Abstract
In a single sample plot, the total amount of heavy metals in the soil could not necessarily reflect the contents of their effective states. This must be considered when attempting to determine the degree of soil heavy-metal pollution in an area. In the present study, the soil around the molybdenum mining area in Huludao, China, was surveyed and sampled to evaluate soil heavy-metal pollution using the Nemerow multifactor pollution index method. The Tessier continuous extraction method was used to analyze the distribution of heavy-metal forms’ and their content changes in the soil of this area. Thus, the bioactivity of heavy metals in the soil, the absorption of heavy metals by plants, and the distribution of heavy metals in plants were explored to provide data supporting the use of phytoremediation technology to treat the heavy-metal pollution in the molybdenum mining area and develop ecological restoration strategies for the area’s wastelands. The pollution index results indicate that heavy-metal pollution in the soil around the tailings pond is severe, mainly due to Pb and Zn heavy metals. Heavy-metal pollution in the surrounding land is mainly due to Cd and Zn. Content analysis of the heavy-metal forms/states in soils shows that exchangeable forms, which are most effective and toxic to plants, of the following metals are highest in the following areas: Cd, Cu, and Zn in the mountains around the stope; Zn, Mo, and Cu in the cultivated land around the dump; and Cd, Zn, and Mo in the cultivated land around the tailings pond. The pollution index analysis provides a basic overview of soil heavy-metal pollution across the entire mining area. However, content analysis of heavy-metal forms/states better reflects the relationship between the availability of heavy metals in the soil and the effectiveness of plants. Thus, the latter analysis can help ensure that phytoremediation strategies are adequately targeted, science-based, and effective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Most heavy metals are transition elements, and their unique electronic layer structure gives them variable valences that allow redox reactions within certain ranges. Large variations exist in the activity and toxicity of heavy metals with variable valences. For example, the toxicities of As3+ and Cr6+ are much greater than those of As5+ and Cr3+ (Liu 2009), respectively. When heavy metals enter the soil, their migration and transformation characteristics, bioavailability, and degree of potential harm differ according to their chemical properties and existing forms. Determining the total amount of heavy metals in soil is crucial for soil-related studies on their ecological risk assessment, biological effects, and environmental effects. However, such data cannot fully reflect the severity of soil heavy-metal pollution and the characteristics of the heavy-metal pollutants. Therefore, it is necessary to study the chemical speciation characteristics of heavy metals in soil (Shi 2010). Tessier proposed a continuous extraction method in 1979 to analyze the combination state of metals in soil or solid waste (Li et al. 2021). As an operational definition, the Tessier continuous extraction method can distinguish the binding states of elements and soil components using specific extraction agents under certain extraction conditions. Although the method is relatively reasonable and has some limitations, a better alternative method has not been found yet. Compared with other analysis methods, the Tessier continuous extraction method can provide results that objectively reflect the complex forms of heavy metals in soil. Accordingly, this method analyzes the distribution of heavy-metal forms in a given research area and explores the biological activity characteristics of heavy metals in soil. Thus, it can provide data that help improve the use of phytoremediation technology to control soil heavy-metal pollution, e.g., in the areas associated with molybdenum ore mining.
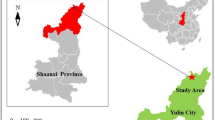
The area of Huludao molybdenum ore district, located at Yangjiazhangzi–Gangtun town, China, is greater than 200 km2. It is in the eastern section of the Yanshan molybdenum belt on the northern margin of North China. Moreover, it is one of the most important molybdenum deposits in China. It includes Gangtun and Lan Jiagou, Lingqian, Songbei, Xintaimen, Yangjiazhangzi, and other large-to-medium-sized molybdenum deposits, with dozens of molybdenum–polymetallic ore deposits.
