Abstract
The present study aims to investigate the physico-chemical structural evolution characteristics of char structure of CO2 atmosphere torrefaction pretreated sludge with Yangchangwan bituminous coal (YC) during co-gasification. The co-gasification reactivity of torrefied sludge and YC was measured using a thermogravimetric analyzer. The co-gasification reactivity of torrefied sludge with YC was thoroughly explored in depth by in situ heating stage microscope coupled with traditional characterization means of char sample (Scanning electron microscope, nitrogen adsorption analyzer, laser Raman spectroscopy). The results show that the gasification reaction rate of sludge treated under CO2 atmosphere and coal blended char was better than other char samples at 1100–1200 °C. The torrefied sludge under CO2 atmosphere promoted its thermal decomposition to the maximum extent, so that it eventually was transformed into a large number of small broken particles. The specific surface area and ID1/IG ratio of blended char of torrefied sludge under CO2 atmosphere and YC were 1.70 and 1.07 times higher than that of YC, respectively. The in situ technique revealed that YC char with the addition of torrefied sludge undergo gasification by shrinking core modes and the presence of obvious ash melting flow phenomenon. It was more obvious than that of YC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the accelerated industrialization of cities, the growth of population and the increasing improvement of people’s living standards, the production of industrial sewage sludge has increased dramatically, e.g. the sludge of effluent system of indirect coal-liquefaction plant. The sludge is a non-homogeneous biomass with high nitrogen and sulfur content, which can form large amounts of NOx and SOx pollutants during thermochemical processes, and these pollutants can further lead to acid rain, photochemical smog, and greenhouse effect (Chen et al. 2011; Liu et al. 2014). Therefore, how to effectively dispose of industrial sludge in a harmless and resourceful way has become a long-term concern for society and one of the important challenges for the sustainable development of urbanization (Kamali et al. 2019). At present, the main disposal methods of industrial sludge are landfill, open dumping, composting or direct incineration. These methods have low utilization efficiency and are prone to cause environmental pollution and ecological damage. If the industrial sludge is applied to entrained flow gasification, it will be useful to find an effective treatment method for the environmental problem of the industrial sludge pollution. Currently, there are many literatures on the study of co-gasification of added herbaceous biomass with coal (Salah et al. 2016, Hernandez et al. 2011, Hernandez et al. 2013, Ding et al. 2017), while there are a few reports on the addition of non-lignocellulosic biomass industrial sludge to coal for gasification and thus resource utilization and harmless treatment. Therefore, the research on this aspect will be very attractive. In view of some shortcomings of high water content and low calorific value energy density of the industrial sludge, it is necessary to pretreat it before adding the industrial sludge to coal co-gasification (Ding et al. 2014; He et al. 2019). The torrefaction pretreatment is an important pretreatment method for the upgrading of biomass solid fuels (Adekunle et al. 2021; Uemura et al. 2011; Atienza-Martínez et al. 2015). The torrefaction (also called mild pyrolysis) is a thermal treatment under conditions of atmospheric pressure, different atmospheres and low temperatures (Zheng et al. 2012; Zhang et al. 2016; Kosov et al. 2014).
