Abstract
The efforts of the world research activities involved in clean coal technologies development focus to a considerable extent on integrated hydrogen and power generation technologies based on coal gasification. As an alternative to combustion processes, gasification offers increased efficiency, lower negative environmental impact as well as wider application range of the main product—synthesis gas—in power generation and chemical syntheses. In order to select the most optimal lignite for the purpose of gasification, it is necessary to determine coal reactivity, the key parameter characterizing how fast the fuel reacts with the gasifying medium and controlling its process ability in thermochemical conversion to energy and/or energy carriers. This paper presents the experimental results of oxygen/steam gasification of lignite coal char in a fixed bed reactor under atmospheric pressure and at the temperature of 700, 800 and 900 °C; the samples come from an open pit lignite mine in the southwest of Poland. The effectiveness of the gasification process was tested in terms of the total gas and hydrogen yields, gas composition, carbon conversion rate and chars reactivity.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
In Poland hard coal and lignite still constitute the main source of electricity production (Jureczka et al. 2019); for example, in 2020, as much as 63.53% of electricity was generated from coal (PSE 2020; Żuk et al. 2021). In the light of the Green Deal approved by the European Commission on December 11th, 2019 (European Commission 2019), it is necessary to remove coal from the energy market as quickly as possible. This is the main contributor to achieve by all the EU member states climate neutrality in 2050 (Brauers and Oei 2020). The primary objective of green transformation is to convert the national economies into new, modern economic structures which are characterized by low environmental impact with energy systems equipped with environmentally friendly technologies mainly based on renewable energy (Cheba et al. 2022). Environment-friendly policy should be based on a transition being nothing else than new production and consumption patterns which imply less negative impacts on the environment, primarily in terms of greenhouse gas emissions (Söderholm 2020; Bekun et al. 2019). Nowadays, the sustainable development is becoming a priority in the economic policy in all the EU countries, which is widely discussed in the literature. Brodny and Tutak (2021) present the methodology which allows to assess the level of sustainable energy development in the Central and Eastern European countries.
The European Union implemented restrictive target in the race for carbon neutrality in Europe (European Commission 2021). The change of electricity systems in the whole Europe constitutes a huge challenge. Aszódi et al. (2021) in their research focus on the investigation of how the conversion of the European electricity system can serve the abovementioned objectives of the security of energy supply and climate protection. Amundsen and Hermansen (2021) focus in their study on the analysis of green transformation in Norwegian primary industries.
The efforts of the European Commission to achieve climate neutrality are important in the fight to reduce greenhouse gas emissions. However, to be successful, it requires a global commitment and solidarity efforts to reduce greenhouse gas emissions. China, which is the biggest world coal producer, established the Green Mining Construction plan, the Chinese national strategy of the environmental regulation of the industry to motivate mining enterprises to improve their resource utilization efficiency, protect the environment and harmonize the relationships between the enterprises and the communities (Qi et al. 2019).
Decarbonization is particularly challenging for the countries that have largely used fossil fuels to meet their energy needs (Papadis and Tsatsaronis 2020; Brauers and Oei 2020). Poland is one of such countries (Gabruś et al. 2021). The process of decarbonisation of the energy sector in Poland will be long and difficult considering the economic as well as social aspects (Tokarski et al. 2021). The application of coal gasification technologies in the Polish energy sector may successfully contribute to facilitate this process (Cabuk et al. 2020; Smolinski et al. 2021; Park et al. 2020) while combined with the CCUS (Urych et al. 2022; Zdeb et al. 2019; Van Bergen et al. 2009;). Bielowicz (2019) presents the deep analysis of the Polish lignite for gasification in the CO2 atmosphere. Coal gasification technologies have been known for more than 100 years. Gasification is a thermochemical process in which the fuel, coal in this case, is converted into synthesis gas composed of carbon monoxide and hydrogen. There are number of papers deal with the lignite co-gasification with biomass/biowaste or Solid Recovered Fuel. Gallucci et al. (2021) tested mixtures of lignite and solid recovered fuel in the oxygen/steam co-gasification oriented on optimization of the process operating conditions. Savuto et al. (2020) evaluate the quality of gas produced from co-gasification of waste materials (lignite blends with solid recovered fuel). Lampropoulos et al. (2021) showed that the highest syngas yield was obtained for steam chars gasification at 800 °C, due to high content in fixed carbon.
