Abstract
Introduction
The evolution of disease-modifying antirheumatic drugs (DMARDs) for the treatment of rheumatoid arthritis (RA) has improved patient prognosis. However, more real-world safety/effectiveness data comparing methotrexate (MTX), tofacitinib, tumor necrosis factor inhibitors (TNFi), and non-TNFi biologic DMARDs (bDMARDs) are warranted.
Methods
The CorEvitas RA Japan registry was used to identify patients with rheumatologist-diagnosed RA who initiated MTX/tofacitinib/TNFi/non-TNFi bDMARDs. Safety outcomes included incidence of major adverse cardiovascular events (MACE), total cardiovascular disease, total serious infections, total herpes zoster, and total malignancies (excluding non-melanoma skin cancer). Effectiveness outcomes included change from baseline (Δ) in Clinical Disease Activity Index (CDAI) and proportion of patients achieving a minimum clinically important difference (MCID) in CDAI at month 6. Adjusted regression models were fit; marginal means were estimated.
Results
Overall, 1972 patients were included in the safety cohort: MTX (N = 298); tofacitinib (N = 253); TNFi (N = 663); non-TNFi (N = 758). Mean follow-up time was 3.8, 2.9, 3.0, and 2.9 years for MTX, tofacitinib, TNFi, and non-TNFi, respectively. Adjusted incidence rates (IRs, patients with events/100 patient-years [95% confidence intervals]) for MACE and total cardiovascular disease, respectively, were numerically lower for MTX (0.34 [0, 0.83]; 0.42 [0, 0.92]) and TNFi (0.09 [0, 0.27]; 0.61 [0.15, 1.07]) versus tofacitinib (0.48 [0, 1.20]; 2.30 [0.38, 4.22]) and non-TNFi (0.77 [0.35, 1.19]; 1.28 [0.73, 1.82]). Serious infections were numerically higher for non-TNFi (4.47 [3.38, 5.56]); herpes zoster was higher for tofacitinib (7.41 [4.52, 10.29]), versus other groups. IRs for malignancies were comparable between groups. Mean ΔCDAI and rates of achieving MCID in CDAI at month 6 were generally greater with tofacitinib versus other groups.
Conclusion
Some variations in incidence of safety outcomes were observed between treatments, while certain effectiveness outcomes favored tofacitinib. Sample size variation between groups and low number of safety events limited the analysis. Further studies are warranted to investigate observed differences.
ClinicalTrials.gov
NCT05572567.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The evolution of disease-modifying antirheumatic drugs (DMARDs) has improved the prognosis for patients with rheumatoid arthritis (RA). |
However, more real-world safety and effectiveness data comparing conventional synthetic, targeted synthetic, and biologic DMARDs (bDMARDs; tumor necrosis factor inhibitors [TNFi] and non-TNFi) are required. |
This study used data from the CorEvitas RA Japan registry to assess the safety and effectiveness of methotrexate, tofacitinib, TNFi, and non-TNFi bDMARDs in patients with RA. |
What was learned from the study? |
The incidence of safety outcomes varied between treatments, while certain effectiveness outcomes favored tofacitinib. |
The results of this study enhance the understanding of real-world outcomes in patients with RA. |
Introduction
Rheumatoid arthritis (RA) is a persistent and systemic inflammatory disease affecting 0.6–1.0% of the Japanese population [1,2,3]. The evolution of disease-modifying antirheumatic drugs (DMARDs) has improved the prognosis for patients with RA; these include conventional synthetic DMARDs (csDMARDs), such as methotrexate (MTX), biologic DMARDs (bDMARDs), such as tumor necrosis factor inhibitors (TNFi) and non-TNFi bDMARDs, and targeted synthetic (tsDMARDs), such as Janus kinase (JAK) inhibitors [2, 4].
An analysis of data from the Institute of Rheumatology, Rheumatoid Arthritis (IORRA) cohort has shown that since the introduction of bDMARDs onto the market in 2003, their use in treating patients with RA increased in Japan through 2018, coinciding with improvements in clinical outcomes over this time period [4]. In Japan, the national health insurance system enables patients with RA to receive advanced therapies (e.g., bDMARDs) more easily compared with those in other countries that do not have universal healthcare [5]. A comparison of data from the CorEvitas registries (2016–2020) from Japan and the USA found that, while MTX was the most common first-line treatment in both countries, a greater percentage of patients with RA in Japan used a non-TNFi as first-, second-, and third-line DMARD therapy versus those from the USA [6]. TNFi use as first- to fourth-line DMARD therapy was less common in Japan versus the USA [6]. However, in patients with RA included within the multi-institutional FIRST Registry cohort in Japan (2013–2020), TNFi was most commonly used as the first b/tsDMARD therapy [7]. Thus, these findings suggest that the treatment guidelines among differing countries and institutions exhibit some variability, possibly due to different strategies for the management of RA.