Nowadays, the torrefaction process of biomass under inert atmosphere has been the focus of research (Atienza-Martínez et al. 2015; Li et al. 2015; Chen et al. 2015), while there are a few studies on torrefaction pretreatment using CO2 as the atmosphere, especially on the torrefaction pretreatment characteristics of industrial sludge. The temperature and time of pretreatment of torrefaction affects the properties of biomass (Isemin et al. 2019; Poudel et al. 2015; Zheng et al. 2020, van et al. 2011, Eseltine et al. 2013). Zhang et al. (2022b) found that the micromorphological development of petrochemical sludge pyrolysis residues with different degrees of pyrolysis went through three main stages, i.e., the appearance of macropore and fissures (500–600 °C), pore development (700–800 °C), and pore blocking (900 °C). The evolution of the solid structure of sludge and coal during co-gasification affects the reactivity. Wei et al. (2018) found that the addition of biomass ash to coal was beneficial in reducing the degree of graphitization of the coal char carbon structure, thus increasing the gasification reactivity. Li et al. (2020) analyzed the structure and reactivity of blended coal and biomass char and found that as the proportion of biomass increased from 0 to 80%, the concentration of reactive K and Mg in the coal char gradually increased and the carbon structure became more disordered. Qin et al. (2017) explored the effect of adding biomass on the carbon structure of coal char during co-gasification and showed that the carbon structure of chars generated by co-gasification of coal and biomass was less ordered than that of coal char. Diao et al. (2021) showed that co-gasification of coal and biomass at high blending ratios can promote the formation of amorphous carbon, which accelerates the co-gasification reaction. Wu et al. (2019) investigated the effect of changes in the structure of co-pyrolysis char on its reactivity and found that the number of pores in coal char increased significantly in the range of 2–7 nm after the addition of biomass. Wang et al. (2020) found that biomass char and coal char follow different reaction pathways in the gasification process due to their different physicochemical properties, and this leads to a different evolution of the chemical structure of the char. Lv et al. (2021) investigated the in situ surface structural characteristics of char particles during co-gasification of biomass and fine residue and clearly found that when the gasification time was 20 min, the area shrinkage ratio of fine residue was only 14.8%, while the area shrinkage ratio of blended char reached 61.7%, further indicating that the biomass increased the gasification rate of fine residue.
In summary, it can be seen that the solid structure evolution during high temperature co-gasification of biomass and coal affects the gasification reactivity, but the characterization of solid structure during gasification is usually done in the off-line state of the reaction, i.e., after the thermal reaction is completed, the samples are cooled to ambient temperature are collected to determine the solid structure parameters. There are a few studies on the real-time changes of solid structure during high-temperature gasification. The high temperature stage is a reaction device capable of providing a high-temperature environment, precise temperature control, and reaction atmosphere. Its combination with optical microscope system enables in situ studies of solid structure evolution characteristics and gasification reaction forms during high-temperature thermochemical transformation of samples.
Therefore, based on the above research background, this study first prepared torrefied sludge char using a horizontal tubular furnace reactor. The co-gasification char samples were prepared by sludge blending with coal in the ratio of 1:1. Then, the raw char and blended char were analyzed by high-temperature gasification experiments using a thermogravimetric analyzer. Finally, the structural properties of the char samples at different temperatures were characterized by combining scanning electron microscope (SEM), nitrogen adsorption analyzer and laser Raman spectrometer. Further, based on the off-line characterization methods, the in situ morphology change of individual char and blended fast pyrolysis char during gasification was studied by heating stage microscope to correlate the interpretation of the gasification reactivity.
2 Materials and methods
2.1 Raw materials
The sludge (abbreviated as SL) from the wastewater system of the polyformaldehyde plant of CHN Energy Ningxia Coal Industry Co., Ltd. and Yangchangwan bituminous coal (abbreviated as YC) were selected as the research objects of this study. The sludge was dried in an oven at 80 °C for 3 d. The SL and YC were crushed and sieved with a particle size of 80–120 μm. The dried SL and YC were ground in a 1:1 ratio with a mortar and pestle. The processed and collected samples were dried in an oven at 105 °C for 12 h. The results of proximate, ultimate and ash composition analysis of the samples are shown in Tables 1 and 2.
2.2 Preparation of torrefied char
The optimal torrefaction temperature and time of SL were determined using a thermogravimetric analyzer. The temperature range is 250–400 °C with 50 °C intervals and the heating rate is 10 °C/min. The optimal torrefaction temperature and time of SL were found in our previous research results (Zhang et al. 2022a). The torrefaction gas media are Ar (as reference) and CO2, respectively. The flow rate was all 50 mL/min. The torrefied SL samples were denoted as SLT, gas medium. The different samples of SLT, gas medium were prepared by the horizontal tube furnace reactor. A certain amount of SL sample was weighed for each experiment and placed into two ceramic boats on a flat surface. Before the start of the experiment, the 100 mL/min of different torrefaction gas medium was introduced to put the reactor interior in the anaerobic environment. The temperature was then increased at a rate of 10 °C/min to a predetermined optimum torrefaction temperature and time to prepare SLT, gas medium samples in different torrefaction gas media.