The synthesis gas generated in the gasification process can be transformed into fuel components or gasoline by further chemical syntheses or, after the separation of carbon monoxide, used to obtain hydrogen (Smolinski et al. 2010; Pio et al. 2020). Considered to be a clean, environmentally friendly energy carrier, hydrogen can be used in fuel cells or in the production of ammonia and fertilizers. In the refineries, such hydrogen can be applied to refine petroleum derivatives for motor fuels (Nikolsky et al. 2022). Apart from the entire path of chemical transformations of synthesis gas, hydrogen can also be used in the power industry in IGCC systems, i.e. integrated gas-steam systems combined with coal gasification (Mahmood et al. 2018). The gasification process can be carried out in various reactors such as a fixed-bed reactor, a fluidized bed reactor or a dispersion bed reactor. In the dispersion reactors, the coal powder reacts under high pressure at temperatures above 1000 °C with gasifying agents, mostly oxygen with the addition of water vapor.
Hydrogen is seen as a new environmentally friendly energy carrier (Aznar et al. 2006; Cormos 2012; Du et al. 2021; Pinto et al. 2007). The European Commission is striving to build a dynamic hydrogen ecosystem in Europe (EU 2020; Espegren et al. 2021; Capros et al. 2019). This requires the solidarity of all actors, both public and private, at all levels including European, national and regional ones; and this cooperation must cover the entire value chain (Moliner et al. 2016; Hanley et al. 2018). The Hydrogen strategy for a climate-neutral Europe (EU 2020) stipulates that the priority of the European Union is the production of the so-called green hydrogen. Such hydrogen could be produced via electrolysis using wind and/or solar energy, which remains in line with the European Commission Green Deal targets for climate neutrality and the EU efforts to achieve zero emissions economy in 2050. This will require a coherent integrated energy system. Currently, renewable hydrogen is expensive (Krawczyk et al. 2016). Therefore, during the transition period, it is assumed that blue hydrogen will be also accepted. Nowadays, blue hydrogen is produced by steam reforming of natural gas (Van Dyk et al. 2009; Verhelst et al. 2011; Polish Hydrogen Strategy to 2030 with the 2040 perspective). Another option of hydrogen production in the midterm of hydrogen economy development is associated with the construction of coal gasification systems which, however, due to their high emission (above 10 kg CO2 eq/1 kg H2), must be combined with the CCS/CCUS technologies (Krawczyk et al. 2016; Urych et al. 2022).
The aim of this study was to present an attractive method of thermal utilization of lignite in the oxygen/steam gasification process oriented on hydrogen rich gas production. Several lignites from a Polish coal mine were tested in terms of their reactivity; based on their values, the lignite samples were selected for further gasification tests oriented on hydrogen production.
2 Materials and methods
The studied lignite coals (denoted as I, II, III and IV) were provided by an open pit mine in the Southwest of Poland. The physical and chemical parameters of the lignite samples tested were determined by the accredited laboratory of the Central Mining Institute in accordance with relevant standards: PN-G-04511:1980 (total moisture), PN-G-04560:1998 and PN-ISO 1171:2002 (ash), PN-G-04516:1998 and PN ISO-562:2000 (volatiles), PN-G-04513:1981 (heat of combustion, calorific value), PN-G-04571:1998 (carbon, hydrogen and nitrogen), and PN-G-04516:1998 (fixed carbon). The results of the analysis of the studied lignite samples in analytical state are presented in Table 1.
In the first part of the research, the reactivity tests of selected lignite coals (I–IV) were carried out in order to select the optimal coals for the study of the oxygen/steam gasification process oriented on hydrogen-rich gas. The reactivity studies were performed using the SDT Q600 thermogravimetric analyzer, TA Instruments. The SDT Q600 device enables the measurement of heat flow and mass changes related to phase changes and/or reactions in the tested samples in the temperature range from ambient temperature to the temperature of 1500 °C.
The measurements can be performed in an inert gas atmosphere (nitrogen, argon or helium) or in a reaction gas (oxygen, air, carbon dioxide). The sample heating speed is 0.1–100 °C/min, and the sensitivity of the microbalance is 0.1 µg.
