Abstract
In this research, molecular structure models were developed respectively for Shenhua coal vitrinite concentrates (SDV) and inertinite concentrates (SDI), on the basis of information on constitutional unit of Shenhau coal and elemental analysis results obtained from 13C-NMR analysis characterization, FTIR analysis characterization, X-ray diffraction XRD and XPS analysis characterization. It can be observed from characterization data and molecular structure models that the structure of SDV and SDI is dominated by aromatic hydrocarbon, with aromaticity of SDI higher than that of SDV; SDV mainly consists of small molecule basic structure unit, while SDI is largely made from macromolecular structure unit. Based on bond-level parameters of the molecular model, the research found through the autoclave experiment that vitrinite liquefaction process goes under thermodynamics control and inertinite liquefaction process under dynamics control. The research developed an efficient directional direct coal liquefaction technology based on the maceral characteristics of Shenhua coal, which can effectively improve oil yield and lower gas yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Generally, direct coal liquefaction process chooses coal as the single feedstock and aims to produce distillable liquids. This process lends itself to coal with low level of inertinite if maximum liquids production is a prerequisite for the selection of process route and appropriate reaction conditions in a reaction system. China Coal Research Institute conducted a study on properties of Chinese coal for direct liquefaction (Shu and Xu 1997) and selected 15 types of coals suitable for direct liquefaction, with inertinite content of the top 10 all less than 7 %. However, Shenhua coal maceral has higher inertinite content level, with some even more than 60 %, than that of any other coals in China or even in the world. Therefore, it is necessary to study the maceral structures of Shenhua coal and consequent differences of their liquefaction performance and then develop an efficient directional direct liquefaction technology based on the characteristics of Shenhua coal maceral.
2 Experimental
2.1 Sample preparation
Shenhua coal samples (SDR) were collected from Shangwan coal mine, Shendong, Inner Mongolia. The raw coal was prepared by hand and a drifting process to obtain vitrinite concentrates (SDV) and inertinite concentrates (SDI). Industrial analysis and elemental analysis of Shenhua coal, vitrinite concentrates and inertinite concentrates are shown in Table 1, as well as petrographical analysis in Table 2.
2.2 13C-NMR analysis characterization
The research adopted an NMR AVANCE400 superconducting spectrometer produced by Bruker company, with a solid double resonance probe, and a 4 mm ZrO2 rotor; magic-angle speed was set at 8,000 Hz, resonance frequency 100.13 MHz, the sampling time 0.05 s, pulse wide 4 μs, cyclic delay time 5 s, and 7,000 scan times. The cross polarization (CP) technology was applied as well.
2.3 FTIR analysis characterization
Instrument type: US Nicolet 6700 FT-IR; test conditions: wavelength range: 4,000–400 cm−1, accuracy: wave number ≤0.1/cm; transmittance ≤0.1, resolution: 4 cm−1, number of scans: 32 times.
2.4 X-ray diffraction XRD
Instrument type: D/MAX 2550 VB/PC, manufacturer: Japan RIGAKU. Test method: wavelength: 1.54056 angstrom; copper target: 40 kV, 100 mA; scanning speed: 12 degrees/min, step: 0.02 degree.
2.5 XPS analysis characterization
XPS measurement is conducted with ESCALAB250 X-ray photoelectron spectroscopy. Use AlKa anode, and the power is 200 W. The full scan penetration power is 150 eV, step length 0.5 eV; narrow scan penetration power 60 eV, step 0.05 eV. Basic vacuum is 10−7 Pa. Take C1s (284.6 eV) as the calibration standard. The ordinate in the XPS spectra represents electronic counting, while the abscissa shows the electron binding energy (Binding Energy, B.E.).