2.3 Preparation of fast pyrolysis char
The different fast pyrolysis chars were prepared by weighing samples with a mass of approximately 2.0 g for 5 min in the self-designed high-frequency furnace reactor at 800 °C at a N2 flow rate of 100 mL/min. The different char samples prepared were labeled as follows: YC char was abbreviated as YCP, YC and SLT, gas medium blended char sample was abbreviated as YCP-SLT, gas mediumP, and YC and SL mixed char sample was abbreviated as YCP-SLP.
2.4 High-temperature gasification reactivity experiments
The high temperature isothermal gasification reactivity of individual pyrolysis char and blended pyrolysis chars under CO2 atmosphere was measured by STA449-F3 thermogravimetric analyzer produced by NETZSCH, Germany. About 10.0 mg of pyrolysis char was heated to the target gasification temperatures of 1100 °C, 1200 °C, 1300 and 1400 °C at a heating rate of 20 °C/min under high purity Ar (50 mL/min), and then cut into 120 mL/min of the gasification agent CO2 for high temperature isothermal gasification experiments. The time of the high-temperature isothermal gasification in the CO2 atmosphere is 20 min.
2.5 Char sample characterization methods
2.5.1 Physical and chemical structure characterization
The microscopic morphology of individual fast pyrolysis char and blended fast pyrolysis char were observed by scanning electron microscope (SEM). The surface properties of different pyrolysis chars were observed at a magnification of 2000x. The char samples were analyzed by BET and BJH using a JW-BK100C-01 nitrogen adsorption analyzer. The char samples were degassed at 300 °C for 6 h to remove the adsorbed water and impurities from the samples. The adsorption isotherms were obtained at a constant temperature of −196 °C using N2 as the adsorption medium.
The chemical structures of different char samples were characterized using a laser Raman spectrometer (Thermo Fisher, DXR) with a laser power of 2 mW, a wavelength of 532 nm and a wave number of 800–2000 cm− 1. The Raman data were fitted to the split peaks based on Wire 3.4 software.
2.5.2 In situ micromorphological characterization
The in situ microscopic morphological changes of individual fast pyrolysis char and blended fast pyrolysis char during high temperature gasification were studied by in situ heating stage microscope. The study of the gasification characteristics of coal char was based on LINKAMTS 1500 heating stage microscope, UK. The instrument components are mainly composed of high temperature heating stage, microscope (DM4500PLED), camera (Leica, Germany), circulating water system, gas supply system, temperature controller and computer. The schematic diagram of the instrument structure is shown in Fig. 1 (Zhang et al. 2022c).
The specific experimental procedure was as follows: firstly, the sapphire substrate was placed in a fixed ceramic crucible. Then, the 0.2 mg of coal char was placed on the sapphire substrate and dispersed uniformly. The ceramic cover piece was placed on the top of the reaction tank to avoid the interference of red light on the image signal at high temperature. The temperature was increased to 1100 and 1400 °C at a heating rate of 50 °C/min under a purge of high-purity N2 at 80 mL/min and constant for 1 min to ensure a constant temperature. After that, the N2 is cut off and the CO2 gasifier of 60 mL/min is introduced to start the isothermal gasification of coal char. And the change of coal char surface morphology was recorded in real time by the camera on top of the instrument.
3 Results and discussion
3.1 Physical structure of torrefied SL under different atmospheres
The surface micromorphology of SL and SL pretreated by torrefaction under Ar and CO2 atmosphere is given in Fig. 2. As can be seen from Fig. 2, the SL has a smooth surface structure and more complete particles. The surface structure of the char samples pretreated by torrefaction SL under Ar and CO2 atmosphere is rough, with the appearance of broken particles. And the surface structure of SL pretreated by torrefaction under CO2 atmosphere is rougher and more broken particles than under Ar atmosphere. This phenomenon indicates that torrefied pretreatment of SL under CO2 atmosphere can make some small molecule gas substances adsorbed on the surface of SL can be released better, so that the surface structure of SL is rougher and there are many broken small particles. Li et al. (2017) found a similar phenomenon when studying the structural changes of Populus tomentosa during CO2 torrefaction.
