Abstract
Introduction
Glucocorticoids (GCs) play a crucial role in the treatment of many rheumatic diseases regarding their anti-inflammatory and immunosuppressive effects. Inappropriate use of GCs can exacerbate GC-related problems besides complex treatment regimens and miscellaneous well-established adverse events. Although several guidelines exist for managing these problems, there is lack of real-life studies evaluating the problems at the patient level. This study aims to identify GC-related problems among patients with rheumatic diseases and address how they have been solved.
Methods
This prospective follow-up study was conducted between January 2021 and June 2022 at a university rheumatology outpatient clinic and included patients using GCs. A clinical pharmacist assessed patients for possible GC-related problems at baseline, 3 months, and 6 months. Identified problems, their causes, interventions to address these problems, and their outcomes were categorized using the Pharmaceutical Care Network Europe (PCNE v9.1) classification system. The resolution of the problems was evaluated at the patient’s next follow-up visit.
Results
A total of 156 patients were included, and 236 GC-related problems were identified in 66% of the patients. Adverse drug events (possible) accounted for the highest proportion of GC-related problems (94.1%), and the most common causes were lack of laboratory monitoring of GC-related adverse events (41.5%) and lack of drug treatment despite existing indications (39.8%). The median cumulative prednisolone dose was higher in patients with GC-related problems (3115 vs. 5455 mg, p = 0.007). The clinical pharmacist suggested 381 interventions: 47.7% (n = 182) at the ‘prescriber level’, 31.8% (n = 121) at the ‘patient level’, and 20.5% (n = 78) at the ‘drug level’. Of those interventions, 98% were accepted, and 80.1% of the problems were solved.
Conclusions
This study showed that the prevalence of GC-related problems is high in patients with rheumatic diseases. Integrating clinical pharmacists into the multidisciplinary rheumatology team provides an advantage in effectively identifying and managing GC-related problems at an early stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Glucocorticoids (GCs) remain the mainstay of treatment for various rheumatic diseases, but inappropriate use can worsen GC-related problems, besides the complex treatment regimens and well-established side effects. |
There has been no previous research on the identification and management of GC-related problems in patients with rheumatic diseases despite the existence of several guidelines and reviews on the management of systemic GC treatment. |
The objective of this study was to identify and manage GC-related problems in patients using GCs in a rheumatology outpatient clinic. |
What was learned from this study? |
This study revealed a high prevalence of GC-related problems in patients with rheumatic diseases, predominantly related to treatment safety. |
The clinical pharmacist's involvement in the healthcare team has had a positive impact on identifying and resolving GC-related problems. |
Detecting GC-related problems at an early stage and managing them appropriately provides an opportunity to minimize possible side effects. |
Introduction
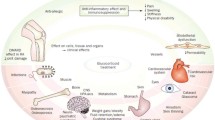
Glucocorticoids (GCs) are highly effective anti-inflammatory and immunosuppressive agents that have been used in the treatment of various rheumatic diseases such as rheumatoid arthritis, vasculitis, connective tissue diseases, and polymyalgia rheumatica. Despite the established efficacy of GCs, their long-term use is associated with well-known adverse events such as hyperglycemia and diabetes mellitus, cardiovascular diseases, osteoporosis, dyslipidemia, ophthalmological, dermatological and gastrointestinal problems, and infections [1, 2]. Depending on investigated adverse events and study design, a prevalence of adverse events has been reported as 10–80% in patients using glucocorticoids. Those troublesome and sometimes severe side effects frequently contribute to drug non-adherence, resulting in treatment failure and disease flares. On the other hand, adverse events, such as weight gain or skin toxicity, can be important for patients and have a negative impact on treatment compliance [3,4,5,6]. A recent study of patients with rheumatoid arthritis on GCs found that 25% of the patients discontinued glucocorticoids due to adverse events [5]. Various glucocorticoid regimens with different tapering instructions for patients with rheumatic diseases are another matter of concern and leads to inappropriate use of glucocorticoids in patients who already receive complex therapies [7].
