mineral (e.g., quartz and clays) input into the paleomire (Ward 2002, 2016; Dai et al. 2017); the presence of these minerals is also confirmed by the petrographic and XRD analyses (Table 2 and Fig. 5). The SiO2 content shows some positive relation with Al2O3, K2O, and TiO2 (Table 5). Furthermore, the abundance of quartz and kaolinite suggests felsic rocks as the major source of inorganic sediments into the paleomire. The TiO2 value suggests that these sediments within the coal are derived from granite or granodiorite rock composition (Dai et al. 2005; Chi and Yan 2007). Similarly, the Na2O content of the studied samples show a positive correlation with CaO, Cr2O3, Fe2O3, MgO, and MnO, and is negatively correlated with SiO2 and Al2O3 (Table 5). This indicates that pore water might be an important source of Na in these Soutpansberg coals, rather than clay minerals (Bai et al. 2015). The Al2O3/TiO2 ratios for most samples are greater than 21 (except sample 15138; Table 3) suggesting that clay minerals in the coals are mostly derived from felsic igneous rocks through chemical weathering processes (Amajor 1983; Imchen et al. 2014; Dai et al. 2016; Zhao et al. 2017; Denge and Baiyegunhi 2021; Biswas et al. 2021). The high CaO contents reflect the occurrence of calcite and/or dolomite in the Soutpansberg coals. In contrast, Fe2O3 shows a positive correlation with sulphide minerals determined during petrography (r2 = 0.91; Table 5), indicating the presence of pyrite which is also evident in the XRD spectra (Fig. 5). However, some of the sulphur is likely also present as organic sulphur as the TS shows a positive relationship with volatile matter yield (r2 = 0.62) and is negatively correlated with ash yield (r2 = 0.43; following Biswas 2021).
Identifying the source of iron in coal is important for understanding paleoenvironment conditions and some Soutpansberg samples have very high Fe2O3 contents (Table 3). Other than syngenetic pyrite, the other source of Fe in coal may be epithermal, hydrothermal, or a H2S-bearing fluid which occurs after peat accumulation (Chou 2012; Wagner et al. 2017; Kusebauch et al. 2018; Dai et al. 2020). The ternary Al–Mn–Fe diagram adapted from Adachi et al. (1986) shows that most samples lie within non-hydrothermal origin area, though sample 15136 from the middle upper (MU) seam of the Voorburg south area plots in the hydrothermal zone (Fig. 10). According to He et al. (2016), Al/(Al+Fe+Mn) values less than 0.4 and (Fe + Mn)/Ti greater than 15 typically indicate hydrothermal origin. For the studied coals, these ratios range between 0.24 and 0.97 (avg. 0.77), and from 0.57 to 70.10 (avg. 12.31), respectively (Table 3). Interestingly, a (Fe + Mn)/Ti ratio of greater than 15 is observed for samples 15136 (MU coal Voorburg south area) and 15140 (BL coal from Makhado). This possibly indicates some mineralizing fluid affected the coals post-peatification (Adachi et al. 1986; He et al. 2016). The presence of massive botryoidal-type pyrite replacing siderite (Figs. 6c-d) in some samples also supports this observation, possibly related to meteoric waters or fluids related to hydrothermal activity (Kusebauch et al. 2018; Yuan et al. 2022).
Ternary Al–Mn–Fe diagram showing hydrothermal activity based on elemental composition (following Adachi et al. 1986)
During deposition, detrital inorganic matter incorporated within peat undergoes various physical and chemical changes due to changes in climatic conditions. These climatic conditions can be deduced from the CIA and CIW values (McLennan et al. 1993; McLennan 2001). The CIW and CIA values for the studied samples range from 92.04 to 97.66 (avg. 95.40), and 98.25 to 99.67 (avg. 99.26), respectively (Table 3). These values indicate that inorganic matter experienced strong chemical weathering during deposition, pointing to wet and warm paleoclimatic conditions (Nesbitt and Young 1982). Similar to the XRD results, the A–CN–K diagram suggests kaolinite is the dominant clay mineral in the samples and was formed by the chemical weathering of K-feldspar from the felsic sediment source region (Patterson 1971; Nesbitt and Young 1982; Mahooana et al. 2022; Fig. 11). The positive relationship of SiO2 and Al2O3 with CIA and CIW (Table 5) also support this observation.