3.2 High temperature isothermal CO2 gasification rate analysis of samples
Figure 3 shows the variation of carbon conversion level with gasification rate (dx/dt) for different fast pyrolysis chars. As seen from Fig. 3, the gasification rate of fast pyrolysis char at different temperatures was increased sharply at the beginning of CO2 gasification reaction, and the gasification rate of coal char at the same carbon conversion level was increased by adding SL and SL pretreated under different atmospheres (Ar and CO2) compared with that of coal char without SL addition. At 1100–1200 °C, the gasification rate of YC-SL300°C,CO2P was better than that of other char samples. The gasification reaction rates of different fast pyrolysis chars were lower at 1300–1400 °C compared to 1100 °C. And the gasification reaction rate of blended chars was overlapped. This may be due to the fact that with the increase of reaction temperature, the melting of ash changed the surface activity of coal char, and the coal char-CO2 gasification reaction was controlled by the diffusion of the ash layer at high temperature ( Zhang et al. 2022c).
3.3 Physical and chemical properties of the char during co-gasification
The specific surface areas of coal char and blended chars are listed in Table 3. As seen from Table 3, the specific surface area values of YC-SL300°C,ArP and YC-SL300°C,CO2P were larger than those of YCP and YC-SLP. The specific surface area of YC-SL300°C,ArP was a little larger than that of YCP and YC-SLP, while YC-SL300℃,CO2P was 1.70 and 1.60 times higher than that of YCP and YC-SLP, respectively. This indicates that YC-SL300°C,CO2P could significantly increase the specific surface area of coal char. This is also consistent with the results of its gasification reactivity.
Figure 4 shows the nitrogen adsorption/desorption isotherms and pore size distribution profiles of different fast pyrolysis char samples. The adsorption/desorption isotherms can provide richer information on pore structure and are generally used to evaluate pore types. From the shape of the nitrogen adsorption/desorption isotherm in Fig. 4a, it can be seen that the gas adsorption grows rapidly in the low relative pressure region, which is due to the enhanced potential energy effect between the adsorbed molecules in the micropores and the surface. It will have a strong ability to capture the adsorbed molecules at low relative pressure. After that, the adsorption capacity is increased continuously with increasing relative pressure, which can be attributed to the multilayer adsorption in the pore structure. In addition, the nitrogen desorption volume was found to be significantly larger than the adsorption volume, and an adsorption hysteresis loop was also observed in the intermediate stage, indicating a system of capillary coalescence in porous adsorption. The analysis of the nitrogen adsorption/desorption isotherms in Fig. 4a shows that the different fast pyrolysis chars has a continuous and complete pore structure. The results of their pore size distribution are shown in Fig. 4b. As shown in Fig. 4b, the pore structure of different fast pyrolytic char was mainly composed of micropores less than 2 nm and mesopores of 2–10 nm. And the mesopore numbers were varied significantly. The YC-SL300°C,CO2P had more micropores than YCP, YC-SLP and YC-SL300°C,ArP. This may be due to the melting and sintering of the holes during the gasification (Zhai et al. 2012).
By fitting the raw Raman spectra to the peaks, it is possible to quantitatively characterize the changes in the different components of the char sample (such as graphite lattice, disordered graphite surface structure, and amorphous carbon) and thus to infer the changes in the chemical structure of the char sample during gasification. The area ratios of some peaks can be used to quantitatively describe the changes in the carbon microcrystalline structure of the char sample during gasification. For example, the D1 peak is generally referred to as the defect peak and represents the defective structure of the carbon microcrystal. The G peak, also called graphite peak, mainly represents the stretching vibration of an ideal graphite lattice. The ratio between D1 and G peaks (ID1/IG) is often used as a quantitative characterization of the degree of ordering of the coal char carbon microcrystalline structure, i.e., the relative number of ordered aromatic rings (mainly large aromatic rings). The higher the ID1/IG ratio, the lower the degree of carbon structure ordering and the higher the gasification reaction rate. Figure 5 shows the ID1/IG Raman parameters for different fast pyrolysis chars. As seen from Fig. 5, the ID1/IG ratios of YC-SLP, YC-SL300°C,ArP, and YC-SL300°C,CO2P were higher than those of YCP, and the ID1/IG ratio of YC-SL300°C,CO2P was greater than that of YC-SL300°C,ArP. The ID1/IG ratio of YC-SL300°C,CO2P was 1.07 times higher than that of YC. This indicates that SL pretreated with CO2 atmosphere can promote the cracking of carbon crystals under high temperature gasification and form small-sized carbon microcrystal structures, resulting in the least ordered carbon microcrystal structure of YC-SL300°C,CO2P.