Supported by data from a phase 3 randomized controlled trial and a long-term extension study which included Japanese patients [8, 9], tofacitinib, an oral JAK inhibitor, has been approved in Japan for the treatment of RA since 2013 [10]. A previous meta-analysis of 26 randomized controlled trials observed no significant changes in the short-term risk of major adverse cardiovascular events (MACE) in patients treated with JAK inhibitors, including tofacitinib [11]. More recently, in the post-authorization safety study, ORAL Surveillance, noninferiority was not shown for tofacitinib vs TNFi for the risk of MACE and malignancies excluding non-melanoma skin cancer (NMSC) in a cardiovascular risk-enriched population [12]. This resulted in a US Food and Drug Administration class label change indicating tofacitinib and other JAK inhibitors for patients with rheumatology and gastroenterology conditions who have had an inadequate response or intolerance to one or more TNFi [13, 14]. Furthermore, an observational study including data from 14 real-world data sources from the USA, Europe, and Japan reported greater risk of venous thromboembolism (VTE), and numerically greater risk (based on incidence rate ratios) of MACE and serious infection for baricitinib (a JAK inhibitor) vs TNFi [15]. Nevertheless, additional post-marketing and real-world data are warranted to fully assess the risk of cardiovascular, malignancy, and infection events with JAK inhibitors.
Given the evolving patterns in therapy usage and strategies in RA treatment [4, 5], more research on the real-world safety and effectiveness of treatments is warranted. Here, we use data from the CorEvitas RA Japan registry to describe the baseline characteristics of patients with RA who initiated MTX, tofacitinib, TNFi, and non-TNFi bDMARDs, and compare the safety and effectiveness of these drugs in routine daily practice.
Methods
Data Source
The CorEvitas RA Japan registry (NCT02737449) is a prospective, multicenter, observational disease-based registry that was launched in February 2016. Longitudinal follow-up data were collected from patients and their treating rheumatologists during routine clinical encounters using questionnaires. As of June 30, 2022, the registry included data from 48 private and academic active clinical sites, with over 219 physicians and 2359 patients with RA across 27 prefectures in Japan.
Study Population
This study (NCT05572567) included patients with rheumatologist-diagnosed RA, who initiated MTX, tofacitinib, TNFi (adalimumab [originator or biosimilar], certolizumab pegol, etanercept [originator or biosimilar], golimumab, infliximab [originator or biosimilar] or any other TNFi biosimilar approved during the study), or non-TNFi bDMARDs (abatacept, tocilizumab, or sarilumab) between March 1, 2016 and June 30, 2022, (October 5, 2022 data extract) and had an index visit (defined as the visit during which the first ever initiation of a drug took place). For patients with more than one initiation in a drug class, the index date was the initiation date of the first drug.
For the analyses of the safety cohort, eligible patients had at least 1 day of registry follow-up time after the index visit unless the individual had an event on the day of initiation. For the analyses of the effectiveness cohort, eligible patients had a 6-month follow-up visit (± 3 months) after initiation of the drug.
This structured, secondary study was conducted within the CorEvitas Registry in accordance with the International Council for Harmonisation Guidelines for Good Clinical Practice and Ethical Guidelines for Medical and Health Research Involving Human Subjects. All patients provided written informed consent for participation in the registry.
Outcomes
Patient Characteristics
In both the safety and effectiveness cohorts, patient characteristics were measured at baseline and included sociodemographic characteristics (age, sex, race, education, employment), lifestyle characteristics (smoking status and alcohol use), and health measures (such as body mass index [BMI], weight, provider-reported depression, provider-reported history of cardiovascular comorbidity, and non-cardiovascular comorbidity). Disease characteristics and some patient-reported outcomes (PROs) were also recorded at baseline.
Primary Outcomes
In the safety cohort, safety events of interest included MACE, total cardiovascular disease (CVD), VTE, total serious infections, total herpes zoster (HZ; non-serious and serious), and total malignancies excluding NMSC. The definitions of these events are listed in the Supplementary Material. Patients were followed according to a risk window, which, for all events except malignancies, began with the index visit and continued until the visit closest to 90 days after the end of therapy, or end of data collection, whichever came first. If the 90-day window overlapped with an initiation of a drug in a different group, then an event during this period was attributed to both therapies. An “any exposure” approach was utilized for analyses of risk for malignancies, in which the risk window began with the index visit and extended until the end of data collection, even in the case of subsequent switching to another therapy. If a malignancy was diagnosed after the start of a subsequent therapy, the event was attributed to both therapies.
Secondary Outcomes
In the effectiveness cohort, discontinuation rates were evaluated at the 6-month follow-up visit. Effectiveness outcomes, also evaluated at the 6-month follow-up visit, included mean change from baseline in Clinical Disease Activity Index (CDAI; 0–76), proportion of patients achieving a minimum clinically important difference (MCID) in CDAI, proportion of patients achieving modified American College of Rheumatology (mACR)20/50/70 responses, and the mean change from baseline in PROs: Japanese version of the Health Assessment Questionnaire (J-HAQ; 0–3), patient pain (Visual Analog Scale [VAS]; 0–100), Patient Global Assessment (VAS; 0–100), patient fatigue (VAS; 0–100), morning stiffness (yes/no; if yes, the patient was asked to state the duration of morning stiffness [0–24 h and 1–60 min]), and EuroQol 5-Dimension 5-Level (EQ-5D-5L; 0–1). MCID in CDAI was based on the CDAI value at initiation (the cut points for improvement were 1 if CDAI at baseline was < 10, 6 if CDAI at baseline was between 10–22 [inclusive], and 12 if CDAI at baseline was > 22) [16].