Evidence-based recommendations have been developed and published for the rational use of systemic GC treatment in rheumatic diseases [8,9,10,11,12,13,14], which emphasize close monitoring and appropriate preventive strategies. In line with these guidelines, it is critical to assess individual risk factors to inform patients about adverse events in order to manage GC-related issues effectively.
Drug-related problems (DRPs) are defined as events or circumstances involving drug therapy that actually or potentially interfere with desired health outcomes that occur at any stage of the treatment [15]. The prevalence of DRPs in the rheumatology population has been reported to be 78–88%, and the majority of these problems are associated with the use of disease-modifying antirheumatic drugs (DMARDs) [16,17,18]. Several studies have shown that medication use in inflammatory rheumatic diseases is suboptimal. A considerable proportion of patients do not take their medication regimen as prescribed, due to adverse effects, concerns about the medication itself, and a lack of information about the disease and the medication [19, 20]. These DRPs can have serious consequences, such as increased disease activity and side effects, which can ultimately lead to increased morbidity, mortality, and healthcare costs [21,22,23]. In addition to DMARDs, glucocorticoids can also cause DRPs; these problems include adverse drug reactions, additional treatment needed, dosing problems, drug use problems, compliance, and unnecessary treatment. Therefore, in order to enhance patient outcomes, the timely identification and resolution of DRPs is critical.
Clinical pharmacists can identify and prevent DRPs to improve health outcomes and reduce treatment-related risks. It has previously been demonstrated that integrating clinical pharmacists into multidisciplinary teams facilitates advanced care provision in primary care and various specialties [24]. In addition, the involvement of pharmacists/clinical pharmacists in rheumatology outpatient clinics have been shown to prevent medication errors [25], detect drug–drug interactions [26], reduce DRPs [16], improve patient compliance, knowledge of disease and medication, and health-related quality of life [27,28,29,30,31,32]. However, only a few studies have focused on the management of GC-related problems in rheumatology settings. Therefore, this study aims to quantify and characterize GC-related problems and present interventions for managing these problems in patients with inflammatory rheumatic diseases who receive GC treatment.
Methods
Study Design and Setting
This prospective study was carried out between January 2021 and June 2022 in the rheumatology outpatient clinic of a tertiary university hospital. Patients aged > 18 years with a diagnosis of inflammatory arthritis, connective tissue disease (CTD) and vasculitides, newly prescribed or currently using GC treatment, were included in the study after informed patient consent was obtained. To ensure accurate medical history, the treatment period with GCs was restricted to a maximum of 2 years. Patients visiting the outpatient clinic for routine follow-up were referred to the clinical pharmacist at baseline (t0), 3 (t3), and 6 (t6) months to be evaluated regarding GC-related problems after consultation by the physician. The clinical pharmacist planned interventions to resolve the identified problems. The interventions that may influence the physician’s treatment decision, such as the requests for laboratory monitoring, adding a new drug to the treatment regimen, or adjusting the dose of an existing drug, were discussed with the physician. In addition, patients were directly provided with interventions, such as counseling for newly prescribed drugs and lifestyle changes. The acceptance rate of the interventions was recorded and the resolution of the problems was assessed at the following outpatient visits. Since the identified adverse events may not be directly related to GCs, all adverse events were considered as potential. Clinical pharmacists are not allowed to make any changes in the prescribing process in Turkey. Thus, in this study, planned interventions were recommended to physicians, even if it was related to drug treatment.
Ethical Approval
The study was approved by the University Clinical Trials Ethics Committee (KA-21026). All patients provided written informed consent to participate in the study. The study was conducted according to the Declaration of Helsinki and the Harmonization of Good Clinical Practice Guidelines.
