Abstract
Introduction
Patients with rheumatoid arthritis (RA) may have an increased malignancy risk versus the general population, potentially elevated by biological disease-modifying antirheumatic drug (bDMARD) use. Using patient registry data, we determined malignancy risk, stratified by bDMARD use, among Japanese patients with RA versus the Japanese general population and investigated whether bDMARD use is a time-dependent risk factor for the development of malignancy.
Methods
Patients aged ≥ 18 years with ≥ 2 data entries of RA in the IORRA (Institute of Rheumatology, Rheumatoid Arthritis) patient registry, enrolled from January 2013–December 2018, were identified (‘All RA’ cohort). Patients were stratified into bDMARD (≥ 1 bDMARD received) or non-bDMARD (no history of bDMARDs) sub-cohorts. Malignancy incidence rates and standardized incidence ratios (SIRs) with 95% confidence intervals (CIs) versus the Japanese general population were calculated. Risk of RA medication use was analyzed using a time-dependent Cox proportional hazards model, after adjusting for covariates.
Results
A total of 8020 patients were identified for the All RA cohort; 2187 and 5833 for the bDMARD and non-bDMARD sub-cohorts, respectively. For all three cohorts, incidence of overall malignancies was similar versus the Japanese general population. Incidence of specific malignancies was also similar, but incidence of lymphoma was higher for all three cohorts (SIRs [95% CIs] 3.72 [2.71–4.93], 5.97 [3.34–9.59], and 2.79 [1.82–4.02], respectively). In the bDMARD sub-cohort, no increase in SIRs was observed for other site-specific malignancies. In the All RA cohort, use of methotrexate, tacrolimus, glucocorticoids, non-steroidal anti-inflammatory drugs, and bDMARDs were not associated with the risk of overall malignancy; the hazard ratio (95% CI) was 1.36 (0.96–1.93) for bDMARD use. Increased disease activity was a time-dependent risk factor of overall malignancy with a hazard ratio (95% CI) of 1.35 (1.15–1.59).
Conclusions
The use of bDMARDs was not a time-dependent risk factor for malignancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Patients with rheumatoid arthritis (RA) may be at an increased risk of malignancies compared with the general population, and this risk is potentially elevated due to the use of biological disease-modifying antirheumatic drugs (bDMARDs) in RA, although evidence remains unclear. |
This analysis used IORRA (Institute of Rheumatology, Rheumatoid Arthritis) patient registry data to determine the risk of malignancies in Japanese patients with RA, stratified by bDMARD use, versus the general Japanese population and to investigate whether bDMARD use is a time-dependent risk factor for the development of malignancy in Japanese patients with RA. |
What was learned from the study? |
The overall incidence of malignancies for patients with RA enrolled in the IORRA patient registry from January 2013–December 2018 was similar to that of the Japanese general population, except for an increase in lymphoma incidence. |
bDMARD use was not a time-dependent risk factor for malignancy, but increased disease activity was. |
This study provides useful insights into the impact of recent progress in RA treatment strategies on malignancy incidences and risk factors in Japanese patients with RA. However, future research in this area is warranted, particularly regarding the impact of Janus kinase inhibitors on malignancies. |
Introduction
Patients with rheumatoid arthritis (RA) may be at an increased risk of malignancies compared with the general population [1]. This risk varies by malignancy type, as well as by race and geographic region [1,2,3,4].
The IORRA (Institute of Rheumatology, Rheumatoid Arthritis) patient registry provides real-world data from the Institute of Rheumatology at Tokyo Women’s Medical University, the largest specialized treatment facility for RA in Japan [5]. Among patients enrolled in the IORRA patient registry between April 2001 and April 2005, an overall increased incidence of malignancy was identified, with particular elevations in lymphoma and lung cancer compared with the general Japanese population [6]. Furthermore, male sex, older age, rheumatoid factor positivity, and disease activity were identified as risk factors for malignancy among patients in the IORRA registry [6, 7].
Evidence from meta-analyses of randomized controlled trials (RCTs) for whether the use of biological disease-modifying antirheumatic drugs (bDMARDs) further elevates the risk of malignancies among patients with RA remains unclear. This is particularly evident in analyses comparing the risk of malignancies in patients with RA or other rheumatic diseases receiving bDMARDs, such as tumor necrosis factor inhibitors (TNFi) or interleukin (IL) inhibitors, versus those receiving placebo, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) such as methotrexate (MTX), or no therapy. For instance, an increased risk of malignancies with IL inhibitors has been reported in pooled results of 74 RCTs across rheumatologic diseases [8], while similar results were reported for TNFi use in an analysis of 9 RCTs of patients with RA [9]. In contrast, other meta-analyses of patients with RA have not shown such an association [10,11,12].