In each reactivity test in TGA, a 10 mg lignite sample was placed in a measurement vessel and heated in an inert gas (nitrogen) atmosphere up to the temperature of the gasification process (700, 800 or 900 °C) with a heating rate of 10 °C/min. Then the gasifying agent—air was fed to the furnace with a flow rate of 100 ml/min. Gasification was carried out until a constant mass of the process residue was obtained. Based on the results of the weight loss over time in the gasification process, the reactivity was calculated at a 50% of coal conversion, R50, and the maximum reactivity, Rmax, in the gasification process:
and
where RX determines the reactivity at the X th degree of carbon conversion, 1/s; m0 is initial char weight, g; ma is ash weight, g; m is instant char weight at time t, g; dm/dt—weight loss of the char at X th degree of carbon conversion. The tests determined the reactivity at 50% carbon conversion (R50) and the maximum reactivity (Rmax).
Based on the results of the reactivity tests, the lignite samples were selected for the second part of the study related to lignite oxygen/steam gasification oriented on hydrogen-rich gas.
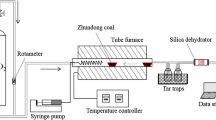
The research on the gasification of the tested lignite samples was carried out with the use of a laboratory installation with a fixed-bed reactor (see Fig. 1).
Photo of the laboratory scale installation for testing the gasification process of solid fuel. 1–Gasification agent supply with steam generator, 2–Fixed bed reactor with resistance furnace, 3–Gas cooling and purifying system: dryer, particulate filter, 4–Gas composition and flow rate measurement system: mass flowmeter and gas chromatograph
The tested 10 g lignite samples in analytical state, were heated in an inert gas (nitrogen) atmosphere to the set temperature of 700, 800 or 900 °C with the heating rate equal to 1.33 °C/s. When the set temperature was stabilized, a mixture of water vapor and oxygen (water vapor: oxygen = 0.5) was injected into the reactor with a flow rate of 5.33 × 10−8 m3/s. The amount of the produced gas was measured with a mass flowmeter.
The concentration of the main gas components was measured using the Agilent 3000A two-channel gas chromatograph with a PLOT U column (8×10−3 m × 0.32 × 10−3 m) equipped with a thermoconductivity detector (TCD) to determine the concentration of carbon dioxide and gases containing 2/5 carbon atoms in the molecule and the MS5A PLOT analytical column (3.2 × 10−3 m) with TCD for determining the concentration of hydrogen, nitrogen, carbon monoxide and methane.
The volumes of the main gas components in the gasification process in each experiment, Vc, were calculated as follows:
where, Vc is the volume of the cth gas component obtained during the experiment, m3; Cci is the volume concentration of the c th gas component in the i th time interval, %vol; Fi is the gas flow rate in the i th time interval, m3/s; ti is the length of the i-time period, s; n is the number of time intervals during the experiment.
The length of the time interval was equal for all the experiments, it resulted from the settings of the chromatographic method, and was 192 s, whereas the number of time intervals during the experiment was 19.
3 Results and discussion
In the first part of the study, the reactivity of 12 lignite samples was tested with the use of a thermogravimetric analyzer. The thermograms of the thermogravimetric analysis are presented in Fig. 2.
On the basis of the obtained thermograms presented in Fig. 2, the values of reactivity in the gasification process of 12 lignite samples at the temperature of 700, 800 and 900 °C, with a 50% degree of coal conversion, R50, and maximum reactivity, Rmax, were determined. The results of the calculations of R50 and Rmax are summarized in Table 2. Reactivity is the basic parameter characterizing how fast the fuel (lignite) reacts with the gasifying medium. The higher reactivity value means that the coal sample reacts better with the gasifying agent. Therefore, lignite with higher reactivity is more suitable for the gasification process.
For all the lignite samples (I-IV), a significant increase in the reactivity values (both R50 and Rmax), was observed with the increase in the temperature of the gasification process. The lowest values of R50 and Rmax at all the tested temperatures were observed for lignite sample III, while the highest values of R50 and Rmax were identified at 700 and 900 °C for lignite sample II, whereas at 800 °C for lignite sample I. Reactivity indicates how fast a given lignite sample reacts with the gasifying medium. The higher value of reactivity means that the lignite reacts faster with the gasifying agent. Apart from the reactivity value, also other physicochemical parameters such as ash, moisture and carbon content have a significant impact on the selection of the optimal coal for the gasification process.