3.4 In situ microscopic morphological changes of fast pyrolysis char
The in situ heating stage microscope was used to observe the microscopic morphology of different fast pyrolysis char samples gasified at 1100 °C for 20 min. The observation results are shown in Fig. 6. As can be seen from Fig. 6c, the fast pyrolysis blended char samples of YC and SL pretreated by the torrefaction method are involved in high temperature gasification by a different reaction form than YCP, i.e., the presence of gasification reaction in the shrinking core modes on the surface of YC-SL300°C,CO2P (as shown in the red area in the figure). This is similar to the participation of a few particles of Meihuajing coal char and Yangchangwan coal char in the shrinking core modes in the gasification reaction at 1200 °C (Zhang et al. 2020). This may be related to the change in the structural properties of the char on the surface when the pretreated SL is co-gasified with YC, and the gasification reaction rate was greater than the diffusion rate of the reaction gas in the nucleus.
Figure 7 shows the in situ microscopic morphology of different fast pyrolysis char samples gasified at 1400 °C for 10 min. As can be seen in Fig. 7, the different fast pyrolysis char samples were gasified at 1400 °C for 10 min, the carbonaceous components were consumed and the residual ash underwent melt flow and agglomeration. This is mainly attributed to the enhanced melt flow properties of coal ash by the active alkali and alkaline earth metal components in the pretreated SL, which explains the non-significant difference in the gasification reactivity of coal char at higher temperatures.
4 Conclusions
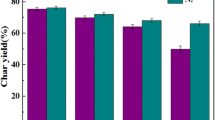
At 1100–1200 °C, the gasification reaction rate of sludge treated under CO2 atmosphere and coal char mixed char was better than other char samples. The addition of CO2 atmosphere torrefaction pretreated sludge to YC can increase the specific surface area of coal char. Its specific surface area was 1.70 higher than that of YC. And the sludge pretreatment under CO2 atmosphere can promote the cracking of carbon crystals of mixed char under gasification and form small size carbon microcrystal structure, thus enhancing the gasification reactivity of coal char. Its ID1/IG was 1.07 times higher than that of YC. The analysis of morphological evolution of coal char high-temperature gasification based on the in situ heating stage microscope, which revealed that the form of co-gasification reaction of torrefied sludge under CO2 atmosphere with YC follows the phenomenon of the shrinking core modes. At 1400 °C, the inorganic ash from different fast pyrolysis chars undergoes melt flow and agglomerates into balls. Comprehensive blended char co-gasification reactivity, physico-chemical structure characteristics, etc. are suitable for high-temperature co-gasification using CO2 atmosphere torrefaction sludge blended with YC to realize resource utilization of coal chemical sludge.