6 Conclusions
Organic petrography and mineralogy were utilized to understand the nature of paleovegetation, paleoenvironment conditions, and depositional setting of the Madzaringwe Formation coal from the Makhado and Voorburg south area of the Soutpansberg Coalfield, South Africa. The coals are high-volatile bituminous B-A in rank with high ash yields; they are characterized by high vitrinite with moderate to high inertinite occasionally and low liptinite contents. The petrofacies indices (i.e., TPI, GI, VI, GWI, and V/I) indicate that forest-swamp vegetations are likely the major source vegetation for the peat formation and was accumulated under mesotrophic-to-rheotrophic hydrological conditions (i.e., wet conditions). The occurrence of structured macerals (i.e., telinite, collotelinite, fusinite, and semifusinite) and the facies reconstruction models indicate that paleomire conditions were conducive to the preservation of plant tissues. The bottom lower (BL) and bottom upper (BU) coals contain relatively high inertinite, mostly inertodetrinite, fusinite, and semifusinite, indicative of oxidation and/or paleofires. Quartz, kaolinite, siderite, muscovite, dolomite, calcite, and pyrite make up the mineralogy of the coals and were likely derived from granite or granodiorite rock composition. The clay minerals are formed as a result of the chemical weathering of felsic igneous. The geochemical indices, CIA, CIW, and A–CN–K plots, show that the paleoclimate conditions were warm and the inorganic components suffered strong weathering. The elemental ratios [i.e., Al/(Al+Fe+Mn), (Fe+Mn)/Ti], Al–Fe–Mn plot, and the presence of massive botryoidal-type pyrite may indicate the influence of meteoric waters or fluids from hydrothermal activity in the post-mire stages. The presence of terrigenous and hydrothermal minerals may have resulted in the occurrence of critical rare earth elements in these Soutpansberg coals.
Data availability
All the data/images used in this manuscript are included in this manuscript.
Code availability
Not applicable.
References
Adachi M, Yamamoto K, Sugisaki R (1986) Hydrothermal chert and associated siliceous rocks from the northern Pacific their geological significance as indication of ocean ridge activity. Sed Geol 47(1–2):125–148
Amajor LC (1983) Major and trace element geochemistry of Albian and Turonian shales from the Southern Benue trough, Nigeria. J Afr Earth Sci 6(5):633–641
Amijaya H, Littke R (2005) Microfacies and depositional environment of Tertiary Tanjung Enim low rank coal, South Sumatra basin, Indonesia. Int J Coal Geol 61(3–4):197–221
ASTM (2011) Standard test methods for proximate analysis of coal and coke by macro thermogravimetric analysis. ASTM, Pennsylvania, pp 1–3
Bai Y, Liu Z, Sun P, Liu R, Hu X, Zhao H, Xu Y (2015) Rare earth and major element geochemistry of Eocene fine-grained sediments in oil shale-and coal-bearing layers of the Meihe Basin, Northeast China. J Asian Earth Sci 97:89–101
Bamford MK (2004) Diversity of the woody vegetation of Gondwanan Southern Africa. Gondwana Res 7(1):153–164
Bennett H, Oliver GJ (1992) XRF analysis of ceramics, minerals and allied materials. Wiley, New York, pp 67–93
Bhatia MR (1983) Plate tectonics and geochemical composition of sandstones. J Geol 91(6):611–627
Biswas S, Varma AK, Kumar M, Mani D, Saxena VK, Mishra V (2020) Influence of geochemical, organo-petrographical and palynofacies assemblages on hydrocarbon generation: a study from upper Oligocene coal and shale of the Makum Coal Basin, Assam India. Mar Pet Geol 114:104206
Biswas S, Varma AK, Kumar M, Saikia BK (2021) Multi-analytical approach of provenance signatures and indication of marine environment in coal and shale bearing sequence from Makum Coalfield, India. Arab J Geosci 14:1–23
Biswas S (2021) Petrographic and geochemical controls on coal and shale for hydrocarbon generation from Makum and Barjora Basins of India: experimental modeling. Ph. D. Thesis. Indian Institute of Technology (Indian School of Mines), Dhanbad, India. (unpublished)
Bock B, McLennan SM, Hanson GN (1998) Geochemistry and provenance of the middle Ordovician Austin Glen member (Normanskill formation) and the Taconian orogeny in New England. Sedimentology 45(4):635–655
Brandl G (1981) The geology of the Messina area. Explanation, Sheet 2230, Messina, Geological Survey of South Africa (35 pp.)
