Abstract
Changes are needed to improve the efficiency and lower the CO2 emissions of traditional coal-fired power generation, which is the main source of global CO2 emissions. The integrated gasification fuel cell (IGFC) process, which combines coal gasification and high-temperature fuel cells, was proposed in 2017 to improve the efficiency of coal-based power generation and reduce CO2 emissions. Supported by the National Key R&D Program of China, the IGFC for near-zero CO2 emissions program was enacted with the goal of achieving near-zero CO2 emissions based on (1) catalytic combustion of the flue gas from solid oxide fuel cell (SOFC) stacks and (2) CO2 conversion using solid oxide electrolysis cells (SOECs). In this work, we investigated a kW-level catalytic combustion burner and SOEC stack, evaluated the electrochemical performance of the SOEC stack in H2O electrolysis and H2O/CO2 co-electrolysis, and established a multi-scale and multi-physical coupling simulation model of SOFCs and SOECs. The process developed in this work paves the way for the demonstration and deployment of IGFC technology in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the past few decades, the tremendous demand for energy caused by industrialization has resulted in a substantial increase in CO2 emissions. Global CO2 emissions are closely correlated with the burning of coal, which has been a primary fossil energy source for centuries and will remain one for decades more. The extensive consumption of coal leads to dramatic CO2 emissions (Xu and Zhang 2012).
There are several options for reducing CO2 emissions: (1) adopting renewable energy sources such as wind, solar, and geothermal energy; (2) improving the power generation efficiency of existing fossil energy sources, particularly coal; and (3) realizing the conversion and utilization of CO2. Integrated gasification fuel cell (IGFC) systems combine coal gasification with high-temperature fuel cells to increase power generation efficiency and improve environmental sustainability compared to conventional coal-fired power generation systems (Peng and Han 2009). IGFC is regarded as the most promising process to achieve near-zero CO2 emissions from coal power generation in the twenty-first century (Li et al. 2018).
In the last decade, both the U.S. and Japan have made tremendous investments in the development and application of IGFC systems (Damo et al. 2019; Discepoli et al. 2012; Li et al. 2010). In 2017, the China energy group launched the IGFC for near-zero CO2 emissions program with support from the National Key R&D Program of China and 11 other organizations. This project focuses on coal gasification purification, high-temperature fuel cells, CO2 capture and conversion, and IGFC system integration. The project will conclude with the demonstration of a megawatt-level (input heat value) IGFC system with near-zero CO2 emissions.
Syngas produced by coal gasification, which has been used as a fuel for solid oxide fuel cell (SOFC) stacks, usually retains a small amount of unreacted CO and H2 after the electrochemical reaction. Both power generation and CO2 enrichment require the complete conversion of CO and H2; thus, the catalytic combustion of the fuel gas is an essential component of the IGFC process (Sung et al. 2018; Kawabata et al 2012). Meanwhile, the thermal efficiency can be increased by using the exhaust heat in the reforming process, which also benefits from the efficient combustion of the fuel gas (Leea et al. 2013; Liese 2010).
Conventional catalysts including Pt and Pd have long been used in flue gas combustion. To mitigate the deactivation caused by water and sulfur compound at high temperature, Pd/Pt bimetallic catalysts that can achieve stable and complete combustion in an SOFC stack were recently developed (Hoque et al. 2012). In addition to the high cost of noble metal catalysts, catalytic deactivation at high vapor content and high temperature is a severe problem (Vepřek et al. 1986; Rudra and Kim 2010; Trembly et al. 2007). Considering these difficulties, we developed perovskite-based catalysts for kW-level catalytic combustion in this work.
To realize zero CO2 emissions and energy storage, the electrochemical transfer of CO2 into chemicals can be implemented using renewable energy resources, including wind and solar energy. Solid oxide electrolysis cells (SOECs), which are based on the inverse process of SOFCs, have been experimentally demonstrated to directly co-electrolyze H2O/CO2 into syngas (CO + H2). Moreover, SOECs have the benefits of low energy consumption and high efficiency (Yang et al. 2019a, b).
One key challenge in the application of SOECs is the unreliability of SOEC stacks, which consist of, at a minimum, a metallic interconnect, sealing material, single SOEC, and electrode contact materials. Research on SOEC stacks is still limited. Ebbesen et al. (2011) reported SOEC stacks for steam electrolysis and CO2/steam co-electrolysis. Zhang et al. (2013) demonstrated the long-term durability of SOEC cells and stacks. In China, SOEC stacks for hydrogen production have only been reported once: Zheng et al. (2014) manufactured and tested 30-cell nickel-yttria-stabilized zirconia hydrogen electrode-supported planar SOEC stacks at 800 °C in steam electrolysis mode.
In this work, we developed kW-level SOEC stacks and evaluated their electrochemical performance for H2O electrolysis and H2O/CO2 co-electrolysis.