Statistical Analyses
Primary Outcomes
Follow-up time was summarized descriptively for each treatment group. For each outcome, only the first event for each patient was analyzed. Incidence rates (IRs; number of patients with events per 100 patient-years) and 95% confidence intervals (CIs) were calculated on the basis of time to the first event for patients with at least one event. For patients without events, the calculation of exposure time included the entire risk period. A Poisson distribution with 95% CIs was computed. A multivariate Poisson model was built for each safety outcome, adjusted for potential a priori-defined confounders, and was used to estimate marginal IRs for each safety outcome within each treatment group. Specifically, a multiplicative Poisson regression model was fitted as a log-linear regression (i.e., a log link and a Poisson error distribution), with an offset equal to the natural logarithm of person-time. Adjusted models included the following potential confounders: age, sex, baseline CDAI, number of prior bDMARDs, BMI, duration of RA, Physician Global Assessment, J-HAQ, and the baseline value of the outcome measurement. Each patient’s observed covariates and the model coefficients were used to compute the average probability of an event by treatment group. Patients with missing covariate data were omitted from both the unadjusted and adjusted analyses. No outcome or missing data for covariates were imputed.
Secondary Outcomes
A separate regression model was fit for each effectiveness outcome, with DMARD group as the exposure variable (primary independent variable of interest). Linear models were used for continuous measures and logistic models were used for binary measures. Unadjusted mean outcomes and 95% CIs were calculated for each treatment group. Adjusted regression models were fit, and marginal means, with associated 95% CIs, were estimated. A priori-defined potential confounders for adjusted models and mean marginal effect computations were the same as for the primary outcomes. Patients with missing covariate data were omitted from both the unadjusted and adjusted analyses. Discontinuations were summarized descriptively for each treatment group. For patients who discontinued the index therapy prior to the 6-month follow-up visit, a non-response was imputed for binary outcome measures, and the last observation carried forward was used as the 6-month value for continuous measures. For patients who discontinued at their 6-month visit, the 6-month outcome was evaluated at the visit.
For the purposes of this analysis, across all safety and efficacy outcomes evaluated, comparisons between treatment groups for marginal mean changes from baseline (continuous outcomes) and proportions (binary outcomes) are described as higher or lower if 95% CIs do not overlap, and numerically higher or lower if 95% CIs do overlap, as appropriate. Multiplicity adjustment was not conducted because of the exploratory nature of the analysis.
Results
Demographics and Baseline Characteristics of Patients in the Safety Cohort
A summary of patient demographics and baseline characteristics is provided in Table 1. In total, 1972 patients met the inclusion criteria for the safety cohort: MTX (N = 298), tofacitinib (N = 253), TNFi (N = 663), or non-TNFi (N = 758). Across treatment groups, most patients were female, with mean age ranging from 59.8 to 65.8 years, while over a third of initiators were college educated. Across groups, most initiators were non-smokers and had a BMI of < 25. Hypertension was the most common prior comorbidity across all groups. A history of malignancy was more common among non-TNFi initiators versus the other groups. A lower percentage of patients in the MTX and TNFi treatment groups had a history of serious infections versus tofacitinib and non-TNFi initiator groups. The duration of RA was longest for tofacitinib initiators (11.6 years) and shortest for MTX initiators (2.3 years).
Tofacitinib initiators used a greater mean number of bDMARDs (1.5) compared with TNFi (0.5), non-TNFi (0.7), and MTX initiators (0.0; Table 1). Use of concomitant therapy with MTX was more common for TNFi initiators followed by tofacitinib initiators and non-TNFi initiators.
Tofacitinib initiators reported higher mean Patient Global Assessment, fatigue, and pain scores at the index visit than those who initiated the other treatments (Table 1). However, non-TNFi initiators had a higher percentage of patients reporting moderate, severe, or extreme difficulty across all EQ-5D-5L domains (mobility, self-care, usual activities, pain/discomfort, and anxiety and depression) compared with MTX, tofacitinib, and TNFi initiators.