Brown D (2013) Makhado definitive feasibility study. www.coalofafrica.com/makhado (Accessed September 2015)
Cadle AB, Cairncross B (1993) A sandy, bed-load dominated fluvial system deposited by lateral-accretion: Permian Karoo Sequence South Africa. Sediment Geol 85(1–4):435–455
Cairncross B (2001) An overview of the Permian (Karoo) coal deposits of southern Africa. J Afr Earth Sci 33:529–562
Cairncross B, Cadle AB (1988) Palaeoenvironmental control on coal formation, distribution and quality in the Permian Vryheid Formation, East Witbank Coalfield, South Africa. Int J Coal Geol 9(4):343–370
Cairncross B, Hart RJ, Willis JP (1990) Geochemistry and sedimentology of coal seams from the Permian Witbank Coalfield, South Africa; a means of identification. Int J Coal Geol 16(4):309–325
Calder JH, Gibbing MR, Mukhopadhyay PK (1991) Peat formation in a Westphalian B piedmont setting, Cumberland Basin, Nova Scotia: implication for the maceral based interpretation of rheotrophic and raised paleomires. Bull Soc Geol Fr 162:283–298
Catuneanu O, Wopfner H, Eriksson PG, Cairncross B, Rubidge BS, Smith RMH, Hancox PJ (2005) The Karoo basins of south-Central Africa. J Afr Earth Sci 43(1–3):211–253
Chi Q, Yan M (2007) Handbook of elemental abundance for applied geochemistry. Geological Publishing House, Beijing, p 148
Chou CL (2012) Sulfur in coals: a review of geochemistry and origins. Int J Coal Geol 100:1–13
Condie KC (1993) Chemical composition and evolution of the upper continental crust: contrasting results from surface samples and shales. Chem Geol 104:1–37
Dai S, Ren D, Tang Y, Yue M, Hao L (2005) Concentration and distribution of elements in Late Permian coals from western Guizhou Province, China. Int J Coal Geol 61:119–137
Dai S, Graham IT, Ward CR (2016) A review of anomalous rare earth elements and yttrium in coal. Int J Coal Geol 159:82–95
Dai S, Xie P, Jia S, Ward CR, Hower HC, Yan X, French D (2017) Enrichment of U-Re-V-Cr-Se and rare earth elements in the Late Permian coals of the Moxinpo Coalfield, Chongqing, China: genetic implications from geochemical and mineralogical data. Ore Geol Rev 80:1–17
Dai S, Bechtel A, Eble CF, Flores RM, French D, Graham IT, Hood MM, Hower JC, Korasidis VA, Moore TA, Püttmann W, Wei Q, Zhao L, O’Keefe JMK (2020) Recognition of peat depositional environments in coal: a review. Int J Coal Geol 219:103383
Denge E, Baiyegunhi C (2021) Geochemical and Petrographical Characteristics of the Madzaringwe Formation Coal, Mudrocks and Sandstones in the Vele Colliery, Limpopo Province, South Africa: implications for Tectonic Setting Provenance and Paleoweathering. Appl Sci 11(6):2782
Dickinson WR, Beard LS, Brakenridge GR, Erjavec JL, Ferguson RC, Inman KF, Knepp RA, Lindberg FA, Ryberg PT (1983) Provenance of North American Phanerozoic sandstones in relation to tectonic setting. Geol Soc Am Bull 94:222–235
Diessel CFK (1992) Coal-bearing depositional systems. Springer, Berlin, p 721
Diessel CFK (1986) On the correlation between coal facies and depositional environments. In: Proc. 20th Newcastle Symp. The University of Newcastle, pp. 19–22
DiMichele WA, Phillips TL (1994) Palaeobotanical and palaeoecological constraints on models of peat formation in the Late Carboniferous of Euramerica. Palaeogeogr Palaeoclimatol Palaeoecol 106:39–90
Falcon RMS (1986) A brief review of the origin, formation, and distribution of coal in southern Africa. In: Anhaeusser CR, Maske S (eds) Mineral Deposits of Southern Africa, vol I. and II. Geological Society of South Africa, Johannesburg, pp 1879–1898
Falcon RMS (1989) Macro-and micro-factors affecting coal-seam quality and distribution in southern Africa with particular reference to the no. 2 seam Witbank coalfield, South Africa. Int J Coal Geol 12(1–4):681–731