2 Characteristics of the study area
The Yanggang area has distinct seasons (cold in winter and hot in summer), and it experiences drought, little rain, and frequent windy and sandy conditions. It has a temperate monsoon continental climate with an average annual temperature of 48.02° (range: −13° to 95°), average annual rainfall of 600 mm, and a frost-free period of 170 days. This area is located on brownfield sites in a mountainous and hilly district. The area contains abundant plant resources, mainly broad-leaved trees dominated by Quercus as well as grass species, such as Stipa bungeana, Arundinella, Setaria viridis, and Cleistogenes squarrosa, and other plants, e.g., Vitex negundo, Corylus heterophylla, Caragana korshinskii, Ziziphus jujuba, Thymus, and Lespedeza daurica.
Since 1996, molybdenum mining in the Yanggang area has been in a state of disorder. This area contains hundreds of mining pits and concentrators. The large number of abandoned tailings ponds left behind by a small dressing plant during the bifurcation of Qingshan Valley has seriously affected the local ecology and environment. In addition, a large amount of wastewater discharged from the tailings pond has polluted the local river, resulting in the closure of a water source near Wujintang Reservoir (Su 2006). Pollution control in the tailings wasteland and the restoration of the surrounding habitats require urgent attention involving detailed studies of the area. To this end, we surveyed the soil in the tailings wasteland and surrounding areas to determine the soil heavy-metal pollution and nutrient statuses, which would lead to the implementation of specific soil improvement and phytoremediation strategies to achieve sustainable development in the area.
The Yangjiazhangzi molybdenum mine tailings pond, in the upper valley of Heiyugou, China, covers a 2.5-km2 area. It consists of two phases and is surrounded by mountains on three sides and a dam on one side. It is a third-class, valley-type, stepped tailings pond (see Fig. 1 for the plan of the pond). Although the mountains around the reservoir were once densely covered with trees and shrubs, a large area of the reservoir’s sedimentary beaches was, in recent years, swept by strong northwest winds during dry spring and autumn seasons. Sandstorms and sand hazards affected hillsides and the cultivated land to the east and south of the reservoir area, substantially reducing the farmland production. Indeed, farmland areas have been severely reduced or lost and the mountains have become severely desertified and barren. Thus, vegetation is lacking across a large area of the entire first-stage reservoir. There are sparse reeds and pampas grasses at the sedimentary beaches but no other plant growth along the inner side of the existing water storage area with the depths of 40–60 cm. On the dam body of the first-stage reservoir, the dam surface below the level 5 subdam has been covered with mountain bark soil many times for artificial vegetation restoration. The main reclaimed plants include sea buckthorn and wild corn grass, which show good growth and coverage (85% in some sections). A small number of shrubs, such as sea buckthorn, thorny plants, and herbaceous plants, have been artificially planted on the dam surface below the sixth-grade dam, among which the diameter of sea buckthorn has reached 15 cm. Between the sixth and ninth grades of the dam, the tailings coverage has become severe; thus, plant coverage on the dam surface is scarce and plant growth is minimal. Notably, plants do not grow between level 9 and the top of the dam. The second-stage reservoir is currently in use. Although most sedimentary beaches are relatively humid, there is no plant growth on these beaches or the dams (Zhou 2004).
3 Materials and methods
3.1 Sample collection and processing
First, we selected typical plots in the abandoned tailings pond and its surrounding area and used a GPS positioning system to determine sampling points. Subsequently, we collected fresh soil from the depths of 0–20 cm at four locations for each sampling point at every 500 m along an S-shaped line. We then mixed samples from the four points using the quartering method to obtain 1 kg of samples, registered their numbers, and placed the final sample in a sample bag. We obtained 64 soil samples: 32 samples from the abandoned tailings pond and 32 samples from the surrounding area. These samples were transported to the laboratory for air drying, grinding, sieving, mixing, and bottling (Bao 2005).