Safety Analyses
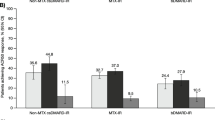
The adjusted marginal IRs and number of patients with safety events (n) across the treatment groups are provided in Fig. 1. For MACE, adjusted IRs (95% CI) for non-TNFi initiators (0.77 [0.35, 1.19]; n = 14) were numerically higher versus MTX (0.34 [0.00, 0.83]; n = 2) and tofacitinib initiators (0.48 [0, 1.20]; n = 2), and higher versus TNFi initiators (0.09 [0.00, 0.27]; n = 1). For total CVD, there were numerically lower adjusted IRs (95% CI) for MTX (0.42 [0.00, 0.92]; n = 3) and TNFi initiators (0.61 [0.15, 1.07]; n = 7) versus tofacitinib (2.30 [0.38, 4.22]; n = 7) and non-TNFi initiators (1.28 [0.73, 1.82]; n = 22). The VTE adjusted IR (95% CI) was numerically higher in tofacitinib initiators (0.55 [0.00, 1.92]; n = 1) versus the other groups. For total serious infections, there were numerically higher adjusted IRs (95% CI) for non-TNFi initiators (4.47 [3.38, 5.56]; n = 69) versus MTX (3.06 [1.41, 4.72]; n = 15), tofacitinib (2.46 [1.04, 3.88]; n = 14), and TNFi initiators (2.63 [1.61, 3.65]; n = 27). The adjusted IR (95% CI) for HZ was higher for tofacitinib initiators (7.41 [4.52, 10.29]; n = 36) than for MTX (1.05 [0.18, 1.91]; n = 6), TNFi (1.64 [0.89, 2.38]; n = 19), and non-TNFi (2.17 [1.42, 2.91]; n = 33) initiators. There were generally similar rates of malignancies (excluding NMSC) across treatment groups.
Adjusted IRs (patients with events per 100 PY) of safety events in MTX, tofacitinib, TNFi, and non-TNFi initiator groups (safety cohort). aMACE includes non-fatal myocardial infarction, non-fatal stroke, and cardiovascular deaths, excluding fatal pulmonary embolism. bTotal CVD is defined as hypertension requiring hospitalization, cardiac revascularization procedure (coronary artery bypass graft, stent, angioplasty), ventricular arrhythmia, cardiac arrest, myocardial infarction, acute coronary syndrome, unstable angina, congestive heart failure requiring hospitalization, stroke, transient ischemic attack, other cardiovascular event (specify), deep vein thrombosis, peripheral arterial thromboembolic event, urgent peripheral arterial revascularization, peripheral ischemia or gangrene (necrosis), and pulmonary embolism. cVTE is defined as deep vein thrombosis and pulmonary embolism. dCIs for events with zero counts are not computed. eTotal serious infections are defined as infections meeting serious adverse event criteria or requiring treatment with intravenous antibiotics. Serious infection types collected in the registry are joint/bursa, cellulitis/skin, sinusitis, diverticulitis, sepsis, pneumonia, bronchitis, gastroenteritis, meningitis/encephalitis, urinary tract infection, upper respiratory infection, active tuberculosis (regardless of seriousness), and other serious infections. fTotal HZ includes both non-serious and serious HZ. gTotal malignancy excluding NMSC includes lymphoma, lung, breast, skin (melanoma), and other cancers. CI confidence interval, CVD cardiovascular disease, HZ herpes zoster, IR incidence rate, MACE major adverse cardiovascular events, MTX methotrexate, n number of patients with events, NMSC non-melanoma skin cancer, PY patient-years, TNFi tumor necrosis factor inhibitors, VTE venous thromboembolism
Similar results were observed in the unadjusted analysis, which is shown in Supplementary Fig. S1 in the electronic supplementary material.
Demographics and Baseline Characteristics of Patients in the Effectiveness Cohort
In total, 1771 initiators who met the inclusion criteria for the effectiveness cohort initiated treatment with MTX (N = 276), tofacitinib (N = 205), TNFi (N = 603), or non-TNFi bDMARDs (N = 687). Generally, most baseline and disease characteristics were similar to those of the safety cohort (Supplemental Table S1 in the electronic supplementary material).
Summary of Discontinuation
Across groups, most initiators in the effectiveness cohort remained on study drug at month 6 (≥ 77.6%; Fig. 2). The proportions of patients who discontinued study drug prior to (12.2–17.2%) or at (3.9–6.2%) month 6 were similar across treatment groups.
Effectiveness Analyses
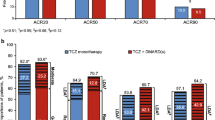
After adjusting for potential confounders, the marginal means for change from baseline in CDAI ranged from − 9.4 to − 13.1 across treatment groups, and achievement of CDAI MCID at 6 months ranged from 55.8% to 72.2%.
Specifically, mean change from baseline (95% CI) in CDAI at month 6 was greater in the tofacitinib treatment group (− 13.1 [− 14.6, − 11.7]) compared with the TNFi (− 9.4 [− 10.3, − 8.6]) and the MTX (− 10.1 [− 11.4, − 8.9]) treatment groups and numerically greater compared with the non-TNFi treatment group (− 12.1 [− 12.9, − 11.3]; Fig. 3). Additionally, the adjusted mean (95% CI) proportion of patients achieving MCID in CDAI at month 6 was higher for tofacitinib initiators (72.2% [66.0, 78.3]) versus MTX initiators (55.8% [49.5, 62.2]) and TNFi initiators (55.9% [51.7, 60.1]), and numerically higher versus non-TNFi initiators (67.5% [63.9, 71.1]). Similarly, at month 6, the proportions of patients achieving mACR20, mACR50, and mACR70 thresholds were generally higher for tofacitinib initiators followed by non-TNFi initiators, TNFi initiators, and MTX initiators, although 95% CIs generally overlapped between tofacitinib initiators and non-TNFi initiators (Fig. 3).