Flores D (2002) Organic facies and depositional palaeoenvironment of lignites from Rio Maior Basin (Portugal). Int J Coal Geol 48:181–195
Glasspool I (2003a) Palaeoecology of selected south African export coals from the Vryheid Formation, with emphasis on the role of heterosporous lycopods and wildfire derived inertinite. Fuel 82:959–970
Glasspool IJ (2003b) Hypautochthonous–allochthonous coal deposition in the Permian, South African, Witbank Basin no. 2 seam; a combined approach using sedimentology, coal petrology and palaeontology. Int J Coal Geol 53:81–135
Gӧtz AE, Ruckwied K (2014) Palynological records of the early Permian postglacial climate amelioration (Karoo Basin, South Africa). Palaeobiodiver Palaeoenviron 94:229–235
Hagelskamp HHB, Snyman CP (1988) On the origin of low-reflecting inertinites in coals from the Highveld Coalfield, South Africa. Fuel 67:307–313
Hancox JP, Gotz PE (2014) South Africa’s coalfields — a 2014 perspective. Int J Coal Geol 132:170–254
Harnois L (1988) The CIW index: a new chemical index of weathering. Sed Geol 55(3):319–322
Hayashi KI, Fujisawa H, Holland HD, Ohmoto H (1997) Geochemistry of ~1.9 Ga sedimentary rocks from northeastern Labrador, Canada. Geochim Cosmochim Acta 61:4115–4137
He C, Ji L, Wu Y, Su A, Zhang M (2016) Characteristics of hydrothermal sedimentation process in the yanchang formation, South Ordos Basin, China: evidence from element geochemistry. Sediment Geol 345:33–41
Hower JC, O’Keefe JMK, Volk TJ, Watt MA (2010) Funginite–resinite associations in coal. Int J Coal Geol 83:64–72
Hower JC, O’Keefe JMK, Eble CF, Raymond A, Valentim B, Volk TJ, Richardson AR, Satterwhite AB, Hatch RS, Stucker JD, Watt MA (2011) Notes on the origin of inertinite macerals in coal: evidence for fungal and arthropod transformations of degraded macerals. Int J Coal Geol 86:231–240
Imchen W, Thong GT, Pongen T (2014) Provenance, tectonic setting and age of the sediments of the Upper Disang Formation in the Phek District, Nagaland. J Asia Earth Sci 88:11–27
International Committee for Coal and Organic Petrology (ICCP) (1998) The new vitrinite classification (ICCP System 1994). Fuel 77(5):349–358
International Committee for Coal and Organic Petrology (ICCP) (2001) The new inertinite classification (ICCP System 1994). Fuel 80:459–471
Jasper A, Guerra-Sommer M, Abu Hamad AMB, Bamford M, Cerruti Bernardes-de-Oliveira ME, Tewari R, Uhl D (2013) The burning of Gondwana: Permian fires on the southern continent – a palaeobotanical approach. Gondwana Res 24(1):148–160
Jiu B, Huang W, Hao R (2021) A method for judging depositional environment of coal reservoir based on coal facies parameters and rare earth element parameters. J Pet Sci Eng 207:109128
Kalkreuth WD, Leckie DA (1989) Sedimentological and petrographical characteristics of Cretaceous strandplain coals: a model for coal accumulation from the North American Western Interior Seaway. Int J Coal Geol 12:381–424
Krzeszowska E (2019) Geochemistry of the Lublin Formation from the Lublin Coal Basin: implications for weathering intensity, palaeoclimate and provenance. Int J Coal Geol 216:103306
Kusebauch C, Oelze M, Gleeson SA (2018) Partitioning of arsenic between hydrothermal fluid and pyrite during experimental siderite replacement. Chem Geol 500:136–147
Mahooana PE, Moroeng OM, Wagner NJ (2022) Petrology of the A and B Seams, Ermelo Coalfield (South Africa): Indications for changing palaeoenvironmental and sedimentary conditions. Int J Coal Geol 263:104135
Malaza N (2013) Basin Analysis of the Soutpansberg and Tuli Coalfields, Limpopo Province of South Africa. Unpublished PhD Thesis, University of Fort Hare, 270 p