References
Adekunle Adeleke J, Odusote P, Ikubanni O, Lasode M, Malathi D Pasawan (2021) Physical and mechanical characteristics of composite briquette from coal and pretreated wood fines. Int J Coal Sci Technol 8(5):1088–1098
Atienza-Martínez M, Fonts I, Lázaro L, Ceamanos J, Gea G (2015) Fast pyrolysis of torrefied sewage sludge in a fluidized bed reactor. Chem Eng J 259:467–480
Chen H, Namioka T, Yoshikawa K (2011) Characteristics of tar, NOx precursors and their absorption performance with different scrubbing solvents during the pyrolysis of sewage sludge. Appl Energy 88:5032–5041
Chen W, Peng J, Bi X (2015) A state-of-the-art review of biomass torrefaction, densification and applications. Renew Sustainable Energy Rev 44:847–866
Diao R, Li S, Deng J, Zhu X (2021) Interaction and kinetic analysis of co-gasification of bituminous coal with walnut shell under CO2 atmosphere: Effect of inorganics and carbon structures. Renew Energ 173:177–187
Ding L, Zhang Y, Wang Z, Huang J, Fang Y (2014) Interaction and its induced inhibiting or synergistic effects during co-gasification of coal char and biomass char. Bioresour Technol 173:11–20
Ding L, Gong Y, Wang Y, Wang F, Yu G (2017) Characterisation of the morphological changes and interactions in char, slag and ash during CO2 gasification of rice straw and lignite. Appl Energy 195:713–724
Eseltine D, Thanapal SS, Annamalai K, Ranjan D (2013) Torrefaction of woody biomass (Juniper and Mesquite) using inert and non-inert gases. Fuel 113:379–388
He Q, Guo Q, Ding L, Wei J, Yu G (2019) Rapid co-pyrolysis of lignite and biomass blends: analysis of synergy and gasification reactivity of residue char. J Anal Appl Pyrolysis 143:1–8
Hernandez AB, Ferrasse J-H, Chaurand P, Saveyn H, Borschneck D, Roche N (2011) Mineralogy and leachability of gasified sewage sludge solid residues. J Hazard Mater 191:219–227
Hernandez AB, Ferrasse J-H, Roche N (2013) Limiting the pollutant content in the sewage sludge producer gas through staged gasification. Chem Eng Technol 36:1985–1996
Isemin R, Klimov D, Larina O, Sytchev G, Zaichenko V, Milovanov O (2019) Application of torrefaction for recycling bio-waste formed during anaerobic digestion. Fuel 243:230–239
Kamali M, Costa ME, Aminabhavi TM, Capela I (2019) Sustainability of treatment technologies for industrial biowastes effluents. Chem Eng J 370:1511–1521
Kosov V, Sinelshchikov V, Sytchev G, Zaichenko V (2014) Effect of torrefaction on properties of solid granulated fuel of different biomass types. High Temp 52:907–912
Li M, Li X, Bian J, Xu J, Yang S, Sun R (2015) Influence of temperature on bamboo torrefaction under carbon dioxide atmosphere. Ind Crops Prod 76:149–157
Li S, Zou J, Li M, Wu X, Bian J, Xue Z (2017) Structural and thermal properties of Populus tomentosa during carbon dioxide torrefaction. Energy 124:321–329
Li X, Zhang H, Liu M, Zhi L, Bai J, Bai Z, Li W (2020) Investigation of coal-biomass interaction during co-pyrolysis by char separation and its effect on coal char structure and gasification reactivity with CO2. J Fuel Chem Technol 48(8):897–907
Liu H, Zhang Q, Hu H, Xiao R, Li A, Qiao Y, Yao H, Naruse I (2014) Dual role of conditioner CaO in product distributions and sulfur transformation during sewage sludge pyrolysis. Fuel 134:514–520
Lv P, Bai Y, Wang J, Song X, Su W, Yu G (2021) Investigation into the catalytic gasification of coal gasification fine slag residual carbon by the leachate of biomass waste: gasification reactivity, structural evolution and kinetics analysis. J Environ Chem Eng 9(6):1–9
Poudel J, Ohm TI, Lee SH, Oh SC (2015) A study on torrefaction of sewage sludge to enhance solid fuel qualities. Waste Manag 40:112–118
Qin Y, Han Q, Zhao Z, Du Z, Feng J, Li W, Vassilev S, Vassileva C (2017) Impact of biomass addition on organic structure and mineral matter of char during coal-biomass co-gasification under CO2 atmosphere. Fuel 202:556–562