2.6 Experiment of coal liquefaction reaction in autoclave
The coal liquefaction experiment used 0.5 L stirred autoclave, and the standard experimental conditions are as follows: coal input is 28 g (dry coal), initial hydrogen pressure is 10.0 MPa, the mass ratio of solvent to the coal is 1.5:1, the catalyst is Fe2O3, the additive amount of Fe is 3 % of the mass of dry coal, sulfur is the co-catalyst, n(S)/n(Fe) = 2. Heating rate is 8 °C/min till the set reaction temperature is reached. After the reaction, the temperature in the autoclave drops to 200 °C within 20 min. Products out of the autoclave include two parts: gaseous phase products, the component of which is analyzed with gas chromatography, and liquid–solid mixture which gets Soxhlet extraction separation successively with n-hexane and then tetrahydrofuran, finding that n-hexane soluble substance is oil, n-hexane insoluble and THF-soluble substances consist of pre-asphaltene and asphaltene (for short, PPA), THF-insoluble substances contains unreacted coal and ash. Analytical extraction procedures can be seen in literature (Shu et al. 2003).
2.7 Bench-scale unit for continuous direct coal liquefaction experiment
The continuous direct coal liquefaction experiment used a small continuous bench-sacle unit (BSU) with daily handling capacity of 120 kg of dry coal. Ordinary process unit has a reaction system of two bubbling bed reactors in series, while the reaction system of Shenhua unit has two reactors in series equipped with a circulating pump at the bottom and a gas–liquid separator on the top (Shu 2009).
3 Results and discussions
3.1 Macromolecular structure model of SDV and SDI
13C-CP/MAS NMR analytical characterization, X-ray diffraction, FTIR analytical characterization, X-ray diffraction XRD, and XPS analytical characterization were applied to the sample.
13C-CP/MAS NMR peak-fitting spectra of Shendong Shangwan SDV and SDI are illustrated respectively in Figs. 1 and 2, with the structure parameters shown in Table 3.
It can be observed from Figs. 1 and 2, 13C spectra of Shendong coal obviously has two peaks, one chemical shift located at aliphatic carbon of 0–60 ppm and the other at aromatic area of 90–165 ppm. However, these two samples at the aromatic area are obviously larger than those at the aliphatic area, and the aliphatic area of inertinite group is obviously smaller than that of vitrinite group.
Aromaticity (fa’) is an important parameter of coal structure. As can be seen from Table 5, aromaticity of inertinite is obviously higher than that of vitrinite. There is a big difference between inertinite at 67.47 % and vitrinit at 61.47 %. The comparison concludes that the biggest difference of aromatic carbon is caused by f ca (carbonyl), f Sa (alkylated aromatic) and f Ba (aromatic bridgehead). The vitrinite has more alkylated aromatic, while the inertinite has more aromatic bridgehead.
The ratio of aromatic bridgehead carbons to total ring carbons serves as an important parameter to research the macromolecular structure of coal, which can be used to calculate the size of aromatic clusters in coal structures. The types and number of aromatic structure units in the molecular structure of coal can be basically determined on the basis of this parameter. The hydrogen aromaticity is used to represent the concentration of aromatic hydrogen in coal structure, with its formula of calculation as below: Ha = (C/H)atom × f Ha . The values of Xb and Ha of these two samples are shown in Table 4.
As can be seen from the Xb values in Table 4, the ratios of aromatic bridgehead carbons to total ring carbons for SDV and SDI are very different. This indicates that these two coal samples have a big difference in macromolecular structure. The ratio of inertinite group is 0.307, much more than vitrinite group (0.154). With respect to Ha, the inertinite group is higher, indicating that its coal structure has more aromatic ring structures. Such result remains consistent with the result of Xb.
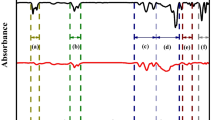
The Fig. 3 illustrates the FTIR spectroscopic analysis spectra of SDV concentrates and SDI concentrates.