Marchioni D, Kalkreuth W (1991) Coal facies interpretations based on lithotype and maceral variations in Lower Cretaceous (Gates Formation) coals of Western Canada. Int J Coal Geol 18:125–162
Matjie RH, Li Z, Ward CR, Kosasi J, Bunt JR, Strydom CA (2015) Mineralogy of furnace deposits produced by South African Coals during Pulverized-fuel combustion tests. Energy Fuel 29(12):8226–8238
McLennan SM (2001) Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem Geophys Geosyst. https://doi.org/10.1029/2000GC000109
McLennan SM, Hemming S, McDaniel DK, Hanson GN (1993) Geochemical approaches to sedimentation, provenance, and tectonics. Geol Soc Am 284:21–40
Mendhe VA, Kumar S, Kamble AD, Mishra S, Varma AK, Bannerjee M, Mishra VK, Sharma S, Buragohain J, Tiwari B (2018) Organo-mineralogical insights of shale gas reservoir of Ib-River Mand-Raigarh Basin, India. J Nat Gas Sci Eng 59:136–155
Moore TA, Shearer JC (2003) Peat/coal type and depositional environment are they related? Int J Coal Geol 56:233–252
Moroeng OM, Wagner NJ, Brand DJ, Roberts RJ (2018) A Nuclear magnetic resonance study: implications for coal formation in the Witbank Coalfield, South Africa. Int J Coal Geol 188:145–155
Moroeng OM, Mhuka V, Nindi MM, Roberts RJ, Wagner NJ (2019) Comparative study of a vitrinite-rich and an inertinite-rich Witbank coal (South Africa) using pyrolysis-gas chromatography. Intern J Coal Sci Technol 6:621–632
Mphaphuli M (2017) Petrographic consideration of the impact of the Tshipise Fault on coal quality in the Soutpansberg Coalfield, South Africa. Unpublished M.Sc. Dissertation, University of Johannesburg (South Africa)
Mukhopadhyay PK (1986) Petrography of selected Wilcox and Jackson group Lignites from the Tertiary of Texas. In: Finkelman, R.B., Casagrande, D.J. (Eds.), Geology of Gulf Coast Lignites. 1986 Annual Meeting of Geological Society of America. Field Trip, pp. 126–145
National Coal Strategy for South Africa (2018) Chamber of Mines of South Africa. Available at: https://www.mineralscouncil.org.za/component/jdownloads/send/25downloads/535-coal-strategy-2018
Nesbitt HW, Young GM (1982) Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 299:715–717
Nesbitt HW, Young GM (1984) Prediction of some weathering trends of plutonic and volcanic rocks based on thermodynamic and kinetic considerations. Geochim Cosmochim Acta 48:1523–1534
Nesbitt HW, Young GM, McLennan SM, Keays RR (1996) Effects of chemical weathering and sorting on the petrogenesis of siliciclastic sediments, with implications for provenance studies. J Geol 104:525–542
O’Keefe JMK, Hower JC (2011) Revisiting Coos Bay, Oregon: a re-examination of funginite-huminite relationships in Eocene subbituminous coals. Int J Coal Geol 85:34–42
Patterson SH (1971) Investigations of ferruginous bauxite and other mineral resources on Kauai and reconnaissance of ferruginous bauxite deposits on Maui. Hawaii U S Geol Surv Prof Pap 656:74
Petersen HI, Lindström S, Nytoft HP, Rosenberg P (2009) Composition, peatforming vegetation and kerogen paraffinicity of Cenozoic coals: relationship to variations in the petroleum generation potential (Hydrogen Index). Int J Coal Geol 78:119–134
Pickel W, Kus J, Flores D, Kalaitzidis S, Christanis K, Cardott BJ, MiszKennan M, Rodrigues S, Hentschel A, Hamor-Vido M, Crosdale P, Wagner N, ICCP (2017) Classification of liptinite – ICCP System 1994. Int J Coal Geol, 169:40–61
Pinetown KL, Ward CR, Van der Westhuizen WA (2007) Quantitative evaluation of minerals in coal deposits in the Witbank and Highveld Coalfields, and the potential impact on acid mine drainage. Int J Coal Geol 70(1–3):166–183
Pinheiro HJ (1999) A techno-economic and historical review of the South African coal industry in the 19th and 20th centuries and analyses of coal product samples of South African collieries 1998–1999. DME, SABS and CSIR Bull 113:78–81
