Abstract
Experimental research was carried out on the manufacturing of bio-coal briquettes from a blend of two different types of low-quality coal and biomass waste in the absence of coal carbonization, where the third blend of the material was fermented by adding a bio-activator solution before pressurizing the components into briquettes. The coal samples from Caringin–Garut Regency (BB–Garut) had a low calorific value and a high sulfur content (6.57 wt%), whereas the coal samples from Bayah–Lebak Regency (BB–Bayah) had a higher calorific value and a lower sulfur content (0.51 wt%). The biomass added to the coal blend is in the form of fermented cow dung (Bio–Kohe), and it had a calorific value of 4192 kcal/kg and a total sulfur content of 1.56 wt%. The main objective of this study is to determine the total decrease in the sulfur content in a blend of coal and biomass in which a fermentation process was carried out using a bio-activator for 24 h. The used bio-activator was made from Garant® (1:40) + molasses 1 wt%/vol, and its used amount was 0.2 L/kg. Also, the total sulfur content in the blend was 1.00 wt%–1.14 wt%, which fulfills the necessary quality requirements for non-carbonized bio-coal briquettes. The pyritic and sulfate content in the raw coal was dominant, and the organic sulfur, when fermented with Garant®, was found to be less in the produced bio-coal briquettes by 38%–58%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Coal is an organic mineral that can burn and produce energy, and it is formed from plant deposits that change in shape overtime because of the natural physical and chemical processes. The formation of coal begins with a biochemical process, followed by geochemical and physical processes in which a change occurs in the coal material, where the young coal changes from peat to lignite and from bituminous to anthracite (Diessel 1992). The factors that play a role in the geochemical and physical processes are mainly the temperature, pressure, and time. Generally, coal deposits can be formed well if they occur in basin areas, which have become less overtime. Also, the coal structure is complex, as it is mainly an organic–containing porous solid, and it is important as an energy source and as chemical source material. The physical and chemical properties of most of the types of coal are extremely sensitive to air oxidation (Gethner 1987), and the quality of coal as a fuel is determined by several factors, namely, the percentage of fixed carbon, moisture, volatile material, calorific value, mineral content, and sulfur content (Deska et al. 2018).
In addition to high-rank coal, Indonesia also has low lignite coal resources with a calorific value of less than 4200 kcal/kg, and they are spread in large quantities across the islands of Kalimantan, Sumatra, and Java (Huda et al. 2012). In Caringin village, Garut Regency, West Java Province, there is low calorific coal with a high content of ash and sulfur, but it is not well-known on the market. On the contrary, the fine coal from Pamumbulan, Bayah Subdistrict, Lebak Regency, Banten Province, has very low selling prices (rejected coal), although it has a higher calorific value in comparison with the coal from Caringin–Garut. Therefore, in order to use low-rank coal, it has to be blended with higher-rank coal to meet the required coal quality specifications according to its use. Coal blending mainly comprises mixing two or more types of coal to combine their properties and qualities so they can match the desired coal specifications by the consumers (Akar et al. 2009). The main benefit of blending low-rank and high-rank coal is meeting the necessary energy content requirements and other specifications regarding the moisture content, ash content, sulfur content, and calorific value so as to properly manufacture bio-coal briquettes. Generally, coal briquettes are made through briquetting coal–based mixed particles, binders, and other additives at a certain pressure, forming certain shapes and sizes. According to Borowski and Hycnar (2013), the process of making coal briquettes consists of drying, homogenization, the addition of binders, and pressurization using mechanical devices.
To improve the quality of the blended coal and biomass and reduce the total sulfur content, a fermentation study trial was conducted using Garant® that contains sp. Bacillus, sp. Lactobacillus, and Saccharomyces as bio-activators. Reducing the sulfur content in the coal can be done by using a desulfurization process that employs different microorganisms (Deska et al. 2018). According to Abdel-Khalek and El-Midany (2013), mycobacterium phlei can improve the process of separating coal from impurities, thus reducing the total sulfur and ash contents by 0.9% and 2.1%, respectively. Also, mesophilic acidithiobacillus ferrooxidans bacteria is most commonly used in coal biodesulfurization, including the bioleaching of pyritic sulfur (Aller et al. 2001; Cardona and Márquez 2009; Kim et al. 2013; Rossi 2014; Abdel Khalek and El-Midany 2013).
