Abstract
Purpose of Review
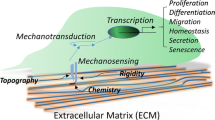
This review summarizes the current insights into stem cell fate determination by biomechanics. We also highlight recent findings that illustrate how mechanotransduction conveys changes in the extrinsic environment to program stem cell fate and how the intrinsic mechanical properties of stem cells regulate their functions.
Recent Findings
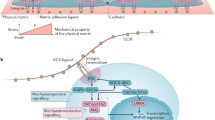
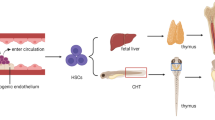
Emerging evidence in stem cell biology has shown that extrinsic mechanical cues, especially the viscoelasticity and topography of the extracellular matrix (ECM), influence many aspects of stem cell behavior, including self-renewal and differentiation. Cell-intrinsic mechanical properties of hematopoietic stem cells (HSCs) play crucial roles in maintaining HSCs attachment to the physical microenvironment (niche), which is critical for preserving HSCs quiescence and hematopoietic regeneration.
Summary
The intrinsic and extrinsic mechanical properties play important roles in controlling stem cell function and fate direction. Using biomechanics as a novel regulator of stem cell fate will provide insight into stem cell biology and aid in understanding the molecular mechanisms and crosstalk between biomechanics and stem cells. Ultimately, advances in the biomechanical regulation of stem cell fate will contribute to the development of regenerative medicine.

Similar content being viewed by others
References
Lee DA, Knight MM, Campbell JJ, Bader DL. Stem cell mechanobiology. J Cell Biochem. 2011;112(1):1–9. https://doi.org/10.1002/jcb.22758.
Zahari W, Hashim SN, Yusof MF, Osman ZF, Kannan TP, Mokhtar KI, et al. Immunomodulatory effect of cytokines in the differentiation of mesenchymal stem cells: a review. Curr Stem Cell Res Ther. 2017;12(3):197–206. https://doi.org/10.2174/1574888x11666160614103404.
Gentile P, Garcovich S. Advances in regenerative stem cell therapy in androgenic alopecia and hair loss: Wnt pathway, growth-factor, and mesenchymal stem cell signaling impact analysis on cell growth and hair follicle development. Cells-Basel. 2019:8(5). https://doi.org/10.3390/cells8050466.
Fukuda S, Bian H, King AG, Pelus LM. The chemokine GRObeta mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftment. Blood. 2007;110(3):860–9. https://doi.org/10.1182/blood-2006-06-031401.
Laterveer L, Lindley IJ, Heemskerk DP, Camps JA, Pauwels EK, Willemze R, et al. Rapid mobilization of hematopoietic progenitor cells in rhesus monkeys by a single intravenous injection of interleukin-8. Blood. 1996;87(2):781–8.
Laterveer L, Lindley IJ, Hamilton MS, Willemze R, Fibbe WE. Interleukin-8 induces rapid mobilization of hematopoietic stem cells with radioprotective capacity and long-term myelolymphoid repopulating ability. Blood. 1995;85(8):2269–75.
Ren R, Ocampo A, Liu GH, Izpisua Belmonte JC. Regulation of stem cell aging by metabolism and epigenetics. Cell Metab. 2017;26(3):460–74. https://doi.org/10.1016/j.cmet.2017.07.019.
Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20(5):303–20. https://doi.org/10.1038/s41580-019-0103-9.
Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20(11):1315–20. https://doi.org/10.1038/nm.3707.
Calvi LM, Link DC. The hematopoietic stem cell niche in homeostasis and disease. Blood. 2015;126(22):2443–51. https://doi.org/10.1182/blood-2015-07-533588.
Crane GM, Jeffery E, Morrison SJ. Adult haematopoietic stem cell niches. Nat Rev Immunol. 2017;17(9):573–90. https://doi.org/10.1038/nri.2017.53.
Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–9. https://doi.org/10.1038/nature04957.
Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–21. https://doi.org/10.1016/j.cell.2005.05.026.
Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–43. https://doi.org/10.1038/nature12612.
Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20(8):833–46. https://doi.org/10.1038/nm.3647.
