Abstract
Purpose of Review
This review highlights recent advances in understanding of the role of tunneling nanotubules, with a focus on cancer: nanotubules are involved in chemoresistance, immunosuppression, and angiogenesis as well as metabolic changes in cancer cells.
Recent Findings
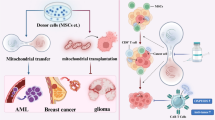
In recent years, we have seen the emergence of the importance of tunneling nanotubule (TNT) formation in intercellular communication of mesenchymal stem cells (MSC) with immune cells and with cancer cells. The formation of these TNT is extremely plastic and dynamic forming in few minutes between MSC and the cancer cell in their microenvironment. Recent work highlights the role of TNT in the migration of membrane material, endosomes, organelles, viral particles, and even proteins or microRNAs. Moreover, mitochondria migrate actively through the tunneling nanotubules from MSC into target cells, significantly impacting the energetic state of the target cells.
Summary
This review reports the role of intercellular communications between stromal cells, immune cells, and cancer cells through tunneling nanotubule. Here, we focus on the essential in the induction of chemo-resistance and in the metabolic changes associated with carcinogenesis through the transfer of mitochondria from MSC to target cells. Future research will have to clarify what are the mechanism involved in the induction of these TNT and how they are modulated in the context of carcinogenesis.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mittal R, Karhu E, Wang JS, Delgado S, Zukerman R, Mittal J, et al. Cell communication by tunneling nanotubes: implications in disease and therapeutic applications. J Cell Physiol. 2019;234(2):1130–46.
Zhang J, Whitehead J, Liu Y, Yang Q, Leach JK, Liu GY. Direct observation of tunneling nanotubes within human mesenchymal stem cell spheroids. J Phys Chem B. 2018;122(43):9920–6.
Babenko VA, Silachev DN, Zorova LD, Pevzner IB, Khutornenko AA, Plotnikov EY, et al. Improving the post-stroke therapeutic potency of mesenchymal multipotent stromal cells by cocultivation with cortical neurons: the role of crosstalk between cells. Stem Cell Transl Med. 2015;4(9):1011–20.
Yao Y, Fan XL, Jiang D, Zhang Y, Li X, Xu ZB, et al. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Rep. 2018;11(5):1120–35.
Zhu S, Bhat S, Syan S, Kuchitsu Y, Fukuda M, Zurzolo C. Rab11a-Rab8a cascade regulates the formation of tunnelling nanotubes through vesicle recycling. J Cell Sci. 2018;131(19):jcs215889.
Escobar P, Bouclier C, Serret J, Bièche I, Brigitte M, Caicedo A, et al. IL-1β produced by aggressive breast cancer cells is one of the factors that dictate their interactions with mesenchymal stem cells through chemokine production. Oncotarget. 2015;6(30):29034–47.
Nzigou Mombo B, Gerbal-Chaloin S, Bokus A, Daujat-Chavanieu M, Jorgensen C, Hugnot JP, Vignais ML. MitoCeption: transferring isolated human MSC mitochondria to glioblastoma stem cells. J Vis Exp 2017;(120). https://doi.org/10.3791/55245.
• Wang J, Liu X, Qiu Y, Shi Y, Cai J, Wang B, et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2018;11(1):11 (this paper demonstrate how transfer of mitochondria to target cells induce resistance of leukemia cells).
Errede M, Mangieri D, Longo G, Girolamo F, de Trizio I, Vimercati A, et al. Tunneling nanotubes evoke pericyte/endothelial communication during normal and tumoral angiogenesis. Fluids Barriers CNS. 2018;15(1):28.
Jash E, Prasad P, Kumar N, Sharma T, Goldman A, Sehrawat S. Perspective on nanochannels as cellular mediators in different disease conditions. Cell Commun Signal. 2018;16(1):76.
Uhl J, Gujarathi S, Waheed AA, Gordon A, Freed EO, Gousset K. Myosin-X is essential to the intercellular spread of HIV-1 Nef throughtunneling nanotubes. J Cell Commun Signal. 2018. https://doi.org/10.1007/s12079-018-0493-z.
Luz-Crawford P, Djouad F, Toupet K, Bony C, Franquesa M, Hoogduijn MJ, et al. Mesenchymal stem cell-derived interleukin 1 receptor antagonist promotes macrophage polarization and inhibits B cell differentiation. Stem Cells. 2016;34(2):483–92. https://doi.org/10.1002/stem.2254.
Luz-Crawford P, Torres MJ, Noël D, Fernandez A, Toupet K, Alcayaga-Miranda F, et al. The immunosuppressive signature of menstrual blood mesenchymal stem cells entails opposite effects on experimental arthritis and graft versus host diseases. Stem Cells. 2016;34(2):456–69. https://doi.org/10.1002/stem.2244.
Luz-Crawford P, Tejedor G, Mausset-Bonnefont AL, Beaulieu E, Morand EF, Jorgensen C, et al. Glucocorticoid-induced leucine zipper governs the therapeutic potential of mesenchymal stem cells by inducing a switch from pathogenic to regulatory Th17 cells in a mouse model of collagen-induced arthritis. Arthritis Rheumatol. 2015;67(6):1514–24.
•• Caicedo A, Fritz V, Brondello JM, Ayala M, Dennemont I, Abdellaoui N, et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci Rep. 2015;5:9073 (this paper visualize and demonstrate transfer of mitochondria to target cells through nanno tunneling).
Vignais ML, Caicedo A, Brondello JM, Jorgensen C. Cell connections by tunneling nanotubes: effects of mitochondrial trafficking on target cell metabolism, homeostasis, and response to therapy. Stem Cells Int. 2017;2017:6917941.
Polak R, de Rooij B, Pieters R, den Boer ML. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood. 2015;126(21):2404–14.
Murray LMA, Krasnodembskaya AD. Concise review: intercellular communication via organelle transfer in the biology and therapeutic applications of stem cells. Stem Cells. 2018;37:14–25. https://doi.org/10.1002/stem.2922.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cell:Cell Interactions in Stem Cell Maintenance
Rights and permissions
About this article
Cite this article
Jorgensen, C. Role of Tunneling Nanotubules in the Cross-Talk Between Mesenchymal Stem Cells and Their Target Cells. Curr Stem Cell Rep 5, 53–56 (2019). https://doi.org/10.1007/s40778-019-00154-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-019-00154-1