Based on an in-depth analysis of the R50 and Rmax, measured for the obtained lignite chars, combined with the analysis of the physical and chemical parameters important from the gasification point of view, all the tested lignite samples are characterized by similar properties for further gasification tests oriented on hydrogen rich gas production. Of course, the lignite samples with the highest R50 and Rmax will relatively more easily react with the gasification medium. Anyway, the differences in the R50 and Rmax observed among the studied lignite samples are not significant enough; based on that, all the studied lignite samples were selected to the second stage of the experimental campaign oriented on hydrogen rich gas production. In that step, the lignite samples were put into the fixed bed reactor and heated to the set temperature of 700, 800 and 900 °C, respectively; when the temperature was stabilized, the O2/steam as a gasification agent was injected (O2:steam = 0.5). In the oxygen/steam gasification process of lignite at all the tested temperatures, hydrogen rich gas was produced. The average total volumes of the main gaseous components of the gas procedure in the gasification of lignite at the temperature of 700, 800 and 900 °C, respectively, are presented in Fig. 3.
During the gasification tests of four lignite samples, it was possible to observe an increase in the total gas yield with the increase of temperature from 700 to 900 °C. Moreover, a significant difference in the total gas yield could be also observed among the studied lignite samples I-IV. Namely, for lignite samples I and II, a relatively lower total gas yield is observed in all the studied temperatures in comparison with the gasification of lignite samples III and IV. The lowest total gas yield is observed for lignite sample I, mainly due to the fact that sample I is characterized by the lowest carbon content as well as the highest ash content and the lowest heating and calorific values. The highest total gas yield is observed for the gasification tests of lignite sample III in all the studied temperatures. It could be explained by the uniqueness of lignite sample III due to the relatively lowest ash content and the highest C content among all the studied samples. Similar heating value is also the highest among all the studied samples.
Similarly, the lowest amount of hydrogen is produced in the gasification of lignite sample I in all the studied temperatures. The highest amount of hydrogen yield occurs in the gasification of lignite III. In that case, the gasification of the 10 g lignite sample I gives 2857 to 2940 cm3 of the total hydrogen yield at the temperature of 700 and 900 °C, respectively. Based on the total gas yield at the studied temperature as well as the composition of the produced gas, it is possible to calculate the carbon conversion in the gasification tests. The value of the calculated carbon conversion is presented in Table 3. On the basis of the presented carbon conversion results, it is possible to observe an increase in the carbon conversion with the increase in the gasification temperatures. The highest carbon conversion is observed at the temperature of 900 °C for all the studied lignite samples.
Based on the average composition of the produced gas and the average total amount generated in the gasification tests, the calorific value of the produced gas (Qg) could be calculated as follows:
where ci describes the concentration of the particular component in the produced gas while Wi describes the heat of combustion in kJ/kg. The calorific value of the gas generated in oxygen/steam gasification of lignite samples I, II, III and IV was calculated on the basis of the experimental data on gas volume and composition (see Table 4). Moreover, the ratio of the energy output in the produced gas to the energy input in lignite (Eout/Einput) was also calculated.
4 Conclusions
In the paper, four lignite samples were gasified with oxygen/steam under atmospheric pressure at the temperature of 700, 800 and 900 °C, respectively. The process was oriented on hydrogen rich gas production. In the first step of the experimental campaign, the reactivities R50 and Rmax were determined in order to select the best lignite for the gasification purpose. The lowest values of R50 and Rmax at all the tested temperatures were observed for lignite sample III, while the highest values of R50 and Rmax were identified at the temperature of 700 and 900 °C for lignite sample II, and at 800 °C for lignite sample I. Based on the reactivity, all the studied lignite samples were selected for oxygen/steam gasification tests oriented on hydrogen rich gas production. In the gasification tests, the total gas yield as well as the hydrogen yield increased with the temperature of the gasification process. Moreover, significant differences in the hydrogen yields were observed in the gasification process of various lignite samples. Namely, lignite samples I and II were characterized by lower hydrogen yield in comparison with the gasification tests of lignite samples III and IV.