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. https://doi.org/10.1038/nature09262.
Scadden DT. Nice neighborhood: emerging concepts of the stem cell niche. Cell. 2014;157(1):41–50. https://doi.org/10.1016/j.cell.2014.02.013.
Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20(11):1321–6. https://doi.org/10.1038/nm.3706.
Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25.
Argentati C, Morena F, Tortorella I, Bazzucchi M, Porcellati S, Emiliani C, et al. Insight into mechanobiology: how stem cells feel mechanical forces and orchestrate biological functions. Int J Mol Sci. 2019;20(21). https://doi.org/10.3390/ijms20215337.
Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. https://doi.org/10.1038/nature11885.
Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18(12):728–42. https://doi.org/10.1038/nrm.2017.108.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. https://doi.org/10.1016/j.cell.2006.06.044.
Chen Y, Ju L, Rushdi M, Ge C, Zhu C. Receptor-mediated cell mechanosensing. Mol Biol Cell. 2017;28(23):3134–55. https://doi.org/10.1091/mbc.E17-04-0228.
Rushdi M, Li K, Yuan Z, Travaglino S, Grakoui A, Zhu C. Mechanotransduction in T cell development, differentiation and function. Cells-Basel. 2020:9(2). https://doi.org/10.3390/cells9020364.
Mathieu S, Manneville JB. Intracellular mechanics: connecting rheology and mechanotransduction. Curr Opin Cell Biol. 2019;56:34–44. https://doi.org/10.1016/j.ceb.2018.08.007.
Wang N. Review of cellular mechanotransduction. J Phys D Appl Phys. 2017;50(23). https://doi.org/10.1088/1361-6463/aa6e18.
Roca-Cusachs P, Conte V, Trepat X. Quantifying forces in cell biology. Nat Cell Biol. 2017;19(7):742–51. https://doi.org/10.1038/ncb3564.
Wolfenson H, Yang B, Sheetz MP. Steps in mechanotransduction pathways that control cell morphology. Annu Rev Physiol. 2019;81:585–605. https://doi.org/10.1146/annurev-physiol-021317-121245.
Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–12. https://doi.org/10.1038/nrm3896.
Macri-Pellizzeri L, De-Juan-Pardo EM, Prosper F, Pelacho B. Role of substrate biomechanics in controlling (stem) cell fate: implications in regenerative medicine. J Tissue Eng Regen Med. 2018;12(4):1012–9. https://doi.org/10.1002/term.2586.
He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93(1):32–9. https://doi.org/10.1161/01.Res.0000080317.92718.99.
Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107(21):2733–40. https://doi.org/10.1161/01.Cir.0000068356.38592.68.
Yuan X, Zhang H, Wei YJ, Hu SS. Embryonic stem cell transplantation for the treatment of myocardial infarction: immune privilege or rejection. Transpl Immunol. 2007;18(2):88–93. https://doi.org/10.1016/j.trim.2007.05.003.
Fonseca SA, Costas RM, Pereira LV. Searching for naive human pluripotent stem cells. World J Stem Cells. 2015;7(3):649–56. https://doi.org/10.4252/wjsc.v7.i3.649.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. https://doi.org/10.1126/science.282.5391.1145.
Lakins JN, Chin AR, Weaver VM. Exploring the link between human embryonic stem cell organization and fate using tension-calibrated extracellular matrix functionalized polyacrylamide gels. Methods Mol Biol (Clifton, NJ). 2012;916:317–50. https://doi.org/10.1007/978-1-61779-980-8_24.
Przybyla L, Lakins JN, Sunyer R, Trepat X, Weaver VM. Monitoring developmental force distributions in reconstituted embryonic epithelia. Methods (San Diego, Calif). 2016;94:101–13. https://doi.org/10.1016/j.ymeth.2015.09.003.
Przybyla L, Lakins JN, Weaver VM. Tissue mechanics orchestrate Wnt-dependent human embryonic stem cell differentiation. Cell Stem Cell. 2016;19(4):462–75. https://doi.org/10.1016/j.stem.2016.06.018.