3.2 Test items and methods
3.2.1 Determination of total soil heavy metals
The contents of Cu, Zn, Pb, and Cd were measured using atomic absorption spectrophotometry, Hg and As were measured using atomic fluorescence spectrophotometry, and Cr was measured via diphenylcarbazide spectrophotometry (Shi et al. 2009). Table 1 shows the sample analysis and measurement results (average values). The soil in the mining area is quite saline and alkaline, and the background values of Hg and As in the soil are high because Huludao is such an old industry city that its industrial structure is heavy.
3.2.2 Analysis of the presence and bioavailability of heavy metals in soil
Owing to the influence of soil components and their physical and chemical properties, heavy metals take different complex forms through various chemical reactions such as dissolution, precipitation, aggregation, complexation, and adsorption. According to the Tessier continuous extraction method (proposed by Tessier in 1979 and subsequently improved) and its extraction steps, the forms of the metals in the soil are divided as follows: exchangeable state (including the carbonate-bound state), iron–manganese (Fe–Mn) oxide–bound state, organic matter–bound state, and residue state. Exchangeable heavy metals are those adsorbed on clay, humus, and other components; these materials are sensitive to environmental change, migrate and transform easily, and can be directly absorbed and utilized by plants. In this form, heavy metals undergo obligate adsorption and can be ion-exchanged; however, they generally account for a small proportion of the total heavy metals in soil. The carbonate-bound state includes precipitated or coprecipitated heavy metals that can be generated via reaction with mild acids; thus, this form of heavy metals is also active or biologically effective. The Fe–Mn oxide–bound state includes heavy metals that undergo obligate adsorption or are coprecipitated. Heavy metals in this state can be released under highly reducing conditions, potentially harming crops. Heavy metals in this state are sensitive to soil environmental conditions, especially pH levels. When the pH level is low, heavy metals are easily released and enter the environment; conversely, high pH is conducive to carbonate formation and the coprecipitation of heavy metals on carbonate minerals. Heavy metals in an organic matter–bound state exist in the organic phase in a coadsorbed manner; thus, their properties are relatively stable. They are easily decomposed and released in an oxidized state. Residual heavy metals are contained in soil crystal lattices, such as silicates and primary and secondary minerals. They have stable properties and minimal activity and toxicity; thus, heavy metals in other states are less involved in the migration and bioavailability of heavy metals in the soil. Indeed, they are not easily absorbed by plants and are relatively safe for the environment. However, when they encounter acids, microorganisms, or chelating agents, residual heavy metals can re-enter the environment and threaten the ecosystem (Shi et al. 2013).
A simplified Tessier continuous extraction method was used to analyze the occurrence and speciation of heavy metals as follows.
-
(1)
Exchangeable state (including the carbonate-bound state): After passing 2 g of a dried soil sample through a 100-mesh sieve, it was placed in a 100-mL centrifuge tube with 40 mL of 0.11 mol/L of HAC solution. After continuous vibration at 77° ± 1.8° for 16 h and centrifugation for 30 min at 3500 revolutions per hour, the supernatant was collected and added to deionized water at a constant volume to produce an atomic absorption solution for further testing. The residue was washed with deionized water and centrifuged for 15 min at 3500 revolutions per hour; the supernatant was then discarded. Thus, the residue was prepared for the next extraction.
-
(2)
Fe–Mn oxide–bound state: The previous residue was added to 20 mL of a 0.04-mol/L 25% (v/v) HAC solution of NH4OH·HCl to a constant volume and covered. This solution was placed in a water bath at 204.8° ± 5.4° and shaken intermittently for 6 h (stirred every 15 min). It was then centrifuged for 30 min at 3500 revolutions per hour, after which the supernatant was removed and deionized water was added to a constant volume. This solution was used as the atomic absorption test solution. The residue was washed with 20 mL of deionized water, shaken for 15 min, and centrifuged for 15 min at 3500 revolutions per hour. Then, the supernatant was removed. Accordingly, the residue was prepared for the next extraction.