The ratio of aliphatic hydrogen (Hal) and aromatic hydrogen (Har) in the coal serves as an important parameter to research the coal structure, and in general the ratio of 2,800–3,000 cm−1 aliphatic–CH stretching vibration absorption area to 700–900 cm−1 aromatic–CH bending vibration absorption area represents the proportion of aliphatic hydrogen to aromatic hydrogen. Peak-fitting information was obtained through peak-fitting analysis of the infrared spectra of two coal samples, and the relevant structure parameter is calculated with the information. See it in Table 5. As can be seen from Table 5, the value of Hal/Har of SDV is 2.18, much higher than that of SDI (1.36), which indicates the structure has more aliphatic structure; it can be seen through A2,800–3,000/A1,600 that SDI is much smaller than SDV, indicating there are more aromatic frame in its structures; the ratio of ν(CH2)/ν(CH3) leads to the conclusion that vitrinite and inertinite have more stretching vibration CH2. These all provide information for establishing macromolecular structure of coal.
The XPS C1s spectra and peak-fitting graphs of SDV and SDI samples are illustrated respectively in Figs. 4 and 5. As can be seen from the figures, carbon has four forms in the coal surface structure. The peak 284.6 eV is attributable to aromatic unit and alkyl-substituted aromatic carbon (C–C, C–H); the peak 286.3 eV is attributable to phenolic carbon or ether carbon (C–O); the peak 287.5 eV is attributable to carbonyl (C=O) and the peak 289.0 eV belongs to carboxyl (COO–). Table 6 shows the XPS C1s results and distribution of samples.
As can be seen from Table 6, the surface structure of SDI has less content of C–C, C–H, indicating less alkyl side chains. A part of alkyl side chains are transformed into phenolic hydroxyl group and ether linkage under the effect of fusainization, which leads to the relatively higher mass fraction of C–O in SDI.
The 5–40 XDR peak-fitting graphs for SDV and SDI samples are illustrated respectively in Figs. 6 and 7. XDR structure parameter is shown in Table 7.
Using the ACD/CNMR predictor combined with data in Table 1, the research established SDV and SDI structure models respectively, as illustrated in Figs. 8 and 9. A 13C NMR calculation of the structure models was carried out, the result of which fitted well with the experimental spectrum of the sample, as illustrated in Figs. 10 and 11.
3.2 Study on reactivity of vitrinite concentrates and inertinite concentrates
By using Dmol3 module of the Materials Studio software, the research did calculations of the structure of the SDV molecular model which had been optimized through molecular dynamics and molecular mechanics calculations, and obtained its bond order, bond length, and electronic layouts charge number. Bond order distribution of SDV is shown in Table 8.
The bond order parameters of molecular structure may determine the active sites where chemical bonds break down, thereby to infer the relations between molecular structure and its pyrolysis. According to the data in Table 8 and in combination of analyzing calculated bond length and electronic layouts number, SDI bond with order parameters smaller than one breaks down under a certain temperature. It is concluded that in SDV molecular structure, weak bridge bonds, ether bond, aliphatic side chain, hydrogenated aromatic ring, carbonyl functional groups and the distorted parts of aromatic layer are more likely to break down, producing CO, CO2, CH4, monocyclic aromatic hydrocarbons and aliphatic hydrocarbons with two carbon atoms, and first-level fragments with larger molecular weights. SDV releases by pyrolysis a large number of small molecular hydrocarbons, and the macromolecular structure of coal is basically destroyed. See SDV active sites and pyrolysis in Fig. 12.
Using Dmol3 module of the Materials Studio software, the research made calculations of the structure of the SDI molecular model, which has been optimized through molecular dynamics and molecular mechanics calculations, and obtained its bond order, bond length, and electronic layouts charge number. Bond order distribution of SDI can be seen in Table 9.