Roser BP, Korsch RJ (1988) Provenance signatures of sandstone-mudstone suites determined using discriminant function analysis of major-element data. Chem Geol 67:119–139
Samad SK, Mishra DK, Mathews RP, Ghosh S, Mendhe VA, Varma AK (2020) Geochemical attributes for source rock and palaeoclimatic reconstruction of the Auranga Basin, India. J Petrol Sci Eng 185:106665
Scott AC (2002a) Coal petrology and the origin of coal macerals: a way ahead? Int J Coal Geol 50:119–134
Scott AC (2002b) Hydrogeologic factors affecting gas content distribution in coal beds. Int J Coal Geol 50:363–387
Sebola MJT, Drennan GR, Wagner NJ (2022) Petrographic and geochemical characteristics of beneficiated metallurgical coal from the No 6 Seam, Tshipise sub-basin, Soutpansberg coalfield, South Africa. J South Afr Inst Min Metall 122(8):461–472
Sen S, Banerjee S (2015) Identifying relationship amongst vitrinite/inertinite ratio (V/I), cleat parameters, vitrinite reflectance, O/C ratio and permeability of coal seams and V/I ratio as exploration tool: study from Raniganj coal bed methane block, Essar Oil Limited, India. Petroleum geosciences: indian contexts. Springer, Cham, pp 205–217
Sen S, Naskar S, Das S (2016) Discussion on the concepts in paleoenvironmental reconstruction from coal macerals and petrographic indices. Mar Pet Geol 73:371–391
Silva MB, Kalkreuth W (2005) Petrological and geochemical characterization of Candiota coal seams, Brazil — implication for coal facies interpretations and coal rank. Int J Coal Geol 64:217–238
Silva MB, Kalkreuth W, Holz M (2008) Coal petrology of coal seams from the Leão-Butiá Coalfield, Lower Permian of the Paraná Basin, Brazil—implications for coal facies interpretations. Int J Coal Geol 73(3–4):331–358
Singh MP, Singh PK (1996) Petrographic characterization and evolution of the Permian coal deposits of the Rajmahal basin, Bihar, India. Int J Coal Geol 29:93–118
South African National Standard (SANS) (1928) (2009) Solid Mineral Fuels – Determination of Gross Calorific value by the Bomb Calorific Method, and Calculation of Net Calorific Value. ISO 1928:2009
South African National Standard (SANS) 334 (2020) (Ed. 2.00). Solid Mineral Fuels - Determination of Total Sulfur - Eschka Method. ISO 334: 2013
South African National Standards (SANS) 7404-2 (2015) Methods for the petrographic analysis of coals Part 2: Methods of preparing coal samples. (ISO 7404-2:2009)
South African National Standards (SANS) 7404–3 (2016) Methods for the petrographic analysis of coals - Part 3: Method of determining maceral group. (ISO 7404–3:2009)
South African National Standards (SANS) 7404–5 (2016) Methods for the petrographic analysis of coals - Part 5: Method of determining microscopically the reflectance of vitrinite. (ISO 7404–5:2009)
Sparrow J (2012) The Soutpansberg Coalfield “The Forgotten Basin”. Presentation at the Inaugural FFF Limpopo Conference, October 2012
Stach E, Mackowsky M-Th, Teichmuller M, Taylor GH, Chandra D, Teichmuller R (1975) Stach’s Textbook of Coal Petrology. Gebruder Borntraeger (Berlin-Stuttgart)
Stach E, Mackowsky M-Th, Teichmiiller M, Tayler GH, Chandra D, Teichmiiller R (1982) Stach's Textbook of Coal Petrology. Borntraeger, Berlin, 3rd ed., 535 pp
Steyn JGD, Smith WH (1977) Coal petrography in the evaluation of South African coals. Coal Gold Base Miner 25(9):107–117
Taylor GH, Teichmüller M, Davis A, Diessel CFK, Littke R, Robert P (1998) Organic Petrology. Gebrüder Borntraeger, Berlin, p 704
Teichmüller M (1989) The genesis of coal from the viewpoint of coal petrology. Int J Coal Geol 12:1–87
Teichmüller M, Teichmüller R (1982) Fundamental of Coal Petrology. In: Stach E, Mackowsky MTH, Teichmüller M, Taylor GH, Chandra D, Teichmüller R (eds) Stach’s Textbook of Coal Petrology, 3rd edn. Gebrüder Borntraeger, Berlin, p 5353