Kumari S, Vermeulen S, van der Veer B, Carlier A, de Boer J, Subramanyam D. Shaping cell fate: influence of topographical substratum properties on embryonic stem cells. Tissue Eng B Rev. 2018;24(4):255–66. https://doi.org/10.1089/ten.TEB.2017.0468.
zur Nieden NI, Turgman CC, Lang X, Larsen JM, Granelli J, Hwang YJ, et al. Fluorescent hydrogels for embryoid body formation and osteogenic differentiation of embryonic stem cells. ACS Appl Mater Interfaces. 2015;7(19):10599–605. https://doi.org/10.1021/acsami.5b02368.
Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, et al. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater. 2009;18:1–13; discussion −4. https://doi.org/10.22203/ecm.v018a01.
Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288(4):H1915–24. https://doi.org/10.1152/ajpheart.00956.2004.
Dasgupta I, McCollum D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. J Biol Chem. 2019;294(46):17693–706.
Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047–59. https://doi.org/10.1016/j.cell.2013.07.042.
Qin H, Hejna M, Liu Y, Percharde M, Wossidlo M, Blouin L, et al. YAP induces human naive pluripotency. Cell Rep. 2016;14(10):2301–12. https://doi.org/10.1016/j.celrep.2016.02.036.
Chung H, Lee BK, Uprety N, Shen W, Lee J, Kim J. Yap1 is dispensable for self-renewal but required for proper differentiation of mouse embryonic stem (ES) cells. EMBO Rep. 2016;17(4):519–29. https://doi.org/10.15252/embr.201540933.
David BG, Fujita H, Yasuda K, Okamoto K, Panina Y, Ichinose J, et al. Linking substrate and nucleus via actin cytoskeleton in pluripotency maintenance of mouse embryonic stem cells. Stem Cell Res. 2019;41:101614. https://doi.org/10.1016/j.scr.2019.101614.
Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, et al. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10(4):429–36. https://doi.org/10.1038/ncb1705.
Chowdhury F, Na S, Li D, Poh YC, Tanaka TS, Wang F, et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 2010;9(1):82–8. https://doi.org/10.1038/nmat2563.
Li D, Zhou J, Wang L, Shin ME, Su P, Lei X, et al. Integrated biochemical and mechanical signals regulate multifaceted human embryonic stem cell functions. J Cell Biol. 2010;191(3):631–44. https://doi.org/10.1083/jcb.201006094.
Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem. 2004;279(40):41263–6. https://doi.org/10.1074/jbc.C400352200.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. https://doi.org/10.1126/science.1151526.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. https://doi.org/10.1016/j.cell.2007.11.019.
Challenging Stem Cells. Cell. 2018:173(5):1063–5. https://doi.org/10.1016/j.cell.2018.05.010.
Ireland RG, Simmons CA. Human pluripotent stem cell mechanobiology: manipulating the biophysical microenvironment for regenerative medicine and tissue engineering applications. Stem Cells. 2015;33(11):3187–96. https://doi.org/10.1002/stem.2105.
Isomursu A, Lerche M, Taskinen ME, Ivaska J, Peuhu E. Integrin signaling and mechanotransduction in regulation of somatic stem cells. Exp Cell Res. 2019;378(2):217–25. https://doi.org/10.1016/j.yexcr.2019.01.027.
Anneren C, Cowan CA, Melton DA. The Src family of tyrosine kinases is important for embryonic stem cell self-renewal. J Biol Chem. 2004;279(30):31590–8. https://doi.org/10.1074/jbc.M403547200.
Liu H, Jiang D, Chi F, Zhao B. The hippo pathway regulates stem cell proliferation, self-renewal, and differentiation. Protein Cell. 2012;3(4):291–304. https://doi.org/10.1007/s13238-012-2919-3.
Rognoni E, Walko G. The Roles of YAP/TAZ and the hippo pathway in healthy and diseased skin. Cells-Basel. 2019:8(5). https://doi.org/10.3390/cells8050411.
Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15(6):642–56. https://doi.org/10.15252/embr.201438638.
Bae SJ, Kim M, Kim SH, Kwon YE, Lee JH, Kim J, et al. NEDD4 controls intestinal stem cell homeostasis by regulating the hippo signalling pathway. Nat Commun. 2015;6:6314. https://doi.org/10.1038/ncomms7314.