This study aims to measure, evaluate, and describe the changes in the characteristics of bio-coal briquette samples during combustion. It also aims to obtain a better understanding of the fermentation process of coal as well as the phenomenon of increasing the quality of bio-coal briquette fuel. The fermentation of low-quality coal blends by adding biomass aims at reducing the total sulfur content and increasing the volatile matter in bio-coal briquettes. However, fermentation using bacteria results in the oxidation of the sulfur in coal and biomass.
2 Experiment
2.1 Geological setting and material, apparatus and experimental procedure
The experimental materials used in this study were a low-quality coal sample taken from Pamubulan, Bayah–Lebak, Banten (BB–Bayah), a coal sample taken from Caringin–Garut, West Java (BB–Garut), and biomass waste in the form of fermented cow dung. PT Cahaya Kharisma Jaya Bandung (Bio–Kohe) and the bio-activator are distributed and produced by PT Makmur Bumi Indah Organik and PT. Hariff Daya Tunggal Engineering, Bandung Indonesia. Garant® is a microbial culture that contains Bacillus sp. etc., and its function is to accelerate the decomposition of organic materials by fermenting both their solid and liquid components.
BB–Bayah is located at coordinates (6°55′5″ S, 106°18′12″ E). Brownish–black, color–black, glazed glass–dull, brown–brown–black, brittle, including sub-bituminous types coal. According to Sujatmika and Santosa (1992), Bayah–Lebak coal is included in the Bayah Formation Conglomerate (Teb). Also, BB–Garut is located at coordinates (07°28′54″ S, 107°30′42″ E) at an elevation of 307–311 m below sea level. The blackish–brown coal, which is easily destroyed (friable), includes brown coal (lignite) with impurity minerals such as iron/pyrite oxides. The deposition of the coal layer thickness was found to be between 5 and 30 cm at a stance of N 283° E with a slope of 28°–34°, where the thickness of the covering soil layer was > 1 m. According to Alzwar et al. (1992), Caringin–Garut coal deposits are part of the Late Miocene Span Formation. The rocks that make up such formations include sandstone tuffs, tuffs pumice, clay stones, conglomerates, and lignite containing mollusks. Typically, this kind of coal contains low fixed carbon of about 49.48 wt%–53.39 wt%, an energy content of 5761–7749 kcal/kg, a high ash content of 17.41 wt%–31.63 wt%, and a sulfur content of 0.5 wt1%–6.57 wt%. In general, selecting coal with a high sulfur content is more suitable for studying the desulfurization of coal using microorganisms (Widodo et al. 2018). The typical properties of the coal and biomass waste from the study site are described in Tables 1 and 2.
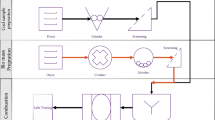
The bio-coal briquette formulation was performed by blending 90% of BB–Bayah, 10% of BB–Garut (BB–91), and Bio–Kohe with different proportions of 9:1, 8:2, and 7:3 (B3T–91, B3T–82, and B3T–73), respectively. Then, they were fermented with a Garant® bio-activator that is made from Garant®, aquadest (1:40), and molasses 1 wt%/vol. Also, the amount of the used bio-activator was 0.2 L/kg, and the fermentation process was carried out for 24 h while observing the changes in temperature and pH every hour.
2.2 Analysis
A diamond saw cut the selected coal sample into pieces of about 2–5 cm2, and their rough surfaces were wet polished with a series of abrasive papers at low speed. The freshly polished coal sample was then quickly dried with purging gas and immediately placed on the measuring place. All the measurements were performed in atmospheric air at room temperature for the petrographic analysis and for determining the maceral and mineral matter content in the sample by using a Nikon Ipol–50 optical microscope with reflected light.
Then, a fuel combustion test was carried out on the bio-coal briquettes using a gas emission detector (Pgas 41). The briquettes were formed by blending fermented coal and biomass (B3T–73, B3T–82, and B3T–91) with BB–Bayah and BB–Garut (BB–91), which were processed as bio-coal briquettes for determining the amount of CO (carbon monoxide), H2S (hydrogen sulfide), O2 (oxygen), and CH4 (methane) emissions.