Data Collection
Patients’ data were collected prospectively by two rheumatologists and a clinical pharmacist at baseline and subsequent visits. Data included demographics (age, sex, weight, height); clinical characteristics; diagnosis of the rheumatic diseases and disease duration, comorbidities; medical treatments including conventional and biologic DMARDs, GCs, time of initiation of GC treatment, and other drugs used for comorbidities. Low-dose GC therapy < 7.5 mg/day; medium dose, 7.5–30 mg/day; high dose was defined as 30–100 mg/day of prednisone equivalent.
To identify GC-related problems, patients’ weight, blood pressure, and muscle strength of both upper and lower extremities were measured, skin and neuropsychiatric toxicity, and possible infections were evaluated at each visit. If the laboratory parameters, such as fasting blood glucose, hemoglobin A1C (HbA1c) and lipid profile, had not been studied within the last 3 months, the physician was consulted to order routine blood tests. Drugs used for comorbidities were recorded, including any changes in drug and/or dosage at each visit. Similarly, if the bone mineral density (BMD) assessment for osteoporosis was not performed within the last year, the physician was consulted, and a BMD assessment was subsequently ordered. If a BMD assessment was performed within the last 2–3 years in patients who were already receiving osteoporosis treatment, they were not ordered for BMD assessment. At each clinical visit, patients were re-evaluated by the clinical pharmacists to ensure whether they were using medical treatments accordingly and adherently.
Classification and Assessment of Glucocorticoid-Related Problems
During the study period, GC-related problems were identified by the clinical pharmacist, and a consensus on the problems was reached in collaboration with a rheumatologist. The GC-related problems were categorized by the Pharmaceutical Care Network Europe (PCNE) classification system version 9.1 [15]. This classification system categorizes GC-related problems as in problem (six subdomains), its causes (38 subdomains), the planned interventions (17 subdomains), acceptance of the intervention proposal (ten subdomains) and outcomes of the intervention (seven subdomains). GC-related problems were considered completely resolved once the physician accepted the pharmacist’s recommendation and implemented the changes for GC treatment before any adverse effects occurred.
Statistical Analysis
Data were analyzed with IBM Statistics SPSS for Windows v23.0 software. The prevalence and types of DRPs are reported as a percentage. Categorical variables were presented as frequency and percentage, and continuous variables were presented as mean and standard deviation (SD) or median and interquartile range (IQR) based on the distribution of the data. The normality of the quantitative variables was assessed using Kolmogorov–Smirnov normality test. The t test for parametric variables and the Mann–Whitney U test for non-parametric variables were used to evaluate the association between continuous variables. Categorical variables were compared with the Pearson chi-square or Fisher’s exact tests. All statistical tests were performed at the statistical significance level of 5%.
Results
Demographics and Clinical Characteristics
A total of 156 patients (57.7% female) were included, with a mean age (± SD) of 49.1 ± 17.1 years. More than half of the patients (62.2%) had primary systemic vasculitides following chronic inflammatory arthritis. A total of 95 (60.9%) patients had at least one comorbidity, the most common ones being hypertension (34.6%) and diabetes mellitus (19.2%), followed by hypothyroidism (14.1%) and hyperlipidemia (10.9%) (Table 1).
Glucocorticoid Use
At baseline (t0), 38.5% of patients were on low-dose (≤ 7.5 mg/day) GCs. Prednisolone was used in 53.2% of patients, and the remaining were using methylprednisolone. Intravenous pulsed methylprednisolone was given for 27.6% of patients during the disease duration and the most common dose regimen was 1000 mg/day for 3 days. At the end of 6 months, patients have received a median (IQR) cumulative prednisolone dose of 4930 mg (7237.5 mg) (Table 1).
Medications Used in the Study Population
Half of the patients were on conventional synthetic DMARDs, the most being methotrexate (38.5%, n = 60), followed by hydroxychloroquine (30.8%, n = 48). Biological DMARDs were only prescribed in 16.7% of patients. Thirteen patients (8.3%) were only on GCs with no additional immunosuppressive therapy (Fig. 1).