Marginal mean change from baseline in CDAI (95% CI) and proportion of patients achieving effectiveness outcomes (95% CI) at month 6 (adjusted effectiveness outcomes) in MTX, tofacitinib, TNFi, and non-TNFi initiator groups (effectiveness cohort). aCDAI represented as mean change from baseline to 6 months. Calculated by subtracting the baseline value from the 6-month value; a negative value indicates improvement. bBinary outcomes represented as percentage achieving the threshold (please see methods for details). CDAI Clinical Disease Activity Index, CI confidence interval, mACR modified American College of Rheumatology, MCID minimum clinically important difference, MTX methotrexate, N number of patients, TNFi tumor necrosis factor inhibitors
At month 6, improvement from baseline was observed in all PROs, across all treatment groups. Adjusted mean reductions from baseline in pain and Patient Global Assessment ranged from 16.9 to 22.3 and from 15.9 to 19.9 points, respectively, across the treatment groups (Table 2). Patient fatigue decreased 9.3–14.5 points across treatment groups. The proportion of patients reporting morning stiffness decreased by 18.2–28.5 points across treatment groups. A small decrease in J-HAQ scores was seen in patients across all treatment groups (0.2–0.3).
There was improvement from baseline in all EQ-5D-5L domains at month 6, with generally similar changes reported across groups (Table 2). The percentage of patients reporting moderate, severe, or extreme problems decreased by 7.8–12.6% for mobility, 6.6–11.8% for self-care, 11.7–15.4% for usual activities, 24.3–28.9% for pain, and 3.9–9.2% for depression/anxiety.
A summary of the unadjusted changes from baseline at month 6 in effectiveness outcomes across the treatment groups is shown in Supplementary Fig. S2 and Table S2 in the electronic supplementary material.
Discussion
In this analysis, we assessed the real-world safety and effectiveness of MTX, tofacitinib, TNFi, and non-TNFi bDMARDs in patients with RA from the CorEvitas RA Japan registry. We observed that MTX and TNFi initiators had numerically lower rates of MACE and total CVD, although the 95% CIs generally overlapped between the assessed treatment groups (95% CIs for TNFi versus non-TNFi did not overlap for MACE). The adjusted marginal IRs were numerically higher for total serious infection in non-TNFi initiators, and higher for HZ in tofacitinib initiators, versus other groups. Adjusted IRs for malignancies (excluding NMSC) were comparable between treatment groups.
The adjusted analyses here build upon earlier work published in 2020 in which an unadjusted analysis was performed on the same four treatment groups. In the current study, effectiveness outcomes were consistent with results observed previously (including changes in mean CDAI scores, and CDAI MCID and mACR20/50/70 achievement across the treatment groups) [17]. Additionally, compared with the current study, similar safety profiles were previously observed for each treatment group with numerically higher IR of serious infections in non-TNFi initiators, and higher IR of HZ for tofacitinib initiators being reported versus other groups [18]. Furthermore, the lower IRs of total CVD observed in MTX and TNFi initiators versus tofacitinib and non-TNFi in the current study are in support of a previous meta-analysis of 28 studies of RA, where MTX and TNFi were also associated with decreased risk of cardiovascular events [19].
The current adjusted safety analysis also demonstrated that tofacitinib initiators had the highest IR of HZ compared with other groups. Elevated rates of HZ with tofacitinib are consistent with prior clinical trial data from Japan; in an open-label, long-term extension study of Japanese patients with RA receiving tofacitinib with or without background MTX, the risk of HZ was observed to be higher in this population compared with that of a global RA population [9]. Additionally, the IR of HZ in Japanese patients with RA treated with tofacitinib has been reported to be 2–3 times higher compared with patients in other geographical regions [20]. Such observations are consistent with the known safety profile of tofacitinib within Japanese patients with RA [21].
The exact mechanism that underlies the increased incidence of HZ with tofacitinib treatment is not fully understood. An analysis of 44 patients with RA from phase 2/3 randomized clinical trials and a long-term extension study observed that tofacitinib suppresses proliferation of CD4+ T lymphocytes, and low CD8+ T lymphocyte levels at baseline were a significant predictor of infectious adverse events, which included 7 cases of HZ [22]. Furthermore, in a study of 125 Taiwanese patients with RA receiving tofacitinib, patients who developed HZ (n = 7) were reported to have prior history of HZ, lower activated T cell counts, and lower plasma interferon-γ (IFNγ) levels at baseline compared with patients who did not develop HZ [23]. Both T lymphocytes and IFNγ play a role in antiviral defense [24]. Thus, the increased risk of HZ with tofacitinib may result from tofacitinib-induced reductions in T cell counts and IFNγ levels which could contribute to impaired cell-mediated immunity and lead to subsequent reactivation of quiescent varicella zoster virus [22, 23].