The primary components and chemical structure of the coal and biomass were analyzed by Shimadzu FTIR (IRTracer–100) using the ATR method, and the JASCO IR Mentor Pro 6.5 software was used for the spectral analysis. Other analysis methods were previously described with their detailed operation techniques, and the band assignments can be found elsewhere (Mursito and Hirajima 2012; Mursito et al. 2010, 2011).
X–ray diffraction (XRD) measurements were carried out with CuKα radiation using the Shimadzu X–ray diffractometer (XRD–7000 Series Maxima), where the radiation was generated at 30 mA and 40 kV, a scanning range of 3°–40°, and a scanning speed of 2°/min.
The sample was chemically analyzed using the standard analytical methods. The proximate analysis of the coal samples was determined according to the ASTM standards (D 3172–89), and the ash analysis of the samples was done using the standard chemical analytical methods. The carbon content was determined using an atomic absorbance spectrophotometer (Perkin–Elmer, model 2400), and the total sulfur content was ascertained by the Eschka method (ASTM D 3177). Also, the sulfur forms were determined by ASTM D 2492.
3 Results and discussion
3.1 Coal sample and products properties
Based on the results of the petrographic analysis, the BB–Garut coal samples consisted of huminite/vitrinite (50%–60%), liptinite (25%–35%), and minerals (10%–15%). The huminite/vitrinite consisted of atrinite/vitrodetrinite, densinite/collodetrinite, and gelinite, and the liptinite comprised kuntinite, resinite, and sporinite. The minerals were composed of calcite, quartz, and pyrite (Fig. 1). Since the BB–Garut coal contained pyrite minerals, there must have been a hydrothermal process in its area, which plays a big role in the formation of coal deposits.
Meanwhile, the results of the petrographic analysis of the BB–Bayah coal were known as coal detritus, which consisted of vitrinite (80%–90%), liptinite (10%–15%), and minerals (4%–6%). The huminite consisted of collotelinite, atrinite, and gelinite, and the liptinite comprised kutinite and sporinite. The minerals were composed of quartz and pyrite (Fig. 1). According to Santoso and Daulay (2007), the Rv max value of the BB–Bayah coal was 0.51%–0.88%, the vitrinite reflectance was 0.53–1.23, and the mineral matter was 5.2%. The vitrinite mineral in the low-rank coal can be easily hydrogenated and burned just like the exinite (liptine) maceral in the low–rank coal, which has a high hydrogen content. However, the inertinite maceral, which existed in all the coal ratings, is low in hydrogen. The low-rank coal, microscopically, is dominated by huminite, whereas the higher–rank coal is dominated by vitrinite.
The XRD analysis of the coal samples aims to determine the mineral matter of the coal. The minerals in this coal were not burned, and they became a part of the ash after the combustion of the briquettes. The results of the XRD analysis showed that the BB–Garut coal contained quartz (SiO2), kaolinite (Al2Si2O5(OH)4), and pyrite (FeS2) (Fig. 2), whereas the BB–Bayah coal contained quartz (SiO2), kaolinite (Al2Si2O5(OH)4), pyrite (FeS2), and halloysite Al2Si2O5(OH)4. However, these minerals were detected in the results of the petrographic analysis with a magnification of 100× (Fig. 1). Also, the Bio–Kohe material contained halloysite Al2Si2O5(OH)4, quartz (SiO2), and calcite (CaCO3). Generally, kaolinite, quartz, and calcite turn into ash after the combustion of bio-coal briquettes. Thus, the higher the levels of these minerals in the coal, the higher the ash content of the remaining combustion.
The results of the study showed that the reduction of the total sulfur content in the coal and biomass blend occurred when they were fermented with a composition of 9/1, 8/2, and 7/3 (Table 2). The total sulfur content in the fermented bio-coal briquette products was about 1.00 wt%–1.14 wt%, which is less than that in the blended coal with the initial biomass of 2.80 wt% and 1.56 wt%, respectively. The organic sulfur content in the bio-coal briquette products was also found to be reduced to blended coal and Bio–Kohe for about 44%–58% and 38%–53%, respectively (Fig. 3). The pyritic and sulfate sulfur content in the raw coal was dominant, and it was also found to be less in the bio-coal briquette products when blended with Bio–Kohe or fermented by Garant®.
