Abstract
Purpose of Review
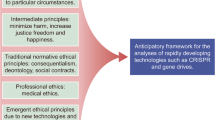
The purpose of this review is to highlight opportunities to incorporate Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) into stem cell research in ways that may alleviate long-standing ethical conflicts in biomedical research.
Recent Findings
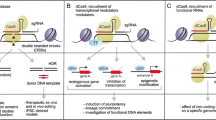
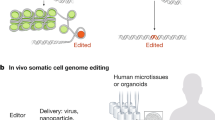
CRISPR has been incorporated into stem cell research that aims to understand developmental biology, model disease, test drugs, and develop therapies. In all of these domains, using CRISPR may alleviate, exacerbate, or create ethical conflicts. This review highlights ways in which CRISPR may facilitate more ethical research.
Summary
Genetically editing stem cells using CRISPR may lead to more ethical research by reducing reliance on animals used in disease modeling and drug screening research and reducing uncertainty ahead of first-in-human uses of stem cell therapies.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Scholefield J, Weinberg MS. The Application of CRISPR/Cas9 Technologies and Therapies in Stem Cells. Curr Stem Cell Rep. 2016;2(2):95–103. This review article provides a thorough summary of the current uses of CRISPR in stem cell basic and therapeutic research.
Waddington SN, Privolizzi R, Karda R, O’Neill HC. A Broad Overview and Review of CRISPR-Cas Technology and Stem Cells. Curr Stem Cell Rep. 2016;2(1):9–20.
Soldner F, Laganière J, Cheng Albert W, Hockemeyer D, Gao Q, Alagappan R, et al. Generation of Isogenic Pluripotent Stem Cells Differing Exclusively at Two Early Onset Parkinson Point Mutations. Cell. 2011;146(2):318–31.
• Johnston J, Zacharias RL. US Stem Cell Research Policy. Principles of Regenerative Medicine. 3rd ed. Cambridge: Academic Press; Forthcoming 2018. This chapter provides a thorough review of the historical and current policy, regulation, and law surrounding stem cell research in the United States and worldwide.
•• Hyun I. Bioethics and the future of stem cell research. New York: Cambridge University Press; 2013. This book provides a comprehensive review of the history and current landscape of stem cell research, ethics, and policy, including the research and clinical uses of stem cells, and the philosophical and practical research ethics issues related to the use of stem cells, chimerism and stem-cell based animal research, testing stem cell-based biologics in patients, and stem cell tourism.
Deleidi M, Yu C. Genome editing in pluripotent stem cells: research and therapeutic applications. Biochem Biophys Res Commun. 2016;473(3):665–74.
Lancaster MA, Knoblich JA. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science. 2014;345(6194).
Ronaldson-Bouchard K, Vujnak-Novakovik G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell. 2018;22(3):310–24.
Cowan CA. Genome editing: from modeling disease to novel therapeutics. Stem Cell Technologies Webinar; 2015. Available from: https://www.stemcell.com/technical-resources/area-of-interest/pluripotent-stem-cell-research/genome-editing/videos-webinars/genome-editing-from-modeling-disease-to-novel-therapeutics.html.
Kim EJ, Kang KH, Ju JH. CRISPR-Cas9: a promising tool for gene editing on induced pluripotent stem cells. Korean J Intern Med. 2017;32(1):42–61.
Park C-Y, Kim Duk H, Son Jeong S, Sung Jin J, Lee J, Bae S, et al. Functional Correction of Large Factor VIII Gene Chromosomal Inversions in Hemophilia A Patient-Derived iPSCs Using CRISPR-Cas9. Cell Stem Cell. 2015;17(2):213–20.
Flynn R, Grundmann A, Renz P, Hänseler W, James WS, Cowley SA, et al. CRISPR-mediated genotypic and phenotypic correction of a chronic granulomatous disease mutation in human iPS cells. Exp Hematol. 2015;43(10):838–48.e3.
Schwank G, Koo B-K, Sasselli V, Dekkers Johanna F, Heo I, Demircan T, et al. Functional Repair of CFTR by CRISPR/Cas9 in Intestinal Stem Cell Organoids of Cystic Fibrosis Patients. Cell Stem Cell. 2013;13(6):653–8.
Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun. 2017;8:14584.
Beauchamp TL, Frey RG. The Oxford handbook of animal ethics. New York: Oxford University Press; 2011.
Begley CG, Ioannidis JPA. Reproducibility in Science. Improving the Standard for Basic and Preclinical Research. 2015;116(1):116–26.
Collins FS. Reengineering Translational Science: The Time Is Right. Sci Transl Med. 2011;3(90):90cm17–7.
•• Russell WMS, Burch RL. The principles of humane experimental technique. London: Methuen; 1959. Russell and Burch propose the concept of the Three Rs in this seminal 1959 book. The Three Rs stand for Replacement, Reduction and Refinement. Over the past 60 years the Three Rs have become widely accepted ethical principles when it comes to animal use in research, and are now embedded in regulations governing animal experimentation all over the world.
• Kimmelman J. A theoretical framework for early human studies: uncertainty, intervention ensembles, and boundaries. Trials. 2012;13(1):173. This article proposes a framework for the ethical and methodological evaluation of early human trials. It usefully distinguishes among types of uncertainty at the outset of early human trials.
Bretzner F, Gilbert F, Baylis F, Brownstone RM. Target Populations for First-In-Human Embryonic Stem Cell Research in Spinal Cord Injury. Cell Stem Cell. 8(5):468–75.
Solbakk Jan H, Zoloth L. The Tragedy of Translation: The Case of “First Use” in Human Embryonic Stem Cell Research. Cell Stem Cell. 2011;8(5):479–81.
Xie F, Ye L, Chang JC, Beyer AI, Wang J, Muench MO, et al. Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res. 2014;24(9):1526–33.
Kearns NA, Genga RMJ, Enuameh MS, Garber M, Wolfe SA, Maehr R. Cas9 effector-mediated regulation of transcription and differentiation in human pluripotent stem cells. Development. 2014;141(1):219–23.
Hyun I. The Ethics of Chimera Creation in Stem Cell Research. Curr Stem Cell Rep. 2018. https://doi.org/10.1007/s40778-018-0136-6.
Glenn Cohen I, Simana S. Regulation of Stem Cell Therapy. Curr Stem Cell Rep. 2018. https://doi.org/10.1007/s40778-018-0134-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Ethics in Stem/Progenitor Cell Therapeutics
Rights and permissions
About this article
Cite this article
Neuhaus, C.P., Zacharias, R.L. Advancing Ethical Stem Cell Research with CRISPR. Curr Stem Cell Rep 4, 248–252 (2018). https://doi.org/10.1007/s40778-018-0137-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-018-0137-5