Besides immunosuppressive treatments, most of the patients (96.8%) were also taking additional medications for comorbid conditions at baseline (t0) (Table 1). The most commonly used drugs were gastroprotective agents and calcium/vitamin D supplements, which were used in 78.2% (n = 122) and 71.2% (n = 111) of patients, respectively (Fig. 2).
Glucocorticoid-Related Problems
Overall, 236 GC-related problems were identified in 103 (66%) patients, with a median of 1 (minimum–maximum: 0–5) problems per patient. Among 103 patients, 25.2% had one, 40.8% had two, and 34% had three or more GC-related problems. The incidence of GC-related problems did not differ between the low (66.7%), medium (64.7%), and high dose (66.7%) of GC using patients (p = 0.97) at the time of enrolment. However, the median cumulative prednisolone dose was higher in patients with GC-related problems (3115 vs. 5455 mg, p = 0.01) (Table 1). The ‘adverse event (possibly) occurring’ was the most common subdomain of problems (n = 222) as ‘treatment safety’, and the remaining (n = 14) were related to ‘treatment effectiveness’.
Among the causes of 236 identified problems, ‘no or inappropriate monitoring’ (fasting blood glucose, HbA1c, lipid profile, BMD measurement for monitoring adverse events of GCs) was the most common (n = 98) and followed by ‘no or incomplete drug treatment despite existing indication’ (lipid-lowering drugs, antihypertensive and antidiabetic drugs, bisphosphonates, calcium/vitamin D supplement) (n = 94) (Table 2).
The most common cause (n = 7) of GC treatment being not optimal was the patient’s inappropriate use of drugs unintentionally. Furthermore, inadequate (wrong, unclear, or missing) instructions for timing of drug usage, patients using the drug less than the prescribed dosage or not taking the drug intentionally and frequent dosage regimens than the intended dose were the other causes identified for suboptimal GC treatment.
Planned Interventions
Amongst identified problems, 381 planned interventions were suggested for 224 problems: 47.7% (n = 182) at the ‘prescriber level’, 31.8% (n = 121) at the ‘patient level’ and 20.5% (n = 78) at the ‘drug level’ and 373 (98%) of them were accepted (Table 3). As a result of those recommendations, drug treatments were initiated (n = 60) (including calcium supplements, bisphosphonates, antihypertensives, lipid-lowering agents, and vitamin D), the dose was changed (n = 9), drug treatments were discontinued (n = 3), drug treatments were changed to alternatives (n = 3) and the instructions for the use of GC were changed (n = 3). No intervention was proposed for 12 out of 236 (5.1%) GC-related problems; all of these were associated with increased lipid levels that did not require treatment.
Outcomes of the Interventions
In total, 182 out of 236 GC-related problems (77.1%) were totally solved, seven (3.0%) were partially solved after the implementation of planned interventions, whereas 20 (8.4%) could not be solved despite the intervention due to patient/physician being non-adhere to the recommended intervention (Table 3). The outcome of the interventions could not be determined in 15 (6.4%) problems due to lost to follow-up.
Discussion
This is the first study particularly to evaluate GC-related problems and their management in patients with rheumatic diseases. In this study, we have shown that although patients experience GC-related problems, these problems can be solved by the interventions of a clinical pharmacist before they cause any harm to the patients. In patients receiving GC therapy, it is important to consider individual risk factors and/or preventive measures and to optimize treatment.
A study conducted in medical wards demonstrated that 71% of patients had GC-related problems [33], which is consistent with the findings of our study. In addition, studies evaluating DRPs in patients with rheumatoid arthritis reported that more than two-thirds of patients had a mean of 1.5 and 1.6 DRP per patient, respectively [16, 17].