Shi J, Farzaneh M, Khoshnam SE. Yes-associated protein and PDZ binding motif: a critical signaling pathway in the control of human pluripotent stem cells self-renewal and differentiation. Cell Rep. 2020;22(2):55–61. https://doi.org/10.1089/cell.2019.0084.
Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12(2):126–31. https://doi.org/10.1038/nrm3049.
Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–50. https://doi.org/10.1002/jor.1100090504.
Gonzalez-Cruz RD, Fonseca VC, Darling EM. Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. Proc Natl Acad Sci U S A. 2012;109(24):E1523–9. https://doi.org/10.1073/pnas.1120349109.
Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7(9):733–6. https://doi.org/10.1038/nmeth.1487.
Benayahu D, Wiesenfeld Y, Sapir-Koren R. How is mechanobiology involved in mesenchymal stem cell differentiation toward the osteoblastic or adipogenic fate? J Cell Physiol. 2019;234(8):12133–41. https://doi.org/10.1002/jcp.28099.
Yang L, Wang L, Geiger H, Cancelas JA, Mo J, Zheng Y. Rho GTPase Cdc42 coordinates hematopoietic stem cell quiescence and niche interaction in the bone marrow. Proc Natl Acad Sci U S A. 2007;104(12):5091–6. https://doi.org/10.1073/pnas.0610819104.
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. https://doi.org/10.1038/nature10137.
Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79(1):144–52. https://doi.org/10.1016/s0006-3495(00)76279-5.
Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci U S A. 2006;103(44):16095–100. https://doi.org/10.1073/pnas.0604182103.
MacQueen L, Sun Y, Simmons CA. Mesenchymal stem cell mechanobiology and emerging experimental platforms. J R Soc Interface. 2013;10(84):20130179. https://doi.org/10.1098/rsif.2013.0179.
McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–95. https://doi.org/10.1016/s1534-5807(04)00075-9.
Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A. 2010;107(11):4872–7. https://doi.org/10.1073/pnas.0903269107.
Mohammed D, Versaevel M, Bruyere C, Alaimo L, Luciano M, Vercruysse E, et al. Innovative tools for mechanobiology: unraveling outside-in and inside-out mechanotransduction. Front Bioeng Biotechnol. 2019;7:162. https://doi.org/10.3389/fbioe.2019.00162.
De Arcangelis A, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 2000;16(9):389–95. https://doi.org/10.1016/s0168-9525(00)02074-6.
Mor-Yossef Moldovan L, Lustig M, Naftaly A, Mardamshina M, Geiger T, Gefen A, et al. Cell shape alteration during adipogenesis is associated with coordinated matrix cues. J Cell Physiol. 2019;234(4):3850–63. https://doi.org/10.1002/jcp.27157.
Hao J, Zhang Y, Wang Y, Ye R, Qiu J, Zhao Z, et al. Role of extracellular matrix and YAP/TAZ in cell fate determination. Cell Signal. 2014;26(2):186–91. https://doi.org/10.1016/j.cellsig.2013.11.006.
Murali A, Rajalingam K. Small rho GTPases in the control of cell shape and mobility. Cell Mol Life Sci. 2014;71(9):1703–21. https://doi.org/10.1007/s00018-013-1519-6.
Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, et al. The hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18(4):579–91. https://doi.org/10.1016/j.devcel.2010.03.007.
Hong W, Guan KL. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol. 2012;23(7):785–93. https://doi.org/10.1016/j.semcdb.2012.05.004.
Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1(8):661–73. https://doi.org/10.1016/1074-7613(94)90037-x.
Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96(6):3120–5. https://doi.org/10.1073/pnas.96.6.3120.
Clevers HSTEMCELLS. What is an adult stem cell? Science. 2015;350(6266):1319–20. https://doi.org/10.1126/science.aad7016.
Zhang P, Zhang C, Li J, Han J, Liu X, Yang H. The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res Ther. 2019;10(1):327. https://doi.org/10.1186/s13287-019-1422-7.