The fermentation process of the fermented coal and biomass blend initially had the same temperature, which was about 28°C (Fig. 4). However, it then gradually rose to 29.6°C for 3–4 h. Afterward, the fermentation temperature tended to fall to 28°C after 4–12 h of the fermentation process, and then, it would remain stable at a temperature of 28°C until the end of the process. From this description, it can be concluded that the fermentation process lasts for 11–12 h with a high fermentation rate occurring for 1–3 h. Also, the amount of the added biomass affected the rise in the temperature during the fermentation process. The material blending of B3T–73 with a higher temperature of 29.3°C was compared with the samples B3T–82 (29.1°C) and B3T–91 (28.8°C).
The pH condition at the beginning of the fermentation experiment was acidic, which was indicated by a scale of 6.3 to 6.5 (Fig. 4). During the fermentation process, the pH gradually increased up to (pH 7) for 1–7 h, which is considered normal, as the condition was stable until the end of the fermentation process. Also, during the process, there was an indication of a sulfuric acid formation reaction occurring in the desulfurization process. The fermentation process took place after 7 h with a balanced acidity level (pH 7), indicating that the sulfuric acid formation reaction in the desulfurization process was over. This increasing pH condition only lasted for 1–7 h because of the influence of the biomass, which functioned as a buffer (the nature of organic fertilizer). The used bacteria for the desulfurization process have a catalytic character, and it is from the genus Bacillus, which uses sulfur as an energy source. Sulfuric acid is the final result of this reaction, which was why the pH of the surrounding environment became acidic. The process took place with the following reaction (Deska et al. 2018):
The decrease in the acidity of the fermentation process of the coal and biomass blend was due to the sulfur decomposition reactions that took place with the help of the bacillus bacterial catalysts, which can be used as a test process parameter in fermentation processes. This can be seen in the parameters of the coal and biomass blend fermentation processes that were carried out (Fig. 4).
The results of the proximate analysis of the BB–Garut coal samples showed that the total moisture content was 6.83 wt% and that the ash content was 31.63 wt%, which are higher than the results of the BB–Bayah coal samples, which had a total moisture content of 4.37 wt% and an ash content of 17.41 wt% (Table 1). This is in line with the ultimate analysis results of the total sulfur content of the BB–Garut coal (6.57 wt%), which was also greater than the total sulfur content of the BB–Bayah coal samples (0.51 wt%). However, the fixed carbon content of the BB–Garut coal was smaller than that of the BB–Bayah coal (7749 kcal/kg). Based on the results of the proximate and ultimate analyses, it is evident that the quality of the BB–Garut coal was lower than that of the BB–Bayah, especially in terms of the fixed carbon content as an energy source and the sulfur as an impurity. This was possible since the used biomass in the process was already fermented, and it was further fermented during the process. As a result, the energy stored in the initial biomass was also decomposed. In addition to the energy decomposition reactions in the hydrocarbon chains presented in the biomass, the sulfur decomposition process in the coal also used the released energy from the decomposition of the hydrocarbons in the biomass. Consequently, the amount of energy contained in the coal and biomass blend would decrease.
The samples of the BB–Garut coal have a calorific value of 5761 kcal/kg calories, including the low-quality coal (lignite), which is commonly used for coal blending (Huda et al. 2012), as it has a calorific value of more than 4200 kcal/kg. Based on the standard quality of the briquette raw materials and according to the regulations of the Indonesian National Standard 4931, 2010, the calorific value requirements of non-carbonized bio-coal briquettes is 4000–5000 kcal/kg for type B and 5000–6000 kcal/kg for type A. For that reason, the BB–Garut coal can be used as a material for producing coal briquettes, but it needs to be blended with coal that has a higher calorific value and a lower ash content.
During production, the oxygen content decreased with the increase in the coal–biomass ratio. Table 2 shows that the chemical properties of the material and the bio-coal briquettes are mainly affected by the coal–biomass ratio. With the increase of the coal–biomass ratio, a significant increase in the C content and a decrease in the O content were observed in the bio-coal briquettes. The oxygen content decreased from 17.83 wt% to 15.04 wt%, and the fermentation process influenced the increase of the carbon content from 71.34 wt% to 75.46 wt%. The variations in the contents of H and N in the bio-coal briquettes were insignificant. Nevertheless, the N content decreased by approximately 28%, which is possible due to the activity of the bio-activator.