A recent interim analysis of a post-marketing surveillance study assessed the real-world safety of tofacitinib in Japanese patients with RA. The safety profile of tofacitinib observed in this current study is generally consistent with the results of the interim analysis [25]. In that study, which included a cohort of patients with similar demographic and baseline characteristics to the current study, similar cumulative IRs of HZ (IR 8.02; 95% CI [7.05, 9.09]) and malignancies (1.45 [1.06, 1.92]), but a higher IR of serious infections (6.91 [6.01, 7.91]) were observed when compared to the current analysis. We observed IRs of 3.10 (1.58, 4.61) and 2.46 (1.04, 3.88) for total serious infections per the unadjusted and adjusted analyses, respectively. Our findings are consistent with those previously reported in analyses of global and Japanese RA populations [9, 26]. However, it should be noted that the interim analysis of the post-marketing surveillance study only assessed adverse events over 6 months (versus a mean follow-up time of 2.9 years for the tofacitinib group in the current study), and the higher IR of total serious infections with tofacitinib, as with other treatment groups in this study (all adjusted IRs ≥ 2.46), are consistent with other post-marketing surveillance studies in Japanese patients assessing bDMARDs over a similar study duration [27, 28]. Thus, these discrepancies may arise because of differences in study duration, among other factors.
In the adjusted effectiveness analysis, tofacitinib initiators had the highest marginal means estimate of achieving MCID in CDAI and the highest percentage of patients achieving mACR20/50/70 at month 6. A study assessing the real-world effectiveness of tofacitinib and TNFi monotherapies and combination therapies (with MTX) in the USA noted that tofacitinib monotherapy achieved a similar standard of effectiveness as that observed with TNFi combination therapy in the third or fourth line [29]. Differences in the effectiveness of tofacitinib versus other treatments between Japan and US real-world populations may be due to differences in their prescribing practices; this warrants further investigation. Interestingly, a meta-analysis of 21 real-world studies of patients with RA worldwide indicated greater effectiveness (determined by risk ratios from fixed/random-effects models) with JAK inhibitors and non-TNFi than with bDMARDs and TNFi, respectively [30].
The current study had a number of limitations. The CorEvitas RA Japan registry is not necessarily representative of all adults with RA in Japan as it includes only patients with RA who attended clinical visits with rheumatologists, and the current findings may not be generalizable to non-Japanese populations. History of medication use prior to enrollment is derived from that reported by patients and their current rheumatologists within the registry. A significant proportion of patients may not have been vaccinated for HZ because Shingrix® was not approved in Japan until 2018 [31]. Also, there were no new MTX and non-TNFi bDMARD initiators after February 2018 and June 2020, respectively, as these cohorts were closed for enrollment. The study, exploratory in nature, was not designed as a comparative study with an a priori hypothesis and the populations were not propensity score-matched. There was variation between the treatment groups regarding the sample size (lowest in the tofacitinib group), the characteristics at the time of treatment initiation, and in the treatment sequence (i.e., line of therapy) for the different therapies. Specifically, TNFi were more likely to be used earlier, while tofacitinib initiators had a larger mean number of prior bDMARDs. We adjusted for line of therapy in our models, as well as for other a priori-defined covariates, but residual confounding was possible. There were a low number of specific safety events, such as MACE and VTE, which limited the certainty of the analysis. Finally, marginal means from multivariable adjusted linear and logistic regression are reported; these represent the average outcomes for a patient in each treatment group. This approach relies on assumptions about the population (e.g., observed covariate values for each patient are used), and marginal means can vary when different assumptions are used. Further research is needed to explore drivers of this result.
Conclusions
MTX and TNFi initiators had numerically lower incidence of MACE and total CVD versus tofacitinib and non-TNFi bDMARD initiators. The incidence of total serious infection was numerically higher in non-TNFi initiators. Although the tofacitinib treatment group had the highest rate for HZ compared with other treatment groups, no new potential safety risks were identified that were unique to Japanese patients treated with tofacitinib or new to the tofacitinib safety profile. Incidence of malignancies was generally similar across treatment groups. Adjusted effectiveness analyses favored tofacitinib for improvements from baseline in CDAI, and the achievement of MCID in CDAI, and mACR20/50/70, although further research is needed to understand the mechanisms underpinning the differences between treatment groups. The differences in sample size between groups, and the low number of safety events for some outcomes limited the analysis. Overall, the results of this study enhance the understanding of real-world outcomes in patients with RA in Japan.
Data Availability
Data are available from CorEvitas, LLC through a commercial subscription agreement and are not publicly available. No additional data are available from the authors.
References
Yamanaka H, Sugiyama N, Inoue E, Taniguchi A, Momohara S. Estimates of the prevalence of and current treatment practices for rheumatoid arthritis in Japan using reimbursement data from health insurance societies and the IORRA cohort (I). Mod Rheumatol. 2014;24:33–40.
Tanaka Y. Rheumatoid arthritis. Inflamm Regen. 2020;40:20.
Nakajima A, Sakai R, Inoue E, Harigai M. Prevalence of patients with rheumatoid arthritis and age-stratified trends in clinical characteristics and treatment, based on the National Database of Health Insurance Claims and Specific Health Checkups of Japan. Int J Rheum Dis. 2020;23:1676–84.
Yamanaka H, Tanaka E, Nakajima A, et al. A large observational cohort study of rheumatoid arthritis, IORRA: providing context for today’s treatment options. Mod Rheumatol. 2020;30:1–6.