Interestingly, real-world data from large registries did not support bDMARDs or TNFi increasing the risk of malignancies. The SafEty of biologics in Clinical Use in Japanese patients with RhEumatoid arthritis (SECURE) nationwide registry of Japanese patients with RA who have ever been treated with bDMARDs versus the Japanese general population concluded that the use of bDMARDs did not increase risk for overall and site-specific malignancy, except for lymphoma [13]. Data from the German biologics RABBIT (Rheumatoide Arthritis: Beobachtung der Biologika-Therapie) register of patients with RA, and the British Society for Rheumatology Biologics Register for RA, support no overall increased risk of cancer with TNFi compared with csDMARDs [14, 15].
Meta-analyses of real-world data also concluded that patients with RA and prior malignancy receiving bDMARDs were at no increased risk of cancer compared with those receiving csDMARDs [16], and among patients with RA, TNFi treatments did not increase risk of malignancies, except skin cancer, when compared with patients not exposed to TNFi [17]. Systematic reviews and meta-analyses indicated a possible increase in the risk of skin cancer in patients with RA receiving TNFi compared with those receiving non-TNFi therapy [17, 18].
A variety of TNFi and non-TNFi bDMARDs have become available in the clinical setting since the launch of the first bDMARD into the Japanese market in 2003, and the proportion of bDMARD users among patients with RA increased from 2012 to 2018 [5, 19]. With mixed reports on the relationship between bDMARD use and the risk of malignancies, in addition to substantial changes in the real-world use of bDMARDs, there is a need for more comprehensive, long-term data—with advanced statistical methods—regarding malignancies in patients with RA treated with a bDMARD compared with the general population.
In this analysis, we used data from patients enrolled in the IORRA patient registry from January 2013 to December 2018 to determine the risk of malignancies in patients with RA, stratified by bDMARD use, versus the general Japanese population, and investigated whether bDMARD use is a time-dependent risk factor for the development of malignancies in patients with RA.
Methods
Study Design
Epidemiologic data are useful to contextualize safety events of interest that cannot be interpreted in clinical trials, for example because they are rare or have long latency periods, such as malignancy events. This retrospective, observational cohort study utilized data from patients with RA enrolled in the IORRA patient registry (based at the Tokyo Women’s Medical University Hospital, Japan) from 2013 to 2018, with follow-up through December 2019.
Data have been collected biannually (in April and October) from the IORRA cohort since it was established in 2000, providing data from 5000–6000 patients [5] with RA diagnosed based on American College of Rheumatology criteria. Collected data include patients’ self-assessment (using the IORRA questionnaire), physician assessment, and laboratory data. Safety data are obtained by patient self-reporting (using the IORRA questionnaire), with data on malignancies also confirmed using the medical records by physicians belonging to IORRA. Only malignancy events that occurred during the observational period were included; events that occurred prior to the index date (as described later) were excluded.
Comprehensive patient consent was obtained at enrollment into the IORRA patient registry [5], and consent for data to be used for studies involving the IORRA patient registry was obtained at each survey. Informed consent for this specific study was not required, although an opt-out approach to consent was used. The final protocol, any amendments, and informed consent documentation were approved by the IORRA committee (2922-R16).
Cohort Selection
Eligible patients with RA, ≥ 18 years of age at index date and with ≥ 2 data entries of RA in the IORRA patient registry since 2013, were identified for the ‘All RA’ cohort. Patients with comorbid malignancies, or prior history of malignancies, were not excluded.
Patients were also stratified into sub-cohorts based on treatments received. In addition to meeting criteria for the All RA cohort, patients selected for the bDMARD sub-cohort were required to have a record of ≥ 1 bDMARD recorded in the IORRA patient registry; patients selected for the non-bDMARD sub-cohort were required to have no bDMARDs recorded within the IORRA patient registry (bDMARDs are described in Supplemental Table S1). A sub-cohort of bDMARD new users (patients who received bDMARDs as naïve) was also identified; however, due to the small numbers of events, these patients were not included in the main analysis, but results are presented in the Supplementary Materials.