3.2 SEM–EDX examination and the Gram-staining identification of selective bacteria results
A preliminary characterization of the isolates was drawn based on the observations of the cell growth on the agar plates, the Gram staining reaction, the microscopic observation of the cell dimensions, and other standard biochemical techniques (Hu et al. 2007). Photographs of the Gram–stained isolated strains of Garant® (B. cereus and B. licheniformis) from the liquid medium with a TPC of 9 CFU/mL were distinguished as Gram–positive bacterium (B. cereus). Also, the gram-negative bacterium (E. cloacae complex) and the K. pneumonlae ssp. pneumonlae strains, according to the micrographs, were found in the Bio–Kohe with a TPC of about 4.6 × 102 CFU/g (Fig. 5). The BB–Bayah and the BB–Garut contain B. megaterium, B firmus, and B. cereus with a TPC of about 3.5 × 102 CFU/g, respectively. The bio-coal briquettes of (B3T–91, B3T–82, and B3T–73) contain an E. cloacae complex, E. ludwigii, K. pneumonlae ssp. pneumonlae, and Escherichia. coli with a TPC of about 5.1 × 102, 2.6 × 102, and 8.6 × 102 CFU/g, respectively. These results indicate that most of the bacteria that were found grew from the used materials..
The selected SEM micrographs of the B3T–73 products and the Bio–Kohe are shown in Fig. 6. From the SEM micrographs, it is possible to determine the cellular dimensions: (2–2.5 µm) (B. firmus) and (< 1 µm) (biomass fibrous), respectively. Mineral matters, such as calcites and pyrites, also appeared in the SEM micrographs of the B3T–91, which indicates that the pyritic sulfur was predominantly produced by the minerals. The reduction of the sulfur content in the product shown in Fig. 7 can also be recognized from the SEM–EDS micrographs of the B3T–73, indicating that sulfur–reducing bacteria appeared and grew in the surrounding material that contains Fe and S. In addition, Si, O, and Al were also predominantly detected in the B3T–73, indicating that clay and quartz minerals can be the main source of ash.
3.3 FTIR examination results
FTIR spectrograms of the low-rank coal samples and biomass waste at different fermentation processes are shown in Fig. 8. The results show the change of the oxygen functional groups and the absorption of the relative intensity of the FTIR spectrograms with an increase in the coal–biomass ratio, where the increase of the coal–biomass ratio resulted in a decrease in the concentration of the oxygen–containing functional groups. The aliphatic hydrocarbon absorbance increased with the increase in the coal–biomass ratio. The unsymmetrical and symmetrical methylene stretching vibration peaks took place at 2915 cm −1and 2845 cm−1, and the relative intensity increased with the increase of the coal–biomass ratio, which shows that the aliphatic hydrocarbons possibly took part in the reactions that occurred during the fermentation process.
The strong bands in the 3600 cm−1 and 3670 cm−1 regions are the result of the Si–OH stretching modes of the silicate clay minerals (kaolinite). The clay is also identified in the OH stretching absorption region, particularly in the band of 3670 cm−1, distinguishing kaolinite in the raw coal (BB–Bayah and BB–Garut). This peak is somewhat diminished when it comes to the relative intensity at all the coal–biomass ratios; however, it can be observed in the FTIR spectrum of the raw coal, which is in agreement with the minerals identified by the XRD (Mursito et al. 2014, 2015, 2018).
The carboxyl band C=O groups at 1595 cm−1 did not change much in the fermentation process, and the aromatic ring C=C stretching absorbance at 1450 cm−1 increased with the rise of the coal–biomass ratio. The carboxyl absorbance became apparent with the coal–biomass ratio, showing the gradual regeneration of the carboxyl groups. This was probably due to the active carboxyl–containing groups, including aldehydes, ketones, and acids, which were easy to transform and difficult to accumulate (Wang et al. 2010). The C–O–R structure of ethers was observed at 1025 cm−1, as the ethers increased with the rise of the coal–biomass ratio. The distribution of the aromatic C–H at the out–of–plane bending modes at 870–800 cm−1 was also observed during the fermentation process. The relative intensity of the aromatic nuclei CH at 750–800 cm−1 tended to decrease at all the tested coal–biomass ratios.