References
Amundsen H, Hermansen EAT (2021) Green transformation is a boundary object: an analysis of conceptualisation of transformation in Norwegian primary industries. Nature Space 4(3):864–885. https://doi.org/10.1177/2514848620934337
Aszódi A, Biró B, Adorján L, Csaba A, Gergely Illés D, Krisztián N, Dávid T, Zalán Z, Zsiborás T (2021) Comparative analysis of national energy strategies of 19 European countries in light of the Green Deal objectives. Energy Convers Manag: X 12:100136
Aznar MP, Caballero MA, Sancho JA, France´s E (2006) Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant. Fuel Process Technol 87:409–420
Bekun FV, Alola AA, Sarkodie SA (2019) Toward a sustainable environment: nexus between CO2 emissions, resource rent, renewable and non-renewable energy in 16-EU countries. Sci Total Environ 657:1023–1029. https://doi.org/10.1016/j.scitotenv.2018.12.104
Bielowicz B (2019) The suitability of Polish lignite for gasification. Clean Technol Environ Policy 21:1115–1130. https://doi.org/10.1007/s10098-019-01695-z
Brauers H, Oei PY (2020) The political economy of coal in Poland: drivers and barriers for a shift away from fossil fuels. Energy Pol 144:111621. https://doi.org/10.1016/j.enpol.2020.111621
Brodny J, Tutak M (2021) Assessing sustainable energy development in the Central and Eastern European countries and analyzing its diversity. Sci Total Environ 801:149745. https://doi.org/10.1016/j.scitotenv.2021.149745
Cabuk B, Duman G, Yanik J, Olgun H (2020) Effect of fuel blend composition on hydrogen yield in co-gasification of coal and non-woody biomass. Int J Hydrog Energy 45:3435–3443. https://doi.org/10.1016/j.ijhydene.2019.02.130
Cheba K, Bąk I, Szopik-Depczyńska K, Ioppolo G (2022) Directions of green transformation of the European Union countries. Ecol Ind 136:108601. https://doi.org/10.1016/j.ecolind.2022.108601
Cormos CC (2012) Hydrogen and power co-generation based on coal and biomass/solid wastes co-gasification with carbon capture and storage. Int J Hydrog Energy 37:5637–5648
Du S, Yuan S, Zhou Q (2021) Numerical investigation of co-gasification of coal and PET in a fluidized bed reactor. Renew Energy. https://doi.org/10.1016/j.renene.2021.03.035
Espegren K, Damman S, Pisciella P, Graabak I, Tomasgard A (2021) The role of hydrogen in the transition from a petroleum economy to a low-carbon society. Int J Hydrog Energy 46:23125–23138. https://doi.org/10.1016/j.ijhydene.2021.04.143
EU (2020) A hydrogen strategy for a climate-neutral Europe. https://ec.europa.eu/energy/sites/ener/files/hydrogen_strategy.pdf
European Commission, Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions, The European Green Deal, Brussels, 11.12.2019 COM (2019) 640 final https://ec.europa.eu/info/sites/default/files/european-green-deal-communication_en.pdf
Gabruś E, Wojtacha-Rychter K, Aleksandrzak T, Smoliński A, Król M (2021) Adsorptive capture of carbon dioxide on Polish hard coals. Sci Total Environ 796:149064. https://doi.org/10.1016/j.scitotenv.2021.149064
Gallucci K, Di Giuliano A, Rapagnà S (2021) Steam O2-enriched air gasification of lignite and solid recovered fuel in fluidized bed. Fuel 303:121271. https://doi.org/10.1016/j.fuel.2021.121271
Jureczka J, Ihnatowicz A, Zdanowski A (2019) Polish hard coal basins: an outline of the research history of the Polish geological institute. Pol Geol Rev 67:578–583
Krawczyk P, Howaniec N, Smolinski A (2016) Economic efficiency analysis of substitute natural gas (SNG) production in steam gasification of coal with the utilization of HTR excess heat. Energy 114:1207–1213. https://doi.org/10.1016/j.energy.2016.08.088
Lampropoulos A, Binas V, Konsolakis M, Marnellos GE (2021) Steam gasification of Greek lignite and its chars by co-feeding CO2 toward syngas production with an adjustable H2/CO ratio. Int J Hydrog Energy 46:28486–28500
Mahmood H, Atta H, Kun U, Muhammad H, Zaman H (2018) Thermo-economic analysis of integrated gasification combined cycle (IGCC) power plant with carbon capture. Chem Eng Process Process Intensif 128:53–62
Moliner R, Lázaro MJ, Suelves I (2016) Analysis of the strategies for bridging the gap towards the Hydrogen Economy. Int J Hydrog Energy 41(43):19500–19508. https://doi.org/10.1016/j.ijhydene.2016.06.202