Data Analysis and Outcomes
Anonymized data on patient demographics and baseline disease characteristics were extracted from the IORRA patient registry. Malignancies were identified by patient self-report and confirmed by physician review of medical records. Recurrent malignancy was not counted as an event. The study observation period was defined as the period from the index date to (1) the occurrence of malignancy of interest (for the analysis of overall malignancy, observation was censored at the occurrence of the first malignancy, and for the analysis of a site-specific malignancy, observation was censored at the first occurrence of the corresponding site-specific malignancy); (2) withdrawal from the IORRA database; or (3) end of study follow-up (December 2019), whichever was earliest. The index date was specified per cohort based on the initial date of entry into the IORRA patient registry for the All RA cohort, or the next IORRA survey after the date of initial bDMARD use for the bDMARD cohort. Baseline was defined as the 6-month period prior to the index date. Events outside of the observation period were excluded. For patients who initiated bDMARD treatment after their entry into the IORRA patient registry, the period between enrollment into the registry to the index date was not included in the bDMARD sub-cohort but was counted in the All RA cohort.
Incidence rates (IRs; cumulative for 2013–2019) stratified by cohort were calculated, with 95% confidence intervals (CIs), for overall malignancies including and excluding non-melanoma skin cancer (NMSC), and for site-specific malignancies with ≥ 20 events in the All RA cohort (i.e., breast, lung, colon, stomach, and lymphoma). IRs were calculated as the number of incident events divided by the total person-years (PY) of observation within each cohort and reported per 100 PY of follow-up. Terms in the IORRA database used for cancer types of interest are described in Supplemental Table S2.
Age- and sex-standardized rates (ASRs) and standardized incidence ratios (SIRs) were calculated with 95% CIs (details of calculation for ASRs and SIRs with 95% CIs are described in the Supplemental Methods) for overall malignancies and site-specific malignancies with ≥ 20 events in the All RA cohort (i.e., breast, lung, colon, stomach, and lymphoma) and compared with the general population, using a Japanese population database of malignancy incidence provided by the Center for Cancer Control and Information Services, National Cancer Center (NCC), Japan. ASRs and SIRs were calculated for the above-mentioned site-specific malignancies, except for those with < 10 events in each treatment sub-cohort. SIRs were calculated from NCC data for the 2013–2019 period [20], as well for each cohort in the IORRA patient registry. For the calculation of IR, SIR, and ASR for overall malignancy, only the first malignancy for each patient was counted as an event. When a patient developed a double malignancy as the first malignancy, it was counted as one event. An SIR of 1.00 indicated no increase or decrease in risk versus the general population.
Risk factor analysis for overall malignancy was conducted using the first malignancy as an event. Hazard ratios (HRs) with 95% CIs were calculated using a multivariable Cox proportional hazards model, with time-dependent covariates including use of MTX, tacrolimus, glucocorticoids, non-steroidal anti-inflammatory drugs (NSAIDs), and bDMARDs. The following characteristics were used as adjusting factors: age, sex, RA duration, body mass index (BMI), smoking status, Disease Activity Score in 28 joints, erythrocyte sedimentation rate (DAS28-4[ESR]) score, Japanese version of the Health Assessment Questionnaire (J-HAQ) score, EuroQol-Five Dimensions (EQ-5D) score, rheumatoid factor (RF) levels, previous history of malignancy, and presence of comorbidities (interstitial pneumonia [IP], liver dysfunction, and diabetes mellitus [DM]). p values of < 0.05 were considered to be evidence of an association between covariates and elevated risk of developing malignancies. Analyses were descriptive and conducted using R version 4.0 software. Adjustments for multiple comparisons were not made and missing data in the multivariable analysis were imputed by the last observed value.
Results
Patients
A total of 8020 patients with RA who met the eligibility criteria were identified in the IORRA patient registry and included in the All RA cohort. The bDMARD and non-bDMARD sub-cohorts comprised 2187 and 5833 patients, respectively. The median duration of observation was 1659 days for the All RA cohort, 1626 days for the bDMARD sub-cohort, and 1644 days for the non-bDMARD sub-cohort. Among the All RA cohort at baseline, 85.0% of patients were female, with a mean age of 59.3 years and a mean disease duration of 12.8 years. Most patients (75.9%) were receiving MTX; 18.8% and 14.5% of patients were receiving bDMARDs and TNFi, respectively. Patient demographics and baseline disease characteristics were generally consistent across the All RA cohort and treatment sub-cohorts (Table 1). At index date, 68% of the patients in the bDMARD sub-cohort were receiving TNFi (Supplemental Table S3). The bDMARD new user sub-cohort comprised 630 patients; the patient demographics and baseline disease characteristics for this sub-cohort are described in Supplemental Table S4.