According to the classification of the problems, the most common GC-related problems observed were related to treatment safety (94.1%), which can be considered as potential adverse drug events. It can be acknowledged that adverse events cannot only be attributed to glucocorticoids because of pre-existing comorbidities (such as hypertension and diabetes), which can be worsened with concurrent GC treatment. It is difficult to establish causality with GCs alone and to distinguish adverse events attributable to GCs from those that occur as a result of the underlying disease, comorbidities or comedications [34].
Although the rate of adverse drug reactions associated with GC treatment was found high in this study (94.1%) compared to the rate found by Chepkonga et al. (39.4%) [33], this difference can be explained by the use of different categorization for DRPs. The PCNE classification system is designed to capture possible adverse drug events, which may increase the rate of adverse drug events (possibly) occurring in this study. Adverse drug events were also found to be the most common DRPs both in rheumatology [16, 17, 35] and other settings [33, 36, 37] in previous studies. This indicates the need for patients to be adequately screened by pharmacists and physicians for the potential risk of adverse events.
Many factors could explain why patients with GC-related problems had higher cumulative doses of prednisolone in this study. Cumulative prednisolone exposure is related to the type, duration, severity and nature of the rheumatic disease. These factors and a higher cumulative dose of prednisolone increase the risk of complications, comorbidities, comedications, adverse events and the need for routine monitoring. This may lead to an increased risk of GC-related problems [38]. However, no difference was found in the incidence of GC-related problems between the patients receiving the low, medium and high doses of glucocorticoids at the time of the enrolment. This finding can be explained by having a significant proportion of the identified problems being related to the lack of laboratory monitoring (such as HbA1c and BMD measurements), and patients did not receive a constant dose of GCs (which was adjusted by the physician according to the disease activation, remission or adverse events) throughout the study. It is important to note that the lack of monitoring can lead to serious consequences for patients. Therefore, regular laboratory monitoring is crucial for patients using GC to detect and manage GC-related problems at an early stage in order to ensure patient safety.
Even though only a small proportion of the problems identified in this study were related to ‘treatment effectiveness’, many of those problems were patient-originated. The complexity of rheumatic diseases and their management strategies (including GCs tapering) and concerns about adverse events may have led to intentional or unintentional inappropriate use of GCs in rheumatology practice. This can result in ineffective treatment, treatment failure, lack of patient adherence and reduced patients’ quality of life. Therefore, interventions that address the educational needs of patients with rheumatic diseases, with a focus on GC treatment, may increase patient’s awareness and improve adherence.
The main causes of the GC-related problems identified in this study were lack of laboratory monitoring for conditions that may have developed by long-term GC use (41.5%) and no drug treatment despite existing indications (mainly for lipid-lowering agents) (39.8%). It has been reported that untreated conditions often occur due to rheumatologists not addressing the patient’s minor conditions, such as anemia, constipation, and diarrhea [37]. Likewise, rheumatologists have primarily concentrated on treating patients' rheumatic diseases rather than monitoring drug side effects. This is due to the high patient load of the outpatient clinic and the time constraints of their practice in this study. It has been known that failure to treat these conditions in patients using GCs can increase the risk of cardiovascular disease and fractures, reduce patients’ quality of life and affect treatment compliance [2].
Almost all interventions proposed by clinical pharmacists were accepted (98%) and 80.1% of problems were solved. This finding was comparable with the results of a similar study conducted in patients with rheumatoid arthritis by Sah SK et al., which found the acceptance rate as 91.3% [17]. This demonstrated that the clinical pharmacist’s recommendations to resolve DRPs were highly appreciated and accepted by the rheumatologists. However, it should be noted that a small number of recommendations were not accepted or accepted but not implemented in this study. The reasons for this may include the patient's clinical condition not allowing the recommendation to be implemented, the patient's or physician's lack of cooperation and the physician postponing the implementation to the next follow-up visit.