3.4 Coal combustion test results
The results of the coal combustion test on the coal and biomass blend, which was fermented at (B3T–73, B3T–82, B3T–91) and (BB–91) and then processed as bio-coal briquettes, could be identified, where the identified resulting emissions were CO (carbon oxide), H2S (hydrogen sulfide), O2 (oxygen), and CH4 (methane). Based on the results of the bio-coal briquette sample fuel test, we came to the conclusion that all the types of coal and biomass, both fermented and unfermented (BB–91) blends, do not produce methane gas with an oxygen content ranging from 20.3 to 20.8 ppm.
The increase in the combustion temperature of the bio-coal briquette samples reduced the CO gas and increased the sulfur gas (Fig. 9). This was caused by the increase in temperature, which oxidized the CO gas, and it was formed from the bio-coal briquette combustion to CO2, resulting in flame. Conversely, with the increase in temperature, the sulfur oxidation process became SO2. The addition of biomass to the bio-coal briquettes material resulted in fewer emissions of H2S, which corresponds to a decrease in the total sulfur content of the bio-coal briquette material analysis of 1.00 wt%–1.14 wt% (Table 2). As a result, the total sulfur content in the coal and biomass blend decreased. However, with the decrease in the total sulfur content, there was also an increase in the volatile matter, and the produced exhaust gas from burning coal biofuel was reduced, whereas with the increase of the volatile matter in the bio-coal briquette fuel, the combustion briquettes became more flammable.
Based on the results of the bio-coal briquette sample fuel combustion test, it was also found that the detected sulfur gas concentration meant that the briquettes with Bayah–Lebak and Caringin–Garut coal materials, without the addition of biomass (BB–91), had a sulfur gas (H2S) content of 2.9 ppm. However, the briquettes that had fermented coal and biomass (B3T–73, B3T–82, and B3T–91) had less sulfur gas (H2S) amounts of 2.2, 2.3, and 2.7 ppm, respectively. The results of this study indicate that there is a tendency that the higher the amount of the added biomass blend to the coal for fermented briquettes, the lower the level of the produced sulfur gas.
4 Conclusion
The decrease in the total sulfur content in the bio-coal briquettes was caused by the desulfurization process, which involved the use of catalysts of microorganisms from the bacillus genus with aerobic fermentation processes in slightly acidic conditions. Based on the analysis of the coal and fermented biomass blend, it became known that the sulfur content was 1.00%–1.14% less than the sulfur content of the coal and biomass blend (1.56%–2.80%). The pyritic and sulfur content in the raw coal was dominant, and the organic sulfur was also found to be less in the bio-coal briquettes products by about 38%–58% when fermented by Garant®. The optimization of the use of bacteria to reduce the sulfur content in this coal has to be further investigated by focusing the research on the reaction mechanisms, process conditions, and end products by products from the reaction results.
References
Abdel khalek M, El-Midany AA (2013) Coal—Mycobacterium phlei interaction and its effect on coal cleaning. Tenside Surfactants Deterg 50(6):414–419
Abdel-Khalek MA, El-Midany AA (2013) Application of Bacillus subtilis for reducing ash and sulfur in coal. Environ Earth Sci 70:753–760
Akar G, Arslan V, Ertem ME, Ipekoglu Ü (2009) Relationship between ash fusion temperatures and coal mineral matter in some Turkish coal ashes. Asian J Chem 21(3):2105–2109
Aller Á, Martínez O, de Linaje JA, Méndez R, Morán A (2001) Biodesulphurisation of coal by microorganisms isolated from the coal itself. Fuel Process Technol 69(1):45–57
Alzwar M, Akbar N, dan Bachri S (1992) Geologi lembar Garut dan Pamengpeuk, Jawa, Lembar 1208-6 dan 1208-3, Sekala 1: 100.000. Pusat Penelitian dan Pengembangan Geologi. Departemen Pertambangan dan Energi
Borowski G, Hycnar JJ (2013) Utilization of fine coal waste as a fuel briquettes. Int J Coal Prep Util 33:194–204