Incidence of Malignancies
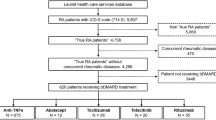
By December 2019, a total of 296 patients with 317 malignancy events were observed in the All RA cohort (Fig. 1). We also observed 73 patients experiencing 80 malignancy events in the bDMARD sub-cohort, and 218 patients with 231 malignancy events in the non-bDMARD sub-cohort by December 2019. Within the All RA cohort, six malignancies were not counted in either treatment sub-cohort due to the definition of the observation period.
Malignancy events in the All RA cohort. All RA cohort: eligible patients with RA, ≥ 18 years of age at index date and with ≥ 2 data entries of RA in the IORRA patient registry since 2013. The observation period for overall malignancy was defined as the period from the index date to (1) the occurrence of the first malignancy (see Methods section for details); (2) withdrawal from the IORRA patient registry; or (3) end of study follow-up, whichever was earliest. The observation period for site-specific malignancy was defined as the period from the index to (1) the occurrence of the first corresponding site-specific first malignancy (see Methods section for details); (2) withdrawal from the IORRA patient registry; or (3) end of study follow-up, whichever was earliest. Malignancy events were self-reported by patients in biannual surveys, and confirmed following medical record review by co-authors (Ryoko Sakai and Naohiro Sugitani). Only events that occurred within the observation period (2013–2019) were included in the analysis. IORRA Institute of Rheumatology, Rheumatoid Arthritis, N number of patients included in the cohort, n number of patients experiencing malignancy events, RA rheumatoid arthritis
The most frequently reported malignancies in the All RA cohort were breast (53 events), lymphoma (45 events), stomach (32 events), colon (31 events), and lung (25 events) (Table 2). The most frequently reported malignancies in the bDMARD sub-cohort were lymphoma (15 events), breast (14 events), and stomach (10 events). The most frequently reported malignancies in the bDMARD new user sub-cohort are shown in Supplemental Table S5, along with all malignancy events in each cohort.
IRs and SIRs for overall malignancies and site-specific malignancies for the All RA cohort and treatment sub-cohorts are shown in Table 2. IRs and ASRs for overall and site-specific malignancies by cohort are shown in Supplemental Table S6. SIRs (95% CIs) for overall malignancies, overall malignancies excluding NMSC, breast cancer, and stomach cancer for the All RA cohort were 0.90 (0.80–1.01), 0.89 (0.79–1.00), 0.87 (0.65–1.13), and 0.83 (0.56–1.15), respectively. SIRs for lymphoma, colon cancer, and lung cancer for the All RA cohort were 3.72 (2.71–4.93), 0.59 (0.40–0.83), and 0.65 (0.42–0.94), respectively (Table 2).
For the bDMARD sub-cohort, SIRs (95% CIs) were 1.06 (0.83–1.33), 1.06 (0.83–1.33), and 5.97 (3.34–9.59) for overall malignancy, overall malignancy excluding NMSC, and lymphoma (Table 2), respectively. For the non-bDMARD sub-cohort, SIRs (95% CIs) were 0.86 (0.75–0.98), 0.85 (0.74–0.97), 0.65 (0.40–0.99), 0.66 (0.43–0.94), and 2.79 (1.82–4.02) for overall malignancy, overall malignancy excluding NMSC, lung cancer, colon cancer, and lymphoma, respectively (Table 2). For the bDMARD new user sub-cohort, the SIR (95% CI) was 1.02 (0.58–1.61) for overall malignancy (Supplemental Table S7).
Risk Factors for Malignancies
HRs (95% CIs) of medication use for overall malignancies are shown in Fig. 2. The use of MTX, tacrolimus, glucocorticoids, NSAIDs, and bDMARDs were not time-dependent risk factors for overall malignancy in the All RA cohort (Fig. 2). HRs of adjusting factors are shown in Supplemental Table S8. Of note, a positive association between DAS28-4(ESR) and overall malignancy was observed with HR (95% CI) of 1.35 (1.15–1.59) and p value of < 0.001 (Supplemental Table S8).
HRs (95% CIs) for developing overall malignancy with medications used in RA (All RA cohort). p values of < 0.05 were considered to be evidence of an association between covariates and elevated risk of developing malignancies. Risk factor reference groups are indicated in parentheses. All RA cohort: eligible patients with RA, ≥ 18 years of age at index date and with ≥ 2 data entries of RA in the IORRA patient registry since 2013. The observation period for overall malignancy was defined as the period from the index date to (1) the occurrence of the first malignancy (see Methods section for details); (2) withdrawal from the IORRA patient registry; or (3) end of study follow-up, whichever was earliest. bDMARD biological disease-modifying antirheumatic drug, CI confidence interval, HR hazard ratio, IORRA Institute of Rheumatology, Rheumatoid Arthritis, MTX methotrexate, NSAID non-steroidal anti-inflammatory drug, RA rheumatoid arthritis
Discussion
This analysis of data from > 8000 Japanese patients with RA enrolled in the IORRA patient registry between 2013 and 2018 found that the overall incidence of malignancies was similar across three cohorts and similar to that of the Japanese general population (SIRs for overall malignancy across cohorts were ~1.00 and the lower limits of the 95% CIs were < 1.00 indicating there was no increased risk of malignancies in patients with RA). Use of bDMARDs was not identified as a time-dependent risk factor for malignancies in the All RA cohort.