-
(3)
Organic-bound state: The previous residue was added to 12 mL of 0.02-mol/L HNO3 and 4 mL of 30% H2O2 to adjust pH to 2. After shaking the resulting mixture intermittently for 2 h in a water bath at 185° ± 3.6°, 20 mL of 30% H2O2 was added to the solution. It was shaken intermittently for 3 h at the same temperature. After cooling to 77° ± 1.8°, 5 mL of 3.2-mo1/L NH4OAC (20% (v/v) HNO3 at a constant volume) was added to the solution, diluted to 20 mL, continuously shaken for 30 min, and centrifuged twice. Hence, the residue was ready for extraction in the next step.
-
(4)
Residual state: The residue was placed in an oven at 167° until it reached a constant weight. Then, 0.25 g of residue, 9 mL of HNO3, and 4 mL of HF were added to a PTFE digestion tank. Following digestion and cooling, the solution was transferred to a PTEE crucible with 3 mL of HCIO4 and heated for evaporation until white smoke was released. After evaporation and removal of excess acid, the digestion solution was transferred to a volumetric flask with 5 mL of lanthanum nitrate solution, and deionized water was added to a constant volume. This solution was tested as the atomic absorption solution (Zhang et al. 2020).
3.3 Nemerow multifactor index method
Seven elements, i.e., Cu, Zn, Pb, Cr, Cd, Hg, and As, were selected as evaluation factors. A single factor index method and the Nemerow multifactor index method were used as evaluation methods (Lu et al. 2019). The background values of Cu, Zn, Pb, Cr, Cd, Hg, As, and other elements in the Huludao area, were used as the evaluation criteria for soil heavy metals in the study area.
Single pollution index:
$${P_{i,\,j}} = {C_{i,\,j}}/{S_i},\,1,\,2,\,3,\, \ldots ,\,m$$where Pi, j is the pollution index of the soil element i at point j, Ci, j is the measured value of element i in the soil at point j, Si is the evaluation standard of element i, and m is the number of sampling points.
Comprehensive pollution index:
$${P_j} = {({I_{j,\,{\rm aver} }} \times {I_{j,\,\max }})^{1/2}}$$where \( {I_{j,\,\max }}\) is the average value of the pollution index of each element in the soil at point j and \( {I_{j,\,\max }}\) is the maximum pollution index of each element at point j.
3.4 Soil heavy-metal risk assessment method
The total amount of heavy metals in the soil only reflects the degree of enrichment and does not reflect the metals’ occurrence status, migration ability, and bioavailability. The biological toxicity of heavy metals depends largely on their chemical forms. The risk assessment code (RAC) is an ecological risk assessment method based on the morphology of heavy metals and is used to evaluate environmental risks according to the analysis of the content of active forms. Compared with other total risk assessment methods, the RAC method more effectively reveals soil heavy metals’ migration activity and bioavailability. The core principle of the RAC method is as follows: the higher the proportion of active forms of heavy metals in the sum of the forms, the greater the risk of harm to the environment. Thus, the proportion of active forms in the sum of all forms is the risk assessment index for evaluating the environmental hazards of heavy metals. The corresponding relationship between the proportion of active forms and the risk level is as follows: RAC ≤ 1%(no risk), 1% < RAC ≤ 10% (low risk), 10% < RAC ≤ 30% (medium risk), 30% < RAC ≤ 50% (high risk), and RAC > 50% (very high risk) (Zhao et al. 2020).
4 Results and discussion
4.1 Soil heavy-metal pollution results
According to the single factor index and Nemerow multifactor index methods, the pollution index results are as shown in Table 2.
According to the soil pollution level standard, the actual degree of heavy-metal pollution in the soil can be calculated as follows: individual evaluation grading standard Pi, j > 1 indicates pollution and Pi, j ≤ 1 indicates the lack of pollution. The comprehensive evaluation grading standards are shown in Table 3.