Cardona IC, Márquez MA (2009) Biodesulfurization of two Colombian coals with native microorganisms. Fuel Process Technol 90(9):1099–1106
Deming WANG, Xiaoxing ZHONG, Junjie GU, Xuyao QI, (2010) Changes in active functional groups during low-temperature oxidation of coal. Mining Science and Technology (China) 20(1):35–40
Deska M, Głodniok M, Ulfig K (2018) Coal enrichment methods by using microorganisms and their metabolites. J Ecol Eng 19(2):213–220
Diessel CFK (1992) Coal bearing depositional systems. Springer, Berlin
Gethner JS (1987) The mechanism of the low-temperature oxidation of coal by O2: observation and separation of simultaneous reactions using in situ FT-IR difference spectroscopy. Appl Spectrosc 41(1):50–63
Hu Q, Dou M, Qi H, Xie X, Zhuang G, Yang M (2007) Detection, isolation, and identification of cadmium-resistant bacteria based on PCR–DGGE. Environ Sci 19:1114–1119
Huda M, Hudaya GK, Ningrum NS, Suganal S (2012) Economics and environmental considerations on the application of coal drying and coal blending technology in Indonesia. Jurnal Teknologi Mineral dan Batubara 8(3):152–163
Kim D-J, Gahan CS, Akilan C, Choi S-Y, Kim B-G (2013) Microbial desulfurization of three different coals from Indonesia, China and Korea in varying growth medium. Korean J Chem Eng 30(3):680–687
Mursito AT, Hirajima T (2012) Hydrothermal treatment of hokkaido peat; an application of FTIR and 13C NMR spectroscopy on examining of artificial coalification process and development. In: Theophile T (ed) Infrared spectroscopy—materials science, engineering and technology. InTech, Rijeka, pp 179–192
Mursito AT, Hirajima T, Sasaki K (2010) Upgrading and dewatering of raw tropical peat by hydrothermal treatment. Fuel 89:635–641
Mursito AT, Hirajima T, Sasaki K (2011) Alkaline hydrothermal deashing and desulfurization of low quality coal and its application to hydrogen-rich gas generation. Energy Convers Manag 52:762–769
Mursito AT, Saepuloh A, Dida EN (2014) Carbonization of Banten coal by adding water steam; changes on carbon functional groups and pore characteristics. Indones Min J 17(3):157–165
Mursito AT, Yuliyanti A, Jakah (2015) Hydrothermal carbonation of K-rich ash, value added energy engineering and CO2 mineral sequestration. Procedia Chem 14:56–65
Mursito AT, Hirajima T, Listiyowati LN, Sudarsono S (2018) Surface physicochemical properties of semi-anthracitic coal from Painan-Sumatra during air oxidation. Int J Coal Sci Technol 5(2):156–166
Rossi G (2014) The microbial desulfurization of coal. Adv Biochem Eng Biotechnol 142:147–167
Santoso B, Daulay B (2007) Comparative petrography of Ombilin and Bayah coals related to their origin. Indones Min J 10(09):1–12
Sujatmiko, Santosa (1992) Peta Geologi Lembar Leuwidamar, Jawa, skala 1:100.000, Peta Geologi Bersistem Indonesia. Pusat Penelitian dan pengembangan Geologi, Bandung
Widodo, Fatimah D, Estiaty LM (2018) Coal blending preparation for non-carbonized coal briquettes. In: IOP conference series: earth and environmental science, vol 118, p 012068
Acknowledgements
The authors express appreciation for the support of this research by the Indonesian Institute of Sciences (LIPI)—National Priority (PN-2019) Program—Center of Excellent for Advanced Functional Material produced from Mineral and Coal Resources (PUI—MFBSDMB) in conjunction with the Research Competitive Program—Productive Innovative Research (RISPRO LPDP) 2019–2021. The authors also acknowledge the support from the PT. Biofarma and R&D for tekMIRA. The publication of this research is supported by the funding scheme under the Deputy of Earth Sciences of the Indonesian Institute of Sciences (LIPI). Financial aid was partly provided by a RISPRO LPDP 2019–2021, PUI—MFBSDMB and In House Research (IHR) Program—Research Centre for Geotechnology—LIPI 2018–2019.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mursito, A.T., Widodo & Arifin, D.N. Characterization of bio-coal briquettes blended from low quality coal and biomass waste treated by Garant® bio-activator and its application for fuel combustion. Int J Coal Sci Technol 7, 796–806 (2020). https://doi.org/10.1007/s40789-020-00309-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-020-00309-0