Since the introduction of TNFi, and subsequently other bDMARDs with different mechanisms of action, the risk of malignancies with their use in RA and other rheumatic diseases has been widely studied, although results vary. The findings of meta-analyses on risk of overall malignancies are inconsistent, with an increased risk being reported in two meta-analyses for TNFi [9] and IL inhibitors [8], but not in other meta-analyses [18, 21,22,23,24,25,26]. Several real-world studies conducted in Japan and other countries have also reported a lack of substantial increased risk of overall malignancies [13,14,15, 27,28,29] or lymphoma [27, 30] with the use of bDMARDs in RA. Reflecting these results, adding a bDMARD to MTX therapy is usually recommended for patients with RA who fail to reach treatment target despite treatment with MTX or csDMARDs in many clinical practice guidelines and recommendations [31,32,33] without a particular concern for the risk of malignancy.
The association between RA disease activity and risk of overall malignancy [7] or lymphoma [34] makes it very difficult to analyze the causal relationship between bDMARD use and malignancy and many of the previous studies do not address this issue. To overcome this, our study used time-dependent Cox regression analysis and found that the use of bDMARDs is not a significant time-dependent risk factor for malignancies after adjusting for various covariates including RA disease activity. We also confirmed that disease activity (i.e., DAS28-4[ESR]) was significantly associated with occurrence of overall malignancy, suggesting that reducing and maintaining RA disease activity within 3 to 6 months after initiating treatment according to the treat-to-target strategy [35] may lead to a reduction in the risk of malignancy in patients with RA. The changes in drug use patterns in RA (i.e., increased use of targeted synthetic DMARDs and bDMARDs, as monotherapy or in combination with MTX, along with decreasing use of certain csDMARDs and NSAIDs), coupled with resultant reductions in disease activity and levels of inflammation [5, 36], may have implications for malignancy risk.
A previous analysis of the overall IORRA cohort from 2000 to 2013 investigated the risk of malignancies over three time periods, stratified by (1) pre bDMARD use (2000–2004); (2) early bDMARD use (2005–2009); and (3) recent bDMARD use (2010–2013) [7]. A similar risk for overall malignancies as that seen in the Japanese general population, and a significantly increased risk of lymphoma, was reported for each time period. Comparing the results of the current analysis of patients enrolled in the IORRA patient registry from 2013 to 2018 with previous cohorts (2001–2005 and 2000–2013), a trend for a decrease in lung cancer incidence over time and an increase in colon cancer over time, relative to the Japanese general population, were noted [6, 7]. The decrease in lung cancer incidence over time may be partly due to a decreasing trend in smoking in the patients of the IORRA cohort, as evidenced by the lower proportion of current smokers in the 2013–2018 cohort compared with the 2000–2013 cohort (9.2% versus 17.3%, respectively) [7]. In the Japanese general population at 60–69 years of age, 34.7% of males and 7.5% of females habitually smoked in 2002, and 30.9% of males and 7.8% of females habitually smoked in 2018 [37]. It is also notable that an increased risk of lung cancer in patients with RA has been reported mainly from Europe and the United States of America [1, 4, 38,39,40]. However, among Asian countries, the risk of lung cancer in patients with RA has been reported to be similar to the general population in China [41] and Taiwan [42], and decreased in Korea [43], which is in line with the present data from the IORRA cohort.
Up-to-date data on the incidence of malignancies are required for proper clinical management of patients with RA, particularly in societies with aging/aged populations, such as Japan. The association between malignancies and JAK inhibitors has gained attention in a recent study [44]. However, the number of patients receiving JAK inhibitors in the present study was limited (All RA cohort n = 4, bDMARD sub-cohort n = 6, non-bDMARD sub-cohort n = 2; Table 1) and we did not evaluate associations between malignancies for this drug class in this analysis. Therefore, future analyses of malignancies among patients with RA in the IORRA database will be warranted to provide further insights into how this drug class impacts the long-term risk of malignancies in patients with RA.