Koike T, Inui K. How can the treatment of rheumatoid arthritis be improved in Japan? Int J Clin Rheumatol. 2015;10:235–44.
Yamanaka H, Kishimoto M, Nakano K, et al. International comparison of Japanese and US cross country utilization of RA medications [abstract]. Arthritis Rheumatol. 2020;72(Suppl 10):0829.
Ochi S, Sonomoto K, Nakayamada S, Tanaka Y. Preferable outcome of Janus kinase inhibitors for a group of difficult-to-treat rheumatoid arthritis patients: from the FIRST Registry. Arthritis Res Ther. 2022;24:61.
van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four–month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–70.
Yamanaka H, Tanaka Y, Takeuchi T, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther. 2016;18:34.
Pfizer Inc. XELJANZ® (tofacitinib citrate) approved in Japan for the treatment of adults with rheumatoid arthritis (RA). 2013. https://www.pfizer.com/news/press-release/press-release-detail/xeljanz_tofacitinib_citrate_approved_in_japan_for_the_treatment_of_adults_with_rheumatoid_arthritis_ra. Accessed Mar 1, 2023.
Xie W, Huang Y, Xiao S, Sun X, Fan Y, Zhang Z. Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis. 2019;78:1048–54.
Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–26.
US Food and Drug Administration. XELJANZ® (tofacitinib): highlights of prescribing information. 2022. https://labeling.pfizer.com/showlabeling.aspx?id=959. Accessed Feb 26, 2024.
US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death. Accessed Mar 28, 2022.
Salinas CA, Louder A, Polinski J, et al. Evaluation of VTE, MACE, and serious infections among patients with RA treated with baricitinib compared to TNFi: a multi-database study of patients in routine care using disease registries and claims databases. Rheumatol Ther. 2023;10:201–23.
Curtis JR, Yang S, Chen L, et al. Determining the minimally important difference in the clinical disease activity index for improvement and worsening in early rheumatoid arthritis patients. Arthritis Care Res (Hoboken). 2015;67:1345–53.
Tanaka Y, Yamanaka H, Harrold L, et al. Real-world DMARD experience and outcomes for rheumatoid arthritis patients in Japan: effectiveness [abstract]. Arthritis Rheumatol. 2020;72(Suppl 10):0802.
Kishimoto M, Tanaka Y, Harrold L, et al. Real-world DMARD experience and outcomes for rheumatoid arthritis patients in Japan: safety [abstract]. Arthritis Rheumatol. 2020;72(Suppl 10):0803.
Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480–9.
Winthrop KL, Curtis JR, Lindsey S, et al. Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol. 2017;69:1960–8.
Pfizer Japan Inc. XELJANZ® package insert for Japan. 2021. https://pins.japic.or.jp/pdf/newPINS/00061355.pdf. Accessed Mar 1, 2023.
Sonomoto K, Yamaoka K, Kubo S, et al. Effects of tofacitinib on lymphocytes in rheumatoid arthritis: relation to efficacy and infectious adverse events. Rheumatology (Oxford). 2014;53:914–8.
Chen YJ, Chen YM, Huang WN, et al. Herpes zoster in rheumatoid arthritis patients receiving tofacitinib, a single center experience from Taiwan. Medicine (Baltimore). 2020;99:e22504.
Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24:439–54.
Kuwana M, Sugiyama N, Momohara S, et al. Six-month safety and effectiveness of tofacitinib in patients with rheumatoid arthritis in Japan: interim analysis of post-marketing surveillance. Mod Rheumatol. 2024;34:272–86.
Wollenhaupt J, Silverfield J, Lee EB, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol. 2014;41:837–52.
Koike T, Harigai M, Inokuma S, et al. Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol. 2014;41:15–23.
Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis. 2011;70:2148–51.
Reed GW, Gerber RA, Shan Y, et al. Real-world comparative effectiveness of tofacitinib and tumor necrosis factor inhibitors as monotherapy and combination therapy for treatment of rheumatoid arthritis. Rheumatol Ther. 2019;6:573–86.
de Castro CT, de Queiroz MJ, Albuquerque FC, et al. Real-world effectiveness of biological therapy in patients with rheumatoid arthritis: systematic review and meta-analysis. Front Pharmacol. 2022;13: 927179.
GlaxoSmithKline. Shingrix approved in Europe and Japan for the prevention of shingles in adults aged 50 and over. 2018. https://www.gsk.com/en-gb/media/press-releases/shingrix-approved-in-europe-and-japan-for-the-prevention-of-shingles-in-adults-aged-50-and-over/. Accessed Dec 10, 2018.
Acknowledgements
We thank the participants of the study. The registry is sponsored by CorEvitas, LLC, and the analyses based on secondary analysis of registry data were funded and sponsored by Pfizer. Access to study data was limited to CorEvitas, and CorEvitas statisticians completed all the analyses; all authors contributed to the interpretation of the results. CorEvitas has been supported through contracted subscriptions in the past 2 years by AbbVie, Amgen, Arena, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Eli Lilly, Genentech, GlaxoSmithKline, Janssen, LEO, Novartis, Ortho Dermatologics, Pfizer Inc, Sun, and UCB.