Telfer CA, Njowa G (2012) Independent geologist specialist report on the principal south african operating and non-operating mineral assets of coal of africa limited. Unpublished Report by Venmyn Deloitte for Coal of Africa Limited, 346 p
Thompson S, Morley RJ, Barnard PC, Cooper BS (1985) Facies recognition of some Tertiary coals applied to prediction of oil source rock occurrence. Mar Pet Geol 2:288–297
Wagner M, Wachowiak J, Kowalczyk J, Natkaniec-Nowak L, Heflik W, Georges C (2017) Petrographic and mineralogical studies of fossil charcoal from Sierra de Bahoruco (Barahona Province, Dominican Republic). Int J Coal Geol 173:142–149
Wagner N, Eble C, Hower J, Falcon R (2019) Petrology and palynology of select coal samples from the Permian Waterberg Coalfield, South Africa. Int J Coal Geol 204:85–101
Wagner N, Malumbazo N, Falcon R (2018) Southern African Coals and Carbons: Definitions and Applications of Organic Petrology. Struik Nature, Cape Town, 248 p
Ward CR (2002) Analysis and significance of mineral matter in coal seams. Int J Coal Geol 50:135–168
Ward CR (2016) Analysis, origin and significance of mineral matter in coal: an updated review. Int J Coal Geol 165:1–27
Wheeler A, Gӧtz AE (2017) Palynofacies as a tool for high-resolution palaeoenvironmental and palaeoclimatic reconstruction of Gondwanan post-glacial coal deposits: no. 2 coal Seam, Witbank Coalfield (South Africa). Palaeobiodiver Palaeoenviron 97:259–271
Widodo S, Oschmann W, Bechtel A, Sachsenhofer RF, Anggayana K, Puettmann W (2010) Distribution of sulfur and pyrite in coal seams from Kutai Basin (East Kalimantan, Indonesia): implications for paleoenvironmental conditions. Int J Coal Geol 91:151–162
Xu FM, Fang AM (2005) The coal facies study of Coal Seam 16 in Yanzhou coal mine. Coal Geol Explor 33:10–15
Yuan W, Liu G, Zhou X, Bulseco A (2022) Linking hydrothermal activity with organic matter accumulation in the chang 7 black Shale of Yanchang Formation, Ordos Basin, China. Frontiers in Earth Science 10:786634
Zhao L, Dai S, Graham IT, Li X, Liu H, Song X, Hower JC, Zhou Y (2017) Cryptic sediment-hosted critical element mineralization from eastern Yunnan Province, southwestern China: mineralogy, geochemistry, relationship to Emeishan alkaline magmatism and possible origin. Ore Geol Rev 80:116–140
Acknowledgements
Sanki Biswas expresses gratitude for the financial assistance granted by the Paleoproterozoic Mineralization Research Group (PPM) within the Department of Geology at the University of Johannesburg in South Africa. Some portions of this study are also integral parts of Maseda Mphaphuli's M.Sc. dissertation. Additionally, we extend our appreciation to CoAL for providing samples. Editor-in-chief, Professor Shimin Liu, and two anonymous reviewers are gratefully acknowledged for providing comments that assisted in improving the quality of the manuscript.
Author information
Authors and Affiliations
Contributions
SB, NJW, and OMM conceived the initial idea. SB analyzed the samples and lab work. All authors contributed to the manuscript writing—review, and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
All authors agree with the content and have provided consent to publish the manuscript.
Competing interests
The authors declare no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Biswas, S., Wagner, N.J. & Moroeng, O.M. Organic petrographic and mineralogical composition of the No. 6 coal seam of the Soutpansberg Coalfield, South Africa: Insights into paleovegetation and depositional environment. Int J Coal Sci Technol 11, 41 (2024). https://doi.org/10.1007/s40789-024-00698-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-024-00698-6