Strengths of this study included the size of the registry and the number of malignancies, the use of time-dependent regression models for risk factor analysis, and the data having been gathered from an unselected, real-world patient population. In addition, a high proportion of patients (> 96%) attending the Institute of Rheumatology outpatient clinic at Tokyo Women’s Medical University participated in the IORRA patient registry between January 2013–December 2018, indicating an absence of any significant bias due to the time of entry. Limitations of this study were aligned with those of observational studies in general, including the potential for unreported malignancies by patients leading to misclassification bias, and that it was not possible to obtain detailed clinical information on malignancies from some patients. In addition, the self-reporting of malignancies by patients may have also led to information bias; however, this was minimized by having physicians confirm the self-reported malignancies through a review of the medical records. Furthermore, this was a single-institute study, with a small number of cases of some site-specific malignancies. Caution should therefore be exercised when generalizing these results. Of patients who received bDMARDs in this analysis, most patients received TNFi; therefore, further studies are warranted to determine if individual classes of bDMARDs are time-dependent risk factors for malignancies in Japanese patients with RA.
Conclusions
The overall incidence of malignancies for patients with RA enrolled in the IORRA patient registry from 2013 to 2018 was similar across the three cohorts and to that of the Japanese general population. Use of bDMARDs was not a time-dependent risk factor for malignancies and a positive association between DAS28-4(ESR) and malignancy was observed. This study provides useful insights into the impact of recent progress in RA treatment strategies on the incidences and risk factors for malignancies in Japanese patients with RA, but future research in this area, particularly with regard to the impact of JAK inhibitors on malignancies, is warranted.
Data Availability
The aggregated data and material that support the findings of this study are available from the corresponding author, Masayoshi Harigai, upon reasonable request.
References
Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212.
Dougados M, Soubrier M, Antunez A, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. 2014;73:62–8.
Parikh-Patel A, White RH, Allen M, Cress R. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control. 2009;20:1001–10.
Smitten AL, Simon TA, Hochberg MC, Suissa S. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther. 2008;10:R45.
Yamanaka H, Tanaka E, Nakajima A, et al. A large observational cohort study of rheumatoid arthritis, IORRA: providing context for today’s treatment options. Mod Rheumatol. 2020;30:1–6.
Yamada T, Nakajima A, Inoue E, et al. Incidence of malignancy in Japanese patients with rheumatoid arthritis. Rheumatol Int. 2011;31:1487–92.
Sugimoto N, Tanaka E, Inoue E, et al. Trends in risks of malignancies in Japanese patients with rheumatoid arthritis: analyses from a 14-year observation of the IORRA cohort. Mod Rheumatol. 2023;33:715–22.
Bilal J, Berlinberg A, Riaz IB, et al. Risk of infections and cancer in patients with rheumatologic diseases receiving interleukin inhibitors: a systematic review and meta-analysis. JAMA Netw Open. 2019;2: e1913102.
Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85.
Maneiro JR, Souto A, Gomez-Reino JJ. Risks of malignancies related to tofacitinib and biological drugs in rheumatoid arthritis: systematic review, meta-analysis, and network meta-analysis. Semin Arthritis Rheum. 2017;47:149–56.
Thompson AE, Rieder SW, Pope JE. Tumor necrosis factor therapy and the risk of serious infection and malignancy in patients with early rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum. 2011;63:1479–85.
de La Forest DM, Gottenberg JE, Salliot C. Safety of biologic DMARDs in RA patients in real life: a systematic literature review and meta-analyses of biologic registers. Joint Bone Spine. 2017;84:133–40.
Harigai M, Nanki T, Koike R, et al. Risk for malignancy in rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs compared to the general population: a nationwide cohort study in Japan. Mod Rheumatol. 2016;26:642–50.
Strangfeld A, Hierse F, Rau R, et al. Risk of incident or recurrent malignancies among patients with rheumatoid arthritis exposed to biologic therapy in the German biologics register RABBIT. Arthritis Res Ther. 2010;12:R5.
Mercer LK, Lunt M, Low ALS, et al. Risk of solid cancer in patients exposed to anti-tumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis. 2015;74:1087–93.
Xie W, Xiao S, Huang Y, et al. A meta-analysis of biologic therapies on risk of new or recurrent cancer in patients with rheumatoid arthritis and a prior malignancy. Rheumatology (Oxford). 2020;59:930–9.
Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895–904.
Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119–30.
Hirata A, Ota R, Hata T, et al. Prescribing trends of biologic disease-modifying anti-rheumatic drugs using a claims database from 6 million people in Japan. Clin Drug Investig. 2021;41:967–74.