According to the data in Table 9, in combination of analyzing calculated bond length and electronic layouts number, SDI with bond order parameters smaller than one breaks down under a certain temperature. SDI bond breaking occurs in the location of hydrogenated aromatic ring and carbonyl functional group, producing CH4, CO2, bicyclic aromatics and first-level fragments with larger molecular weights. SDV and SDI vary greatly in this phase of pyrolysis. Bond breaking points of SDV are far more than that of SDI; SDV releases through pyrolysis large amounts of small molecule hydrocarbons, leading to coal macromolecular structure basically being destroyed, while SDI releases less small molecules and obtains through pyrolysis first-level fragments with larger molecular weights. See SDI active sites and the schematic representation of pyrolysis in Fig. 13.
The analysis of maceral bond order parameters demonstrates that liquefaction mechanisms for SDV and SDI are different. For SDV, it produces pyrolysis products with small molecular weights and has a high conversion rate. To improve the yield rate of its liquefaction is to quickly stabilize active free radicals and prevent the subsequent secondary pyrolysis. The whole process is subject to thermodynamics control.
For SDI, it produces pyrolysis products with large molecular weights. To improve the yield rate of its liquefaction is to increase hydrocracking activity and severity of its macromolecule products. The whole process is subject to dynamics control.
3.3 Autoclave experiment of SDV and SDI
To prove the difference of liquefaction reaction mechanisms between SDI and SDV, hydrogenation liquefaction experiments in an autoclave were conducted respectively. Autoclave heating rate remained at 8 °C/min, and the temperature was kept constant when it reached 455 °C. The results are shown in Table 10.
As can be seen from Table 10, when it reaches the reaction temperature with a constant heating rate, i.e. the reaction time is “0”, SDI oil yield (hexane solubles) is 43.03 %, rate of gasification is 7.45 %; in the same conditions, SDV oil yield goes up to 61.55 %, 18.52 % points higher than SDI, which indicates that small molecule structure are dominant in the molecular structure of SDV, and most of its pyrolysis products are small-molecule ones.
At the initial stage of the reaction, when the reaction is at its 30 min, SDI oil yield improves by 8.29 %, rate of gasification by 2.09 %; while for SDV within the same amount of time, the oil yield increases by 11.61 %, and rate of gasification is equal to that of SDI. The reason behind this is there is mainly pyrolysis happening in the initial stage of the reaction, i.e. the pyrolysis process which has not been finished in the heating process will continue in this stage. This experiment also shows that SDV is dominated by small molecule structure and its liquefaction is subject to thermodynamics control.
In the middle and late stage of the reaction, between 30 and 60 min of the reaction time, SDI oil yield improves by 7.63 %, reaching nearly 59 %, while rate of gasification increases only by 1.25 %; meanwhile, SDV oil yield increases only by 0.89 %, while rate of gasification is up 3.94 %, much higher than that of SDI.
The experiment results further demonstrate that SDI pyrolysis products have large molecular weights; the way to improve the yield rate of its liquefaction is therefore to increase the hydrocracking activity and severity of its macromolecule products. SDI liquefaction process is subject to dynamics control. On the other hand, SDV pyrolysis products have small molecular weights; the target products will have secondary pyrolysis and rate of gasification increases as reaction time and severity increases.
3.4 Efficient directional direct coal liquefaction technology fit for the macerals characteristics of Shenhua coal
Through bond order parameter analysis of molecular structure of SDV and SDI and with results of autoclave hydrogenation liquefaction experiment, the research has proved that liquefaction mechanisms of SDV and SDI are different.
As vitrinite and inertinite have different liquefaction mechanisms, and different products come from different processes, so there will inevitably be increase of gas yield if general direct coal liquefaction process is applied to Shenhua coal, which has a high content of inertinite.
Process that suits the macerals characteristics of Shenhua coal should be as follows: For vitrinite, a shorter reaction time is appropriate; reaction mechanism is mainly pyrolysis; there must be adequate active hydrogen to prevent the condensation of radical fragments of small molecules produced by pyrolysis. As regards inertinite, it requires longer reaction time and higher severity of the reaction, and the reaction mechanism is mainly hydrocracking, hence the need for high-activity catalysts and high-activity hydrogen-donating solvents.