In regards to the interventions, the initiation of lipid-lowering agents was the main recommendation, which was not implemented. This can be attributed to the factors of; conservative approaches of the rheumatologists in this study, an asymptomatic nature of dyslipidemia [39] and atherosclerotic cardiovascular disease being developed slowly over time [40, 41]. Therefore, physicians may be reluctant to add a new drug to a patient's already complex treatment, which may have led them to prioritize dyslipidemia as later in the list of problems [42, 43].
It is important to highlight the strengths of this study. Firstly, to the best of our knowledge, there has been no previous research on the identification and management of GC-related problems in the rheumatology setting, despite the availability of therapeutic guidelines. The study's prospective nature has provided an opportunity to demonstrate the clinical impact of interventions following the identification of GC-related problems. Despite its strengths, this study has some limitations. As the patient’s comorbidities and heterogeneity of rheumatic diseases may contribute to the development of identified adverse events, it cannot be fully regarded as GCs-related. The outcomes of the interventions could not be determined in 15 problems due to patients being lost to follow-up. Finally, the study was conducted in a single outpatient clinic, which may limit the generalizability of study findings.
Conclusions
This study showed that GC-related problems are common in patients with rheumatic diseases, and the integration of a clinical pharmacist into the routine clinic plays an important role in facilitating the identification of GC-related problems and assisting physicians to resolve these problems. Given the fact that most common problems were related to inadequate monitoring or GC-induced untreated indications, pharmacists can be well-positioned to support individualized patient care by reviewing patients' medications, identifying DRPs, and providing safe and effective medication use.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Petta I, Peene I, Elewaut D, Vereecke L, De Bosscher K. Risks and benefits of corticosteroids in arthritic diseases in the clinic. Biochem Pharmacol. 2019;165:112–25.
van der Goes MC, Jacobs JW, Bijlsma JW. The value of glucocorticoid co-therapy in different rheumatic diseases—positive and adverse effects. Arthritis Res Ther. 2014;16(Suppl 2):S2.
Costello R, Patel R, Humphreys J, McBeth J, Dixon WG. Patient perceptions of glucocorticoid side effects: a cross-sectional survey of users in an online health community. BMJ Open. 2017;7(4): e014603.
McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008;20(2):131–7.
Venter G, Tieu J, Black R, Lester S, Leonardo N, Whittle SL, et al. Perspectives of glucocorticoid use in patients with rheumatoid arthritis. ACR Open Rheumatol. 2021;3(4):231–8.
Zerah L, Arena C, Morin AS, Blanchon T, Cabane J, Fardet L. Patients’ beliefs about long-term glucocorticoid therapy and their association to treatment adherence. Rev Med Interne. 2012;33(6):300–4.
Buttgereit F, Palmowski A. How to taper glucocorticoids in inflammatory rheumatic diseases? A narrative review of novel evidence in rheumatoid arthritis, systemic lupus erythematosus, and giant cell arteritis. Jt Bone Spine. 2022;89(1): 105285.
Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30.
Alten R, Mischkewitz M. New concepts to reduce glucocorticoid toxicity. Jt Bone Spine. 2019;86(6):715–23.
Hoes JN, Jacobs JW, Boers M, Boumpas D, Buttgereit F, Caeyers N, et al. EULAR evidence-based recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2007;66(12):1560–7.
Duru N, van der Goes MC, Jacobs JW, Andrews T, Boers M, Buttgereit F, et al. EULAR evidence-based and consensus-based recommendations on the management of medium to high-dose glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2013;72(12):1905–13.
Humphrey MB, Russell L, Danila MI, Fink HA, Guyatt G, Cannon M, et al. 2022 American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023;75(12):2088–102.
van der Goes MC, Jacobs JW, Boers M, Andrews T, Blom-Bakkers MA, Buttgereit F, et al. Monitoring adverse events of low-dose glucocorticoid therapy: EULAR recommendations for clinical trials and daily practice. Ann Rheum Dis. 2010;69(11):1913–9.