Salah Akkache A-B, Hernández G, Teixeira F, Gelix N, Roche, Ferrasse Jean Henry (2016) Co-gasification of wastewater sludge and different feedstock: feasibility study. Biomass Bioenergy 89:201–209
Uemura Y, Omar WN, Tsutsui T, Yusup SB (2011) Torrefaction of oil palm wastes. Fuel 90:2585–2591
van der Stelt MJC, Gerhauser H, Kiel JHA, Ptasinski KJ (2011) Biomass upgrading by torrefaction for the production of biofuels: a review. Biomass Bioenerg 35:3748–3762
Wang M, Shen Y, Guo P, Kong J, Wu Y, Chang L, Wang J, Xie W (2020) A comparative study on the intrinsic reactivity and structural evolution during gasification of chars from biomass and different rank coals. J Anal Appl Pyrolysis 149:1–8
Wei J, Gong Y, Ding L, Yu J, Yu G (2018) Influence of biomass ash additive on reactivity characteristics and structure evolution of coal char-CO2 gasification. Energy Fuels 32(10):10428–10436
Wu Z, Ma C, Jiang Z, Luo Z (2019) Structure evolution and gasification characteristic analysis on co-pyrolysis char from lignocellulosic biomass and two ranks of coal: Effect of wheat straw. Fuel 239:180–190
Zhai Y, Peng W, Zeng G, Fu Z, Lan Y, Chen H, Wang C, Fan X (2012) Pyrolysis characteristics and kinetics of sewage sludge for different sizes and heating rates. J Therm Anal Calorim 107(3):1015–1022
Zhang S, Hu B, Zhang L, Xiong Y (2016) Effects of torrefaction on yield and quality of pyrolysis char and its application on preparation of activated carbon. J Anal Appl Pyrolysis 119:217–223
Zhang X, Bai Y, Wei J, Song X, Wang J, Yao M, Yu G (2020) Study on char-ash-slag-liquid transition and its effect on char reactivity. Energy Fuels 34(3):3941–3951
Zhang X, Ma M, Bai Y, Su W, Song X, Lv P, Wang J, Yu G (2022a) Torrefaction of sludge under CO2 atmosphere to improve the fuel properties for high temperature gasification with coal. Thermochim Acta 713:1–11
Zhang W, He Y, Wang Y, Li G, Chen J, Zhu Y (2022b) Comprehensive investigation on the gasification reactivity of pyrolysis residue derived from Ca-rich petrochemical sludge: roles of microstructure characteristics and calcium evolution. Energy Convers Manage 253:1–11
Zhang X, Ma M, Bai Y, Song X, Wang J, Lv P, Yu G (2022c) Study on high temperature gasification kinetics of coal char by TGA and in situ heating stage microscope. J Therm Anal Calorim 147:8997–9008
Zheng A, Zhao Z, Chang S, Huang Z, He F, Li H (2012) Effect of torrefaction temperature on product distribution from two-staged pyrolysis of biomass. Energy Fuels 26:2968–2974
Zheng A, Li L, Tippayawong N, Huang Z, Zhao K, Wei G, Zhao Z, Li H (2020) Reducing emission of NOx and SOx precursors while enhancing char production from pyrolysis of sewage sludge by torrefaction pretreatment. Energy 192:1–10
Acknowledgements
The present work was supported by the Scientific Research Fund Project of Yunnan Provincial Department of Education (2022J0756) and the National Natural Science Foundation of China (32260321, 21968024).
Author information
Authors and Affiliations
Contributions
Xinsha Zhang: Investigation, Conceptualization, Methodology, Data curation, Validation, Writing - original draft. Yonghui Bai: Supervision, Visualization. Jie Qin: Supervision, Writing - review & editing. Shengli Shi: Supervision, Visualization. Jiazhong Liu: Methodology, Validation, Writing - review & editing. Shuaibing Wang: Investigation, Resources. Minhui Zhao: Supervision, Writing - review & editing. Guiming Shi: Methodology, Validation, Writing - review & editing. Changbing Ye: Supervision, Visualization. Guangsuo Yu: Conceptualization, Writing - review & editing, Resources, Supervision, Project administration.
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., Bai, Y., Qin, J. et al. Structural characterization of char during co-gasification from torrefied sludge and Yangchangwan bituminous coal. Int J Coal Sci Technol 10, 56 (2023). https://doi.org/10.1007/s40789-023-00638-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-023-00638-w