2 Project introduction
2.1 Objective
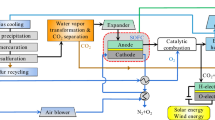
The aims of the IGFC for near-zero CO2 emissions program include coal gasification, syngas purification, CO2 capture & utilization, and IGFC system integration, as schematically shown in Fig. 1.
The objectives of the IGFC for near-zero CO2 emissions program are to: (1) further develop coal gasification and syngas purification technology; (2) realize the efficient conversion of chemical energy from syngas in fuel cells; (3) establish a kW-level catalytic combustion system with a ≥ 99% conversion rate of combustible components; (4) explore a new method for CO2 conversion; and (5) establish a kW-level SOEC verification platform. The ultimate goal is near-zero CO2 emissions.
2.2 Scope of work
This research mainly focuses on catalytic combustion and SOFC/SOEC technology with the following objectives: (1) establish a simulation model for SOFC and SOEC to analyze the mechanisms of internal heat and mass transfer, optimize the fuel cell working mode, and improve fuel utilization; (2) investigate the mechanism of catalytic combustion involving exhaust gas, catalyst materials, and catalytic burners; (3) develop new exhaust gas catalysts; (4) study the catalytic combustion characteristics of exhaust gas to inform the construction of high-performance exhaust gas catalytic combustors; (5) establish a kW-level SOEC verification platform that optimizes the surface and interface microstructures of the electrode catalysts, reduces the polarization electromotive force and resistance of the CO2 reduction reaction, improves the reaction efficiency, and reduces energy consumption; and (6) develop a SOEC module for CO2/H2O co-electrolysis.
3 Major progress in CO2 capture and utilization
3.1 SOFC/SOEC model
By establishing the simulation model for SOFC and SOEC, it is possible to deeply analyze the mechanism of internal heat and mass transfer and improve fuel utilization. The primary function of the simulation was to optimize the design of the electrodes, cells, and stacks. The microscopic model was used to optimize the electrode mirostructure and analyze the long-term stability of the cell.
The main progress is summarized in Fig. 2 and elaborated as follows:
-
(1)
A comprehensive steady-state model was developed to investigate the effects of electrode structure on SOFC performance, specific heat and mass transfer, and electronic and ionic charge transport. Percolation theory was used to evaluate the transport properties of the electrodes. The results show that for small particle sizes less than 0.4 μm, the optimal thickness of the functional layer is between 5 and 30 μm. For thicker functional layers, a relatively larger diameter results in better cell performance. The uniform and non-uniform distributions of electronic/ionic conducting materials in the anodic/cathodic functional layers were comprehensively compared. The findings also provide an alternative microstructural design in consideration of the non-uniform distribution of conducting materials, which is meaningful for fuel cell optimization.
-
(2)
An enhanced quasi-two-dimensional, non-isothermal model for SOFC parametric simulation and optimization was proposed. The dependence of electric power generating efficiency on microstructural parameters is fully considered in this model. In addition, an elementary effect approach based on Monte Carlo experiments was adopted to comprehensively evaluate the sensitivity of all parameters. Subsequently, a feasible non-uniform distribution method in allusion to the functional layers was proposed to further improve cell performance along the channel direction.
-
(3)
Three-dimensional (3D) SOFC/SOEC models were developed, and the effects of the new flow field structure of the oxygen electrode were analyzed. The effects of different cell operating parameters on the CO2/H2O co-electrolysis characteristics in SOECs were studied, and corresponding optimization strategies were proposed. The adoption of a new type of porous material could alter the flow field to reduce the electrolysis voltage (0.026 V at 25,000 A/m2) and increase the electrolysis efficiency (4.78% at 25,000 A/m2). In addition, from the theoretical point of view, the mechanism of CO2 enrichment and conversion in SOFCs/SOECs has been clarified.
-
(4)
A phase-field model was established to describe the morphological evolution of a porous electrode in a SOFC. The reductions in the three-phase boundary (TPB) density and performance caused by Ni coarsening at high temperature were evaluated using this model. At the operating current density of 4000 A/m2, the total overpotential increased from the initial value of 0.176–0.191 V after 3750 h, and the activation overpotential increased by 0.012 V at 850 °C. In addition, the effects of the Ni content and other microstructural parameters were considered. Using this model, the real microstructure of the porous electrode and the related parameters can be characterized.
-
(5)
A 3D kinetic Monte Carlo (KMC) model was developed to study the sintering kinetics and microstructural evolution of SOFC composite electrodes during the sintering process. The catalytic activity of the cathode materials, which based on the triple-point-boundary length, porosity, and tortuosity were calculated during KMC sintering. This model also provides a real microstructure for further research on the effects of microstructure on fuel cell electrochemistry and performance.