Tables 2 and 3 show that the comprehensive pollution index, Pj, of both the abandoned tailings pond and its nearby cultivated land is greater than 3, indicating that the soil and crops in this area are severely polluted.
Tables 1 and 4 indicate that the heavy metals Cd, Pb, and Zn in the abandoned tailings pond and the cultivated land near the tailings pond exceeded the standard values. Therefore, the area is currently unsuitable for planting crops to ensure food safety from agricultural production. Priority should instead be given to the development of forestry and the planting of heavy-metal–tolerant plants; crop planting should be considered after the soil environmental quality has improved.
4.2 Pollution statuses of various forms of heavy metals in the soil
Different forms of heavy metals in the soil have different effects on microorganisms. According to the bioavailability of each form, heavy metals in the soil are classified as exchangeable (including carbonate-bound) heavy metals (available state; toxic to plants), Fe–Mn oxide–bound heavy metals (potentially available state; relatively low bioavailability and biotoxicity), organic-bound heavy metals (potentially available state; relatively low bioavailability and biotoxicity), and residual heavy metals (unusable state; inert and cannot be used by microorganisms). The heavy metals detected in these forms in the soil of the study area are shown in Figs. 2, 3 and 4.
The following results were obtained for Pb: residual state > Fe–Mn oxide–bound state > organic matter–bound state > exchangeable state. Pb was mainly distributed in the residue and Fe–Mn oxide–bound states and was at very low levels in the exchangeable state (< 0.3% of all the Pb content). Pb is closely bound in the Fe–Mn oxide–bound state; thus, it is unlikely to migrate under the alkaline conditions found in the study area. However, these results are not related to the high content of Pb in the crops in the study area. Approximately 90% of the Pb content in plants comes from the atmosphere rather than being absorbed from the soil by the roots (Shi et al. 2013). Nevertheless, the soil Pb in the study area should be considered potentially harmful under reducing conditions.
The following results were obtained for Cr: residual state > Fe–Mn oxide–bound state > exchangeable state. Cr existed mainly in the residue state (89.6%–98.28% of the total Cr content) and was not found in the exchangeable state, indicating that Cr hardly migrates and transforms in the soil of the study area and is unlikely to be used by plants.
The following results were obtained for Hg: residual state > Fe–Mn oxide–bound state > organic matter–bound state > exchangeable state. Hg was mainly distributed in the residue and the Fe–Mn oxide–bound states (accounting for 70.6%–89.4% of the total Hg amount). At the same time, the proportion of Hg in the Fe–Mn oxide–bound state was relatively high, (29.3%–47.1% of the residue state and Fe–Mn oxide–bound states). However, in the cultivated land around the dumpsite, the proportion of Hg residues was especially low (~ 23.5%). Although Hg did not exist in the exchangeable state, its potential harm cannot be ignored.
The following results were obtained for As: residual state > organic matter–bound state > Fe–Mn oxide–bound state > exchangeable state. As was mainly distributed in the residue state (67.5%–86.0% of the total As content) and the organic matter–bound state (13.3%–32.2% of the total As content). The Fe–Mn oxide–bound state accounted for only a small percentage of the total content, and As was not found in the exchangeable state. Thus, As has poor mobility and will be unlikely to cause harm to crops or plants in the study area.
The following results were obtained for Mo: residue state > organic matter–bound state > Fe–Mn oxide–bound state > exchangeable state. Mo was found in the residue state at high levels (reaching 94.5% of the total Mo content). The exchangeable state of Mo accounted for a small proportion (0.06%–0.35% of the total); thus, Mo has poor mobility, an inactive form, and is unlikely to harm plants.