Messina OD, Vidal LF, Wilman MV, Bultink IEM, Raterman HG, Lems W. Management of glucocorticoid-induced osteoporosis. Aging Clin Exp Res. 2021;33(4):793–804.
PCNE Classification for Drug-Related Problems V9.1. https://www.pcne.org/upload/files/417_PCNE_classification_V9-1_final.pdf. Accessed 14 Mar 2024.
Ma SN, Zaman Huri H, Yahya F. Drug-related problems in patients with rheumatoid arthritis. Ther Clin Risk Manag. 2019;15:505–24.
Sah SK, Ramaswamy S, Ramesh M. Frequency and risk factors for the development of drug related problems among rheumatoid arthritis patients. Clin Epidemiol Glob Health. 2022;13: 100969.
Ernst ME, Iyer SS, Doucette WR. Drug-related problems and quality of life in arthritis and low back pain sufferers. Value Health. 2003;6(1):51–8.
Burkhart PV, Sabaté E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh. 2003;35(3):207.
van den Bemt BJ, van den Hoogen FH, Benraad B, Hekster YA, van Riel PL, van Lankveld W. Adherence rates and associations with nonadherence in patients with rheumatoid arthritis using disease modifying antirheumatic drugs. J Rheumatol. 2009;36(10):2164–70.
Leendertse AJ, Van Den Bemt PM, Poolman JB, Stoker LJ, Egberts AC, Postma MJ. Preventable hospital admissions related to medication (HARM): cost analysis of the HARM study. Value Health. 2011;14(1):34–40.
Nauman J, Soteriades ES, Hashim MJ, Govender R, Al Darmaki RS, Al Falasi RJ, et al. Global incidence and mortality trends due to adverse effects of medical treatment, 1990–2017: a systematic analysis from the global burden of diseases, injuries and risk factors study. Cureus. 2020;12(3): e7265.
Magdelijns FJ, Stassen PM, Stehouwer CD, Pijpers E. Direct health care costs of hospital admissions due to adverse events in The Netherlands. Eur J Public Health. 2014;24(6):1028–33.
Sah SK, Subramanian R, Ramesh M, Chand S. Impact of pharmacist care in the management of autoimmune disorders: a systematic review of randomized control trials and non-randomized studies. Res Soc Adm Pharm. 2021;17(9):1532–45.
Yailian AL, Revel E, Tardy C, Fontana A, Estublier C, Decullier E, et al. Assessment of the clinical relevance of pharmacists’ interventions performed during medication review in a rheumatology ward. Eur J Intern Med. 2019;59:91–6.
van Roon EN, van den Bemt PM, Jansen TL, Houtman NM, van de Laar MA, Brouwers JR. An evidence-based assessment of the clinical significance of drug-drug interactions between disease-modifying antirheumatic drugs and non-antirheumatic drugs according to rheumatologists and pharmacists. Clin Ther. 2009;31(8):1737–46.
Gutermann L, Dumas S, Lopez-Medina C, Boissinot L, Cotteret C, Perut V, et al. Impact of a pharmacist-led programme on biologics knowledge and adherence in patients with spondyloarthritis. Clin Exp Rheumatol. 2021;39(4):811–8.
Clifford S, Barber N, Elliott R, Hartley E, Horne R. Patient-centred advice is effective in improving adherence to medicines. Pharm World Sci. 2006;28(3):165–70.
Fields TR, Rifaat A, Yee AMF, Ashany D, Kim K, Tobin M, et al. Pilot study of a multidisciplinary gout patient education and monitoring program. Semin Arthritis Rheum. 2017;46(5):601–8.
Sandhu VK, Tuico A, Hum J, Zachary B. The impact of clinical pharmacist integration in a community rheumatology clinic. Am J Health Syst Pharm. 2023;80(10):551–7.
Stockl KM, Shin JS, Lew HC, Zakharyan A, Harada AS, Solow BK, et al. Outcomes of a rheumatoid arthritis disease therapy management program focusing on medication adherence. J Manag Care Pharm. 2010;16(8):593–604.