Ni F, Yu WM, Wang X, Fay ME, Young KM, Qiu Y, et al. Ptpn21 controls hematopoietic stem cell homeostasis and biomechanics. Cell Stem Cell. 2019;24(4):608–20.e6. https://doi.org/10.1016/j.stem.2019.02.009.
Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol. 2010;28(10):1123–8. https://doi.org/10.1038/nbt.1687.
Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463(7280):485–92. https://doi.org/10.1038/nature08908.
Svitkina TM. Ultrastructure of the actin cytoskeleton. Curr Opin Cell Biol. 2018;54:1–8. https://doi.org/10.1016/j.ceb.2018.02.007.
Svitkina T. The actin cytoskeleton and actin-based motility. Cold Spring Harb Perspect Biol. 2018;10(1). https://doi.org/10.1101/cshperspect.a018267.
Shin JW, Spinler KR, Swift J, Chasis JA, Mohandas N, Discher DE. Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. Proc Natl Acad Sci U S A. 2013;110(47):18892–7. https://doi.org/10.1073/pnas.1304996110.
Shin JW, Buxboim A, Spinler KR, Swift J, Christian DA, Hunter CA, et al. Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell Stem Cell. 2014;14(1):81–93. https://doi.org/10.1016/j.stem.2013.10.009.
Sedzinski J, Biro M, Oswald A, Tinevez JY, Salbreux G, Paluch E. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature. 2011;476(7361):462–6. https://doi.org/10.1038/nature10286.
Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–35. https://doi.org/10.1038/nature01148.
Mostowy S, Cossart P. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13(3):183–94. https://doi.org/10.1038/nrm3284.
Lam M, Calvo F. Regulation of mechanotransduction: emerging roles for septins. Cytoskeleton (Hoboken, NJ). 2019;76(1):115–22. https://doi.org/10.1002/cm.21485.
Mavrakis M, Azou-Gros Y, Tsai FC, Alvarado J, Bertin A, Iv F, et al. Septins promote F-actin ring formation by crosslinking actin filaments into curved bundles. Nat Cell Biol. 2014;16(4):322–34. https://doi.org/10.1038/ncb2921.
Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104(24):10158–63. https://doi.org/10.1073/pnas.0703478104.
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–8. https://doi.org/10.1038/367645a0.
Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17(3):313–9. https://doi.org/10.1038/nm.2304.
Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7. https://doi.org/10.1038/nm0797-730.
Diehn M, Clarke MF. Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst. 2006;98(24):1755–7. https://doi.org/10.1093/jnci/djj505.
Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12(2):133–43. https://doi.org/10.1038/nrc3184.
LeBleu VS, Neilson EG. Origin and functional heterogeneity of fibroblasts. FASEB J. 2020;34(3):3519–36. https://doi.org/10.1096/fj.201903188R.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–98. https://doi.org/10.1038/nrc.2016.73.
Roy Choudhury A, Gupta S, Chaturvedi PK, Kumar N, Pandey D. Mechanobiology of cancer stem cells and their niche. Cancer Microenviron. 2019;12(1):17–27. https://doi.org/10.1007/s12307-019-00222-4.
Zanotelli MR, Reinhart-King CA. Mechanical forces in tumor angiogenesis. Adv Exp Med Biol. 2018;1092:91–112. https://doi.org/10.1007/978-3-319-95294-9_6.
Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196(4):395–406. https://doi.org/10.1083/jcb.201102147.
Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16(3):225–38. https://doi.org/10.1016/j.stem.2015.02.015.
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. https://doi.org/10.1016/j.cell.2009.10.027.
Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of Cancer. Cancer Cell. 2016;29(6):783–803. https://doi.org/10.1016/j.ccell.2016.05.005.
Funding
This work is supported in part by the National Key Research and Development Project to FN (No. 2019YFA0801800) and the National Natural Science Foundation of China to FN (No. 32070916).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Linlin Jin, Ping Wang, and Fang Ni declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Role of Classical Signaling Pathways in Stem Cell Maintenance
Rights and permissions
About this article
Cite this article
Jin, L., Wang, P. & Ni, F. Biomechanical Regulation of Stem Cell Fate. Curr Stem Cell Rep 7, 30–38 (2021). https://doi.org/10.1007/s40778-020-00183-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-020-00183-1