The distribution of heavy metals in various regions revealed relatively high content for the following forms in the mountains surrounding the stope: exchangeable state: Cd, Cu, and Zn; Fe–Mn oxide–bound state: Cd, Hg, Pb, and Zn; organic matter–bound state: Mo, Cd, and As; and residue state: Cr, Cu, As, and Zn. Thus, Cd, Cu, and Zn are the most effective and toxic elements to plants in this area while Hg, As, and Mo have relatively high bioavailability and potential biotoxicity. The content of the following forms was relatively high in the cultivated land around the dumpsite: exchangeable state: Zn, Mo, and Cu; Fe–Mn oxide–bound state: Hg, Cd, and Pb; organic matter–bound state: Mo, Cd, and Hg; and residue state: Cr, Zn, and As. These results suggest that Zn, Mo, and Cu are the most effective and toxic elements to plants in the soil of this area while Hg, Cd, and Pb have relatively high bioavailability and potential biotoxicity. The content of the following forms was relatively high in the cultivated land around the tailings pond: exchangeable state: Cd, Zn, and Mo; Fe–Mn oxide–bound state: Hg, Cd, and Pb; organic matter–bound state: As, Hg, and Cd; and residue state: Cr, Mo, Cu, and Zn. Hence, Cd, Zn, and Mo are the most effective and toxic elements to plants in the soil of this area while Hg, As, and Pb have relatively high bioavailability and potential biotoxicity.
Our data show that Cu, Zn, Pb, and Cd exist in exchangeable states and exhibit relatively strong bioavailability. Cu has a strong binding capacity with organic matter, and the proportion of organic binding states is relatively high for this element. Pb is tightly bound to the Fe–Mn oxide–bound state and is unlikely to migrate under the alkaline conditions found in the study area. Cr did not exist in the exchangeable state, indicating that it hardly migrates or transforms and is unlikely to be used by plants. Compared with other heavy metals, Cd was found at higher proportions in the exchangeable state, i.e., always more than 5%, and in the Fe–Mn oxide–bound state. Although Hg was undetected in the exchangeable state, it could still be harmful; however, it has poor mobility and will cause limited harm to crops or plants. Although heavy metals mainly existed in the residue state, the content of elements in the Fe–Mn oxide–bound and organic matter–bound states was also high. This result indicates that heavy metals highly contaminate the soil in the study area owing to the development and use of mines. Therefore, to control environmental pollution and restore the ecosystem of the Yangjiazhangzi molybdenum mining area, attention should be given to the biological toxicity of heavy metals such as Cu, Zn, and Cd in the soil.
4.3 Ecological risk assessment of heavy metals in soil
To obtain the RAC, the three-step BCR extraction method (the weak acid extraction state, reducible state, oxidizable state, and residue state) is usually used as a reference for morphological classification and the weak acid extraction state is used as the active form for evaluation. In the present study, the morphological classification was based on Tessier’s five-step sequential extraction method, which was conducted according to the method proposed by Wang et al. (2005). They proposed that a relationship existed between the BCR and Tessier methods, i.e., the weak acid extraction state of the BCR method corresponds to the exchangeable and carbonate-bound states of the Tessier method and both methods have the same geochemical significance. In addition, some RAC evaluation methods use the exchangeable and carbonic acid-bound states as active forms (Sun et al. 2015; Feng et al. 2017). The current study used the percentage of exchangeable states (including carbonate-bound states) from the total amount as the RAC value calculation. Table 5 shows the risk levels for heavy metals in the mining area’s soil.
The heavy metal RAC risk coefficients are ordered from strong to weak, e.g., Cd (6.78) > Cu (1.96) > Zn (1.53). From the soil collected from various sampling points, the ecological risk ranking of various elements is as follows: Cu: mountains around the stope > cultivated land around the dump > cultivated land around tailings pond, Cd: cultivated land around tailings pond > cultivated land around the dump > mountains around the stope, Pb: cultivated land around tailings pond > cultivated land around the dump > mountains around the stope, and Zn: cultivated land around tailings pond > mountains around the stope > cultivated land around the dump.