If vitrinite and inertinite of Shenhua coal can be separated respectively and the subsequent two hydrogenation processes be set, one for vitrinite, the other for inertinite, then the problem of high rate of gasification caused by different reaction mechanisms of vitrinite and inertinite can be properly addressed. But it is industrially impossible.
Process that suits the maceral characteristics of Shenhua coal is to control the reaction time of SDV and SDI in the same reaction system to avoid secondary pyrolysis of SDV light products.
Shenhua direct coal liquefaction process is bespoke developed on the basis of the maceral characteristics of Shenhua coal. It sets a gas–liquid separator in the upper part of the reactor, thus light oils produced from one-way pyrolysis of vitrinite can be separated in time at the top of the reactor to prevent secondary pyrolysis thanks to short one-way reaction time of forced circulation reactor. Macromolecule fragments produced from inertinite pyrolysis will again be put into the reactor by circulating pump for catalytic hydrogenation in order to obtain more liquid products. The whole process realized efficient, directional, and effective conversion of the vitrinite and inertinite of Shenhua coal.
Under the same reaction conditions, the research conducted a comparative test on Shenhua coal with a small 0.12 t/d direct coal liquefaction continuous unit (BSU). The results are shown in Table 11, and the data of which clearly demonstrates that gas yield of Shenhua process is significantly lower than that of foreign process as Shenhua process avoids the secondary pyrolysis of generated oil, hence a significantly higher oil yield compared to that of foreign process; meanwhile, Shenhua process extends the reaction time of heavy oil and bitumen whilst avoiding the secondary pyrolysis of generated oil, so the conversion rate of Shenhua process is higher than that of foreign process.
4 Conclusions
-
(1)
From the constructed SDV and SDI molecular structure models, it can be seen that SDV is largely made of small molecules, while SDI is made of macromolecules.
-
(2)
According to data about SDV and SDI molecular model bond order, bond length and electronic layouts number, they are significantly different during pyrolysis, bond breaking points of SDV are far more than that of SDI; SDV releases through pyrolysis large amounts of small molecule hydrocarbons with its macromolecular structure basically being destroyed, while SDI doesn’t release through pyrolysis so much small molecules as fragments with larger molecular weights.
-
(3)
Findings of the autoclave experiment show that SDI pyrolysis products have large molecular weights. The way to improve the yield of its liquefaction is to increase hydrocracking activity and severity of its macromolecule products. SDI liquefaction process is subject to dynamics control. Since the molecular weights of SDV pyrolysis products are relatively small, the target products will have secondary pyrolysis as reaction time and severity increases, leading to a consequent increase in gas yield. Vitrinite liquefaction can be categorized as the process of thermodynamics control.
-
(4)
Shenhua direct coal liquefaction process that is developed on the basis of the maceral characteristics of Shenhua coal can effectively reduce its gas yield and improve oil yield.
References
Lin HL, Li KJ, Zhang XW, Li YL (2013a) Study on Shendong Shangwan coal and the structure characteristics of its maceral concentrates. Coal Convers 36(2):1–5
Lin HL, Li KJ, Zhang XW (2013b) Structural characteristics and model constructing of inertinite concentrates of Shangwan coal. J Fuel Chem Technol 41(6):641–648
Shu GP (2009) History and significance of the development of Shenhua coal direct liquefaction process. Shenhua Sci Technol 27(1):78–82
Shu GP, Xu ZG (1997) Direct coal liquefaction technology. Chin Coal 10:21–24
Shu GP, Shi ShD, Li KJ (2003) Coal liquefaction technology. Coal Industry Press, Beijing, pp 97–98
Acknowledgments
Supported by the National Engineering Laboratory of Direct Coal Liquefaction (MZY-16).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Shu, G., Zhang, Y. Research on the maceral characteristics of Shenhua coal and efficient and directional direct coal liquefaction technology. Int J Coal Sci Technol 1, 46–55 (2014). https://doi.org/10.1007/s40789-014-0003-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-014-0003-8