Medical Writing/Editorial Assistance
Medical writing support, under the direction of the authors, was provided by Yasmin Parr, PhD, and Lewis C Rodgers, PhD, CMC Connect, a division of IPG Health Medical Communications, and was funded by Pfizer, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022;175:1298–304).
Funding
The registry is sponsored by CorEvitas, LLC, and the analyses based on secondary analysis of registry data were funded and sponsored by Pfizer. The journal’s Rapid Service Fee was funded by Pfizer.
Author information
Authors and Affiliations
Contributions
Conceptualization of study and design: Yoshiya Tanaka, Mitsumasa Kishimoto, Koichi Amano, Alina Onofrei, Jacqueline O’Brien, Christine Barr, Yasushi Mizuno, Ekta Agarwal, Naonobu Sugiyama, and Hisashi Yamanaka. Data analysis: Yoshiya Tanaka, Mitsumasa Kishimoto, Alina Onofrei, and Zachary Margolin. Data interpretation: Yoshiya Tanaka, Mitsumasa Kishimoto, Koshiro Sonomoto, Koichi Amano, Masayoshi Harigai, Alina Onofrei, Jacqueline O’Brien, Zachary Margolin, Christine Barr, Yasushi Mizuno, Ekta Agarwal, Naonobu Sugiyama, and Hisashi Yamanaka. All authors were involved in drafting the article or reviewing it critically for important intellectual content, and all authors approved the final version to be submitted for publication.
Corresponding author
Ethics declarations
Conflict of Interest
Yoshiya Tanaka has received speaker fees and/or honoraria from AbbVie, Asahi Kasei, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Eisai, Eli Lilly, Gilead, GlaxoSmithKline, Pfizer Inc, Taiho, and Taisho, and has received research grants from Asahi Kasei, Chugai, Eisai, Mitsubishi-Tanabe, and Taisho. Yoshiya Tanaka is an Editorial Board member of Rheumatology and Therapy. Yoshiya Tanaka was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Mitsumasa Kishimoto has received consulting fees and/or honoraria from AbbVie, Amgen, Asahi Kasei Pharma, Ayumi Pharma, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Novartis, Ono Pharma, Pfizer Inc, Tanabe-Mitsubishi, and UCB Pharma. Koshiro Sonomoto has received speaking fees from AbbVie, Astellas, Chugai, Eli Lilly Japan, Gilead Sciences, Janssen, and Taisho, and has received research funding from UCB Japan. Koichi Amano has received speaker fees and/or honoraria from AbbVie GK, Chugai, Eisai, GlaxoSmithKline, and Pfizer Japan Inc, and has received research grants from Chugai Pharmaceutical Co. Ltd. Masayoshi Harigai has received research grants from AbbVie Japan GK, Asahi Kasei Corp., Ayumi Pharmaceutical Co., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Eisai Co., Ltd., Eli Lilly Japan K.K., Mitsubishi Tanabe Pharma Co., Nippon Kayaku Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., and Teijin Pharma Ltd, has received speaker’s fees from AbbVie Japan GK, Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., and Teijin Pharma Ltd, and is a consultant for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb Co., and Teijin Pharma Ltd. Masayoshi Harigai was an employee of Tokyo Women’s Medical University School of Medicine at the time of this study and is now employed at Sanno Hospital and International University of Health and Welfare. Alina Onofrei is an employee of CorEvitas, LLC. Jacqueline O’Brien is an employee of CorEvitas, LLC. Zachary Margolin is an employee of CorEvitas, LLC. Christine Barr is an employee of CorEvitas, LLC. Yasushi Mizuno is an employee and shareholder of Pfizer Inc. Ekta Agarwal is an employee and shareholder of Pfizer Inc. Naonobu Sugiyama is an employee and shareholder of Pfizer Inc. Hisashi Yamanaka has received speaker fees or consultant fees from AbbVie, Asahi Kasei, Ayumi, Bristol Myers Squibb, Chugai, CorEvitas LLC, Eli Lilly, Fuji Yakuhin, Mochida, Pfizer Inc, Sato Seiyaku, Tanabe-Mitsubishi, Teijin Pharma, and YL Bio.
Ethical Approval
This structured, secondary study was conducted within the CorEvitas Registry in accordance with the International Council for Harmonisation Guidelines for Good Clinical Practice and Ethical Guidelines for Medical and Health Research Involving Human Subjects. All patients provided written informed consent for participation in the registry.
Additional information
Prior Presentation: This manuscript is based on work that has been previously presented at the American College of Rheumatology Convergence in 2020 (virtual).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tanaka, Y., Kishimoto, M., Sonomoto, K. et al. Methotrexate, Tofacitinib, and Biologic Disease-Modifying Antirheumatic Drug Safety and Effectiveness Among Patients with Rheumatoid Arthritis in Japan: CorEvitas Registry Observational Study. Rheumatol Ther 11, 1237–1253 (2024). https://doi.org/10.1007/s40744-024-00700-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-024-00700-2