The RAC for the mining area showed that Cd in the soil presents a low ecological risk and Cu and Zn are very low-risk heavy metals in the soil. In terms of sampling point types, the ecological risk presented by Cd in the farmland soil near the tailings pond was low while the risk presented by Zn and Mo was very low. In addition, the ecological risk presented by Mo, Zn, Cu, and Pb in the farmland soil around the dump was very low. Moreover, the ecological risk presented by Cd in the soil of the mountain area around the stope was low and the risk presented by Cu, Pb, Mo, and Zn in this soil was very low.
5 Conclusions
-
(1)
Implications for the concentration and pollution status of heavy metals in soil according to the total amount of heavy metals.
In the contaminated farmland around the molybdenum tailings pond, the soil’s physical structure and nutritional status are good; thus, the soil is suitable for plant growth. However, the contents of Cd and Zn heavy metals in the soil and those of Pb and Cd in crops exceed the national secondary soil standard values. To ensure food hygiene and safety and prevent the heavy metals Pb, Cd, and Zn from entering the food chain, some cultivated land is considered temporarily unsuitable for growing crops. Therefore, the traditional view of land use must change; this land should be considered a new resource, and its utilization mode should be redesigned according to new resource attributes.
-
(2)
Scientific ecological restoration measures determined based on the occurrence form and migration and transformation characteristics of soil heavy metals.
A series of chemical characteristics of heavy metals determines the variability of their dissolution characteristics under the soil environment, which in turn affects the variability of their migration characteristics under the same environment. Heavy metals usually exist in the soil in mineral particles or are adsorbed on the surface of soil colloids; these metals form complexes or chelates and undergo migration and transformation through dissolution and precipitation. The physical and chemical migration of heavy metals is mainly affected by factors such as soil pH and Eh values, moisture, organic matter, and soil environment. Plants absorb certain forms of heavy metals from the soil during their metabolic activities, and the metals accumulate in the plant tissues. In addition, the absorption of heavy metals by soil microorganisms and the metabolic activities of these microorganisms are the main pathways for the biological migration of heavy metals.
The soil improvement measures that should be implemented in the alkaline or slightly alkaline area of the abandoned molybdenum mining area in Huludao are as follows. The organic improvement method, which mixes conifers or hardwood soil into the existing soil, would change the soil pH value by introducing acidic soils to neutralize the alkalinized soil. Moreover, after the organic matter itself decays, it provides the nutrients necessary for the growth of various plants and loosens the soil, thereby maintaining good air and water permeability. At the same time, to avoid the long-term alkalization of the soil and the formation of saline–alkali land, organic fertilizer is added to improve the content of organic matter in saline–alkali land, increase the soil fertility level, make the soil pH value close to neutral, which is conducive to the good growth of most plants, and speed up the process of vegetation restoration in the abandoned mine land.
For variable-value heavy-metal (metalloid) pollutants, different soil redox conditions directly affect the ecological toxicity, bioavailability, and mobility of heavy metals in the soil. Therefore, given the severe Cd pollution in the surrounding farmland of the mining area, it may be possible to increase the soil moisture and reduce the redox potential through reasonable irrigation or by replacing dry land with paddy fields, thereby reducing the activity of heavy metal Cd and reducing Cd-induced damage to crops.
By determining the effectiveness and toxicity of soil heavy metals to plants, vegetation restoration measures can be taken in the study area. Owing to the exchangeable states of Cu, Zn, Mo, and Cd, these heavy metals exhibit relatively strong bioavailability. Therefore, the forestry restoration model should be adopted at the initial stage of restoration. After years of biological transformation, the heavy-metal content in the soil will be reduced and agricultural land can be used for agricultural purposes when the soil meets the standards for agricultural land use. For example, when planting nonedible plants, the land can first be reclaimed for developing nurseries and forestry or to plant turfgrasses, such as tall fescue, bluegrass, and ryegrass, with the ability to accumulate heavy metals, including Cu, Zn, Mo, and Cd, or plant varieties, e.g., sea buckthorn with strong nitrogen fixation abilities. Ultimately, crops such as corn, soybean, millet, and fruit trees and vegetables will be planted after the soil environmental quality has improved.