-
(6)
A pore-scale Lattice–Boltzmann model was established to simulate the reactive transport processes in the cathode functional layer of the SOFC. The coupled effects of oxygen diffusion and charge transport in the nanometer-scale functional layer are fully considered. The effects of microstructure on cell performance were investigated in terms of species distribution, reactive area, and reaction rate, thereby providing a theoretical basis for optimizing the electrode structure.
3.2 Exhaust gas catalytic combustion technology
Water- and CO2-resistant perovskite and hexaaluminate catalysts (La0.8Sr0.2Al0.5Mn0.5O3−δ and LaMnAl11O19, respectively) for SOFC off-gas combustion were successfully synthesized and deposited on a honeycomb ceramic substrate using a dip-coating technique. Figure 3 shows a schematic diagram of the preparation process. To reduce the difficulty in sealing the SOFC stack, the pressure drop of the catalytic combustor was reduced to the greatest extent possible by using a monolithic catalyst with a hole through it.
A kW-level SOFC catalytic combustion burner and the testing system were established, as shown in Fig. 4.
The conversion rates of H2 and CO achieved using the self-developed monolithic catalyst and catalytic burner are shown in Fig. 5 for typical SOFC simulated exhaust gas with high water content. During the operation period, the conversion rates of H2 and CO were higher than 99% and 95%, respectively, without any significant decay.
3.3 SOEC materials, cells, and stacks for CO2 conversion
3.3.1 SOEC materials
New perovskite electrode materials such as La0.4Sr0.6Co0.2Fe0.7Nb0.1O3−δ (LSCFN) and Sr2Fe1.3Co0.2Mo0.5O6−δ have been developed via the in situ precipitation of nanoparticles (Yang et al. 2019a, b) and demonstrated to facilitate CO2 reduction in SOECs. The precipitation process is shown in Fig. 6. In our previous work, a high current density of 0.442 A/cm2 and a low polarization resistance were obtained at 1.5 V with pure CO2 at 800 °C due to the excellent CO2 adsorbability of LSCFN.
Yang et al. (2020) reported a one-step synthetic method to fabricate Sr2Fe1.3Co0.2Mo0.5O6−δ-Gd0.1Ce0.9O2−δ composite electrode materials for symmetrical SOFCs. These materials also show great promise for application in SOECs.
3.3.2 Anode-supported single cells
Anode-supported SOFC/SOEC single cells with sizes of 156 mm × 70 mm were successfully fabricated with good quality and high yield by tape casting and sintering (Fig. 7). The active area of the oxygen electrode was approximately 85 cm2. The cell production process can also be scaled up for stack development.
3.3.3 Stack development and performance
We have developed a unique stack design with an open-air structure that can be integrated into a more extensive SOFC/SOEC system. As shown in Fig. 8, the kW-level stack for SOEC applications consists of 30 cells.
Figure 9a shows the electrolysis results obtained with different steam contents. Higher steam contents resulted in lower electrolytic voltage. At 750 °C, the electrolytic voltage was only 35.6 V with 90%H2O/10%H2 under 29 A, a hydrogen electrode flux of 11.62 L/min, and an oxygen electrode flux of 83 L/min. The calculated rates of H2O conversion and hydrogen production are shown in Fig. 9b. The conversion rate of H2O was 58%, and the hydrogen production rate reached 6.06 L/min. The electrolytic efficiency \(\eta_{LHV}\) was determined using the following equation:
where, VH2 is the electrolyzing rate of H2, LHVH2 is the lower heating value of H2 (LHVH2 = 3.00 kWh/Nm3 for electrolysis), Pel is the electric energy consumption, Uc is the applied voltage per single cell, and \(\eta_{\rm F}\) is the Faraday efficiency, which was close to 100% in our experiments. The electrolytic efficiency during hydrogen production in this study reached 105.3%. The results indicate that that as the steam content increased, the electrolytic efficiency increased, while the conversion rate of H2O slightly decreased.
Figure 10 shows the electrochemical performance of the stack for H2O/CO2 co-electrolysis. The results indicate that the electrolytic voltage of H2O/CO2 co-electrolysis was negatively correlated with the steam content. At 750 °C, the electrolytic voltage was 37, 38.1, or 38.8 V for 60%H2O/30%CO2/10%H2, 45%H2O/45%CO2/10%H2, or 30%H2O/60%CO2/10%H2 under 32 A, respectively.
4 Conclusions
In this work, a set of multi-scale SOFC and SOEC models was established to analyze the mechanisms of internal heat and mass transfer and optimize the structure and operating parameters. SOFC stack flue gas catalytic combustion and SOEC technology were evaluated for CO2 capture and conversion in IGFCs. Perovskite and hexaaluminate catalysts were successfully fabricated. The kW-level catalytic combustion burner, the perovskite electrode materials, and a single SOEC were developed. Furthermore, the kW-level stack was assembled, and the electrochemical performance was evaluated. In future studies, the conversion rates of H2 and CO in the catalytic combustion burner will be improved, and the long-term stability of the SOEC system will be verified.