National Cancer Center Japan Center for Cancer Control and Information Services. Cancer statistics. 2021. https://ganjoho.jp/reg_stat/index.html. Accessed March 3, 2023.
He B, Li Y, Luo WW, et al. The risk of adverse effects of TNF-α inhibitors in patients with rheumatoid arthritis: a network meta-analysis. Front Immunol. 2022;13:814429.
Leombruno JP, Einarson TR, Keystone EC. The safety of anti-tumour necrosis factor treatments in rheumatoid arthritis: meta and exposure-adjusted pooled analyses of serious adverse events. Ann Rheum Dis. 2009;68:1136–45.
Bonovas S, Minozzi S, Lytras T, et al. Risk of malignancies using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf. 2016;15:35–54.
Michaud TL, Rho YH, Shamliyan T, Kuntz KM, Choi HK. The comparative safety of tumor necrosis factors in rheumatoid arthritis: a meta-analysis update of 44 trials. Am J Med. 2014;127:1208–32.
Liu Y, Fan W, Chen H, Yu MX. Risk of breast cancer and total malignancies in rheumatoid arthritis patients undergoing TNF-α antagonist therapy: a meta-analysis of randomized control trials. Asian Pac J Cancer Prev. 2014;15:3403–10.
Moulis G, Sommet A, Béné J, et al. Cancer risk of anti-TNF-α at recommended doses in adult rheumatoid arthritis: a meta-analysis with intention to treat and per protocol analyse. PLoS ONE. 2012;7: e48991.
Seror R, Lafourcade A, De Rycke Y, et al. Risk of malignancy in rheumatoid arthritis patients initiating biologics: an historical propensity score matched cohort study within the French nationwide healthcare database. RMD Open. 2022;8: e002139.
Huss V, Bower H, Wadström H, Frisell T, Askling J. Short- and longer-term cancer risks with biologic and targeted synthetic disease-modifying antirheumatic drugs as used against rheumatoid arthritis in clinical practice. Rheumatology. 2022;61:1810–8.
Setoguchi S, Solomon DH, Weinblatt ME, et al. Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:2757–64.
Hellgren K, Di Giuseppe D, Smedby KE, et al. Lymphoma risks in patients with rheumatoid arthritis treated with biological drugs—a Swedish cohort study of risks by time, drug and lymphoma subtype. Rheumatology (Oxford). 2021;60:809–19.
Kawahito Y, Morinobu A, Kaneko Y, et al. Drug treatment algorithm and recommendations from the 2020 update of the Japan College of Rheumatology clinical practice guidelines for the management of rheumatoid arthritis-secondary publication. Mod Rheumatol. 2022;33:21–35.
Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82:3–18.
Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73:1108–23.
Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54:692–701.
Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–7.
Tanaka Y. Recent progress in treatments of rheumatoid arthritis: an overview of developments in biologics and small molecules, and remaining unmet needs. Rheumatology. 2021;60:12–20.
Foundation JHPF. Adult smoking rate (Ministry of Health, Labour and Welfare National Health and Nutrition Survey). 2020. https://www.health-net.or.jp/tobacco/statistics/kokumin_kenkou_eiyou_report.html. Accessed 19 January 2024.
Chatzidionysiou K, di Giuseppe D, Soderling J, Catrina A, Askling J. Risk of lung cancer in rheumatoid arthritis and in relation to autoantibody positivity and smoking. RMD Open. 2022;8: e002465.
Khurana R, Wolf R, Berney S, Caldito G, Hayat S, Berney SM. Risk of development of lung cancer is increased in patients with rheumatoid arthritis: a large case control study in US veterans. J Rheumatol. 2008;35:1704–8.
Beydon M, Pinto S, De Rycke Y, et al. Risk of cancer for patients with rheumatoid arthritis versus general population: a national claims database cohort study. Lancet Reg Health Eur. 2023;35:100768.
Wang HL, Zhou YM, Zhu GZ, Yang Z, Hua BJ. Malignancy as a comorbidity in rheumatic diseases: a retrospective hospital-based study. Clin Rheumatol. 2018;37:81–5.
Huang WK, Chiou MJ, Kuo CF, Lin YC, Yu KH, See LC. No overall increased risk of cancer in patients with rheumatoid arthritis: a nationwide dynamic cohort study in Taiwan. Rheumatol Int. 2014;34:1379–86.
Ko KM, Moon SJ. Prevalence, incidence, and risk factors of malignancy in patients with rheumatoid arthritis: a nationwide cohort study from Korea. Korean J Intern Med. 2023;38:113–24.
Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–26.
Acknowledgements
The authors are grateful to Masato Hoshi and Michika Mochizuki for their contributions to this work.
Medical Writing, Editorial, and Other Assistance
Medical writing support, under the direction of the authors, was provided by Karen Thompson, PhD, and Maisie Camm, BSc, CMC Connect, a division of IPG Health Medical Communications, and was funded by Pfizer, New York, NY, USA in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022;175:1298–304).
Funding
This study was sponsored by Pfizer Japan Inc. The publication costs and Rapid Service Fee were funded by Pfizer Japan Inc.
Author information
Authors and Affiliations
Contributions
Masayoshi Harigai, Eiichi Tanaka, Ryoko Sakai, Shigeyuki Toyoizumi, and Hisashi Yamanaka conceived or designed the study. Masayoshi Harigai, Eiichi Tanaka, and Hisashi Yamanaka were involved in patient recruitment. Eisuke Inoue and Ryoko Sakai performed the data analysis, Masayoshi Harigai, Eiichi Tanaka, Ryoko Sakai, Naohiro Sugitani, Shigeyuki Toyoizumi, Naonobu Sugiyama, and Hisashi Yamanaka were involved in data interpretation. All authors had access to the data, critically reviewed the manuscript, and approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of Interest
Masayoshi Harigai has received research grants from AbbVie Japan GK, Asahi Kasei Corp., Boehringer Ingelheim Japan, Inc., Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Nippon Shinyaku Co., Ltd., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., UCB Japan Co., Ltd., and Viatris Japan; speaker fees from AbbVie Japan GK, Asahi Kasei Corp., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., Gilead Sciences Inc., Janssen Pharmaceutical K.K., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nippon Shinyaku Co., Ltd., Ono Pharmaceutical Co., Ltd., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd, and UCB Japan; and has acted as a consultant for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb Co., Ltd., and Teijin Pharma. Masayoshi Harigai was an employee of Tokyo Women’s Medical University School of Medicine at the time of this study and is now employed at Sanno Hospital and International University of Health and Welfare. Eiichi Tanaka is a member of the speakers’ bureau for AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., GlaxoSmithKline K.K., Janssen Pharmaceutical K.K., Kyowa Pharma Chemical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Pfizer Japan Inc, Takeda Pharmaceutical Co., Ltd., and Teijin Pharma Ltd; has received lecture fees or consulting fees from AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., Gilead Sciences, Inc., GlaxoSmithKline K.K., Janssen Pharmaceutical K.K., Kyowa Pharma Chemical Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Pfizer Japan Inc, Takeda Pharmaceutical Co., Ltd., Teijin Pharma Ltd, and Viatris Inc; and has received research funding from Pfizer Inc and UCB Japan Co. Ltd. Eisuke Inoue is a member of the speakers’ bureau for Bristol Myers Squibb and Pfizer Japan Inc, and has received consulting fees from Nippontect Systems Co., Ltd. Ryoko Sakai is a member of the speakers’ bureau for Bristol Myers Squibb. Naohiro Sugitani declares that they have no competing interests. Shigeyuki Toyoizumi is an employee of Pfizer R&D Japan. Naonobu Sugiyama is an employee and shareholder of Pfizer Japan Inc. Hisashi Yamanaka is a member of the speakers’ bureau for Teijin Pharma Ltd and YLBio, and has received speaker or consultant fee from Astellas, Bristol Myers Squibb, CorEvitas, LLC, Eisai, Pfizer, Mitsubishi Tanabe, Teijin Pharma, and YLBio.
Ethical Approval
Comprehensive patient consent was obtained at enrollment into the IORRA patient registry [5], and consent for data to be used for studies involving the IORRA patient registry was obtained at each survey. Informed consent for this specific study was not required, although an opt-out approach to consent was used. The final protocol, any amendments, and informed consent documentation were approved by the IORRA committee (reference number: 2922-R16).
Additional information
Masayoshi Harigai’s affiliation has changed since the time of this study.
Prior Presentation: Some of the data in this manuscript were presented at EULAR 2021, 2–5 June, virtual congress (Harigai et al., Ann Rheum Dis. 2021; 80 [Table 1]).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Harigai, M., Tanaka, E., Inoue, E. et al. Incidence of Malignancies and the Association with Biological Disease-Modifying Antirheumatic Drugs in Japanese Patients with Rheumatoid Arthritis: A Time-Dependent Analysis from the IORRA Patient Registry. Rheumatol Ther (2024). https://doi.org/10.1007/s40744-024-00689-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40744-024-00689-8