Nikolsky V, Dychkovskyi R, Cabana CE, Howaniec N, Jura B, Widera K, Smolinski A (2022) The hydrodynamics of translational−rotational motion of incompressible gas flow within the working space of a vortex heat generator. Energies 15:1431. https://doi.org/10.3390/en15041431
Papadis E, Tsatsaronis G (2020) Challenges in the decarbonization of the energy sector. Energy 205:118025. https://doi.org/10.1016/j.energy.2020.118025
Park SW, Seo YC, Lee SY, Yang WS, Ohd JH, Gue JH (2020) Development of 8 ton/day gasification process to generate electricity using a gas engine for solid refuse fuel. Waste Manage 113:186–196. https://doi.org/10.1016/j.wasman.2020.06.004
Pinto F, Lopes H, Andre RN, Gulyurtlu I (2007) Effect of catalysts in the quality of syngas and by-products obtained by co-gasification of coal and wastes. Tars Nitrogen Compd Abatement Fuel 86:2052–2063
Pio DT, Tarelho LAC, Tavares AMA, Matos MAA, Silva V (2020) Co-gasification of refused derived fuel and biomass in a pilot-scale bubbling fluidized bed reactor. Energy Convers Manag 206:112476. https://doi.org/10.1016/j.enconman.2020.112476
Polish hydrogen strategy to 2030 with the 2040 perspective. https://www.teraz-srodowisko.pl/media/pdf/aktualnosci/9801-Projekt-Polskiej-Strategii-Wodorowej-do-roku-2030-z-perspektywa-do-2040-r.pdf
Polskie Sieci Elektroenergetyczne (PSE) (2020) Raport 2020 PSE. https://www.pse.pl/dane-systemowe/funkcjonowanie-kse/raporty-roczne-z-funkcjonowania-kse-za-rok/raporty-za-rok-2020#t1_1
Qi R, Liu T, Ji Q, Sun L, Liu J (2019) Simulating the sustainable effect of green mining construction policies on coal mining industry of China. J Clean Prod 226:392–406
Savuto E, Di Carlo A, Gallucci K, Di Giuliano A, Rapagnà S (2020) Steam gasification of lignite and solid recovered fuel (SRF) in a bench scale fluidized bed gasifier. Waste Manag 114:341–350. https://doi.org/10.1016/j.wasman.2020.07.016
Smoliński A, Stańczyk K, Howaniec N (2010) Steam gasification of selected energy crops in a fixed bed reactor. Renew Energy 35:397–404. https://doi.org/10.1016/j.renene.2009.06.005
Smolinski A, Howaniec N, Gasior R, Polański J, Magdziarczyk M (2021) Thermal conversion of low rank coal, flotation concentrate and refuse derived fuel in the process of steam co-gasification to hydrogen-rich gas. Energy 235:121348. https://doi.org/10.1016/j.energy.2021.121348
Söderholm P (2020) The green economy transition: the challenges of technological change for sustainability. Sustain Earth. https://doi.org/10.1186/s42055-020-00029-y
Tokarski S, Magdziarczyk M, Smoliński A (2021) Risk management scenarios for investment program delays in the Polish power industry. Energies 14(16):5210. https://doi.org/10.3390/en14165210
Urych T, Checko J, Magdziarczyk M, Smolinski A (2022) Numerical simulations of carbon dioxide storage in selected geological structures in North-Western Poland. Front Energy Res 10:827794. https://doi.org/10.3389/fenrg.2022.827794
Van Bergen F, Krzystolik P, Van Wageningen N, Pagnier H, Jura B, Skiba J, Winthaegen P, Kobiela Z (2009) Production of gas from coal seams in the Upper Silesian Coal Basin in Poland in the post injection period of an ECBM pilot site. Int J Coal Geol 77:175–187
Van Dyk JC, Benson SA, Laumb ML, Waanders B (2009) Coal and coal ash characteristics to understand mineral transformations and slag formation. Fuel 88:1057–1063
Verhelst S, Turner J, Sileghem L, Vancoillie J (2011) Clean liquid fuels from direct coal liquefaction: chemistry, catalysis, technological status and challenges. Energy Environ Sci 4:311–345
Zdeb J, Howaniec N, Smoliński A (2019) Utilization of carbon dioxide in coal gasification—An experimental study. Energies. https://doi.org/10.3390/en12010140
Żuk P, Żuk P, Pluciński P (2021) Coal basin in Upper Silesia and energy transition in Poland in the context of pandemic: the socio-political diversity of preferences in energy and environmental policy. Resour Policy 71:101987. https://doi.org/10.1016/j.resourpol.2021.101987
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. This research received no external funding.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smolinski, A., Wochna, S. & Howaniec, N. Gasification of lignite from Polish coal mine to hydrogen-rich gas. Int J Coal Sci Technol 9, 77 (2022). https://doi.org/10.1007/s40789-022-00550-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-022-00550-9