References
Damo U, Ferrari M, Turan A, Massardo A (2019) Solid oxide fuel cell hybrid system: a detailed review of an environmentally clean and efficient source of energy. Energy 168:235–246
Discepoli G, Cinti G, Desideri U, Penchini D, Proietti S (2012) Carbon capture with molten carbonate fuel cells: experimental tests and fuel cell performance assessment. Int J Greenh Gas Control 9:372–384
Ebbesen SD, Høgh J, Nielsen KA, Nielsen JU, Mogensen M (2011) Durable SOC stacks for production of hydrogen and synthesis gas by high temperature electrolysis. Int J Hydrog Energy 36(13):7363–7373
Hoque MA, Lee S-b, Park N-K, Kim K (2012) Pd–Pt bimetallic catalysts for combustion of SOFC stack flue gas. Catal Today 185:66–72
Kawabata M, Kurata O, Iki N (2012) Analysis of IGFC with energy recuperation and corbon dioxide seperation unit. Proc ASME Turbo Expo 6:11–15
Leea S, Ahn G, Kim K (2013) Optimum GHSV for the catalytic combustor of an 1.1 kW solid oxide fuel cell (SOFC) system. ECS Trans 51:117–127
Liese E (2010) Comparison of preanode and postanode carbon dioxide seperation for IGFC systems. J Eng Gas Turbine Power 132:1–8
Li M, Rao AD, Brouwer J, Samuelsen G (2010) Design of highly efficient coal-based integrated gasification fuel cell power plants. J Power Sources 195:5707–5718
Li P, Liu C, Huang B, Fan W, Wang Q, Li C, Singh S, Ba L (2018) Process simulation and energy analysis for IGFC system. Comput Appl Chem 35:988–996
Peng S, Han M (2009) Development of coal/carbon based solid oxide fuel cell. Chin J Nat 31:187–192 ((in Chinese))
Rudra M, Kim HT (2010) A simulation study of SOFC for IGFC power generation using aspen plus. J Energy Clim Change 5:24–35
Sung JG, Kim T, Jung HK, Kim H, Chung JS (2018) Catalytic combustion of SOFC stack flue gas over CuO and Mn2O3 supported by La0.8Sr0.2Mn0.67Cu0.33O3 perovskite. AIChE J 64:940–949
Trembly JP, Gemmen RS, Bayless DJ (2007) The effect of IGFC warm gas cleanup system conditions on the gas–solid partitioning and form of trace species in coal syngas and their interactions with SOFC anodes. J Power Sources 163:986–996
Vepřek S, Cocke DL, Kehl S, Oswald HR (1986) Mechanism of the deactivation of Hopcalite catalysts studied by XPS, ISS, and other techniques. J Catal 100:250–263
Xu M, Zhang X (2012) Present situation and optimization of energy consumption structure in China. Henan Sci 30:1157–1162
Yang Z, Ma C, Wang N, Jin X, Jin C, Peng S (2019a) Electrochemical reduction of CO2 in a symmetrical solid oxide electrolysis cell with La0.4Sr0.6Co0.2Fe0.7Nb0.1O3−δ electrode. J CO2 Util 33:445–451
Yang Z, Wang N, Ma C, Jin X, Lei Z, Xiong X, Peng S (2019b) Co-electrolysis of H2O-CO2 in a solid oxide electrolysis cell with symmetrical La0.4Sr0.6Co0.2Fe0.7Nb0.1O3−δ electrode. J Electroanal Chem 836:107–111
Yang YR, Li SS, Yang ZB, Chen Y, Zhang PP, Wang YH, Chen FL, Peng SP (2020) One step synthesis of Sr2Fe1.3Co0.2Mo0.5O6−δ–Gd0.1Ce0.9O2−δ for symmetrical solid oxide fuel cells. J Electrochem Soc 167:084503
Zhang X, O’Brien JE, O’Brien RC, Hartvigsen JJ, Tao G, Housley GK (2013) Improved durability of SOEC stacks for high temperature electrolysis. Int J Hydrog Energy 38:20–28
Zheng Y, Li Q, Guan W, Xu C, Wu W, Wang W (2014) Investigation of 30-cell solid oxide electrolyzer stack modules for hydrogen production. Ceram Int 40:5801–5809
Acknowledgements
This work was financially supported by the National Key R&D Program of China (2017YFB0601904).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Z., Lei, Z., Ge, B. et al. Development of catalytic combustion and CO2 capture and conversion technology. Int J Coal Sci Technol 8, 377–382 (2021). https://doi.org/10.1007/s40789-021-00444-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-021-00444-2