Petkova VB. Education for arthritis patients: a community pharmacy based pilot project. Pharm Pract (Granada). 2009;7(2):88–93.
Chepkonga, CJ. Drug therapy problems associated with corticosteroid use among patients admitted in medical wards at Kenyatta National Hospital (a thesis study). 2021.
Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68(7):1119–24.
Haegens LL, Huiskes VJB, Smale EM, Bekker CL, van den Bemt BJF. Drug-related problems reported by patients with rheumatic diseases: an observational study. BMC Rheumatol. 2023;7(1):7.
Zhang S, Zhang GB, Huang P, Ren Y, Lin B, Shao YF, et al. Drug-related problems in hospitalized patients with chronic kidney diseases and clinical pharmacist interventions. BMC Geriatr. 2023;23(1):849.
Greeshma M, Lincy S, Maheswari E, et al. Identification of drug related problems by clinical pharmacist in prescriptions with polypharmacy: a prospective interventional study. J Young Pharm. 2018;10(4):460–5.
Yasir M, Goyal A, Sonthalia S. Corticosteroid adverse effects. In: StatPearls. Treasure Island: StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK531462/.
Chee YJ, Chan HH, Tan NC. Understanding patients’ perspective of statin therapy: can we design a better approach to the management of dyslipidaemia? A literature review. Singap Med J. 2014;55(8):416–21.
Scott J. The pathogenesis of atherosclerosis and new opportunities for treatment and prevention. J Neural Transm Suppl. 2002;63:1–17.
Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Nerem RM. The pathogenesis of atherosclerosis: an overview. Clin Cardiol. 1991;14(2 Suppl 1):I1-16.
Kara E, Tecen Yucel K, Bayraktar-Ekincioglu A, Demirkan K, Tokgozoglu L, Unal S. Evaluation of internal medicine physicians’ attitudes toward the treatment of dyslipidemia. Postgrad Med. 2020;132(6):538–43.
Durack-Bown I, Giral P, d’Ivernois JF, Bazin C, Chadarevian R, Benkritly A, et al. Patients’ and physicians’ perceptions and experience of hypercholesterolaemia: a qualitative study. Br J Gen Pract. 2003;53(496):851–7.
Acknowledgements
We thank the all the staff and care team at the rheumatology clinic and the study participants.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Conceptualization: [Melda Bahap-Kara, Aygin Bayraktar-Ekincioglu]; Methodology: [Melda Bahap-Kara, Emine Sariyildiz, Gozde K. Yardimci, Omer Karadag, Aygin Bayraktar-Ekincioglu]; Formal analysis and investigation: [Melda Bahap-Kara, Emine Sariyildiz]; Writing—original draft preparation: [Melda Bahap-Kara, Emine Sariyildiz]; Writing—review and editing: [Aygin Bayraktar-Ekincioglu, Omer Karadag]; Resources: [Melda Bahap-Kara, Emine Sariyildiz]; Supervision: [Aygin Bayraktar-Ekincioglu, Omer Karadag]. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Melda Bahap-Kara, Emine Sariyildiz, Gozde K. Yardimci, Omer Karadag, and Aygin Bayraktar-Ekincioglu have nothing to disclose.
Ethical Approval
The study was approved by the University Clinical Trials Ethics Committee (KA-21026). All patients provided written informed consent to participate in the study. The study was conducted according to the Declaration of Helsinki and the Harmonization of Good Clinical Practice Guidelines.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bahap-Kara, M., Sariyildiz, E., Yardimci, G.K. et al. Addressing Glucocorticoid-Related Problems with the Clinical Pharmacist Collaboration in Rheumatology Practice: A Prospective Follow-Up Study. Rheumatol Ther (2024). https://doi.org/10.1007/s40744-024-00692-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40744-024-00692-z