References
Bao SD (2005) Soil Agrochemical Analysis. China Agriculture Press, Beijing, pp 66–68
Feng YH, Zheng LP, Ying RR, Lin YS, Wang GQ (2017) Forms of Heavy Metals in Soils of Zinc Mining Area in Northwestern Guizhou Province and their environmental risks. J Ecol Rural Environ 02:142–149
Li D, He L, Sheng PP (2021) Transformation of Cr(VI)-Cr(III) and application suitability in Tessier sequential extraction of soil chromium. Chin J Environ Eng 15(7):2368–2378
Liu L, Xiao YB (2009) Advance in the study on Recovery and Remediation of Heavy Metals Pollution in Soil. J Changchun Inst Technol (Natural Sci Edition) 03:73–78
Lu YF, Xie L, Sun H, Gu W (2019) Criterion Selection in Assessment of Soil Heavy Metal Pollution in Farmland on County Scale. China Environ Sci 11:4743–4751. https://doi.org/10.19674/j.cnki.issn1000-6923.2019.0553
Shi P, Wei ZY, Wang ED, Yang ZQ (2009) Investigation on the Soil Heavy Metal Contamination of an abandoned Tailing Land and its Soil Remediation. Metal Mine 02:158–162 doi: CNKI:SUN:JSKS.0.2008-02-033
Wu YY (1994) Background values of soil elements in Liaoning Province. China: Environmental Science Press, Beijing. (in Chinese)
Shi P (2010) Research on Evaluation of Soil Heavy Metal Pollution and Phytoremediation in Representative Nonferrous Metal Mine Area of Liaoning Province. Dissertation. Northeastern University
Shi P, Zhang GX, Fu YH, Guo SH (2013) Research on effect of plants on the Heavy Metals Bioavailability in Urban Derelict Land of Huludao City. North Hortic 09:196–200
Su M (2006) Icebreaking in the Sun—Record of Integration of Molybdenum Resources in Yanggang Area, Huludao, Liaoning Province. Land and Resources 12:20–29
Sun RR, Chen QH, Li DK (2015) Comparison of Ecological Risk Assessment Methods based on the chemical forms of lead in Soil. Saf Environ Eng 22(5):47–51. https://doi.org/10.13578/j.cnki.issn.1671-1556.2015.05.008
Wang YP, Huang Y, Wang SM, Xu CX, Liu M (2005) Chemical speciation of elements in sediment sand soils and their sequential extraction process. Chin J Geol 24(8):728–734 doi: CNKI:SUN:ZQYD.0.2005-08-010
Zhang S, Yu Y, Wang DH, Wang W, Zhang HG (2020) Forms distribution of Heavy Metals and their ecological risk evaluation soils of Ion Adsorption type in the Rare Earth Mining Area of Southern Jiangxi, China. Mineral Anal 39(5):726–738. https://doi.org/10.15898/j.cnki.11-2131/td.201911050152
Zhao YM, Qin YW, Cao W, Wen Q, Shi Y, Ma YQ (2020) Speciation and ecological risk of Heavy Metals in Surface sediments of Dongting Lake. Res Environ Sci 33(3):572–580. https://doi.org/10.13198/j.issn.1001-6929.2019.07.28
Zhou XY (2004) Environmental Geochemistry in the Metal Mining and Smelter Area of West Coastal Plain of Bohai. Dissertation. Northeastern University
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (51504066).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, P., Huang, J., Wu, Z. et al. Differences between total amount of heavy metals and their occurrence form contents in the wastelands of a molybdenum mine area. Int J Coal Sci Technol 10, 19 (2023). https://doi.org/10.1007/s40789-023